Macadamia Nut Bio-Waste: An Agricultural Waste with Potential to Be Used as Carbon Support Material in Fuel Cell Applications
Abstract
:1. Introduction
2. Types of Nuts and Their Profiles
2.1. Macadamia Nuts
2.2. Walnut
2.3. Cashew
2.4. Peanut
3. Nuts Derived for Nanomaterials Catalysis
4. Metal Electrocatalysts and Their Impacts
4.1. Oxygen Reduction Reaction (ORR)
4.2. Methanol Oxidation Reaction (MOR)
5. Difficulties, Prospects, and Views
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosales Martinez, A.; Rodríguez-García, I.; López-Martínez, J.L. Green reductive regioselective opening of epoxides: A green chemistry laboratory experiment. J. Chem. Educ. 2022, 99, 2710–2714. [Google Scholar] [CrossRef]
- Lim, J.S.; Li, C.; Van Fan, Y.; Klemeš, J.J. How circular economy and green technology can address Sustainable Development Goals? J. Clean. Prod. 2022, 333, 130161. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Yu, J.; Song, B.; Feng, H.; Shen, M.; Zhang, H.; Zou, J.; Zeng, G.; Tang, L.; et al. Waste valorization: Transforming the fishbone biowaste into biochar as an efficient persulfate catalyst for degradation of organic pollutant. J. Clean. Prod. 2021, 291, 125225. [Google Scholar] [CrossRef]
- Goodman, B.A. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Ashrafi, G.; Nasrollahzadeh, M.; Jaleh, B.; Sajjadi, M.; Ghafuri, H. Biowaste-and nature-derived (nano) materials: Biosynthesis, stability and environmental applications. Adv. Colloid Interface Sci. 2022, 301, 102599. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Sahu, A.; Shukla, P.; Rai, A.; Singh, M.; Rai, V.K. Bio-inspired unprecedented synthesis of reduced graphene oxide: A catalytic probe for electro-/chemical reduction of nitro groups in an aqueous medium. New J. Chem. 2018, 42, 2067–2073. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Cai, Y.; Wang, S.; Hu, B.; Li, B.; Ding, X.; Zhuang, L.; Wang, X. Application of covalent organic frameworks and metal–organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies. Carbon Res. 2023, 2, 8. [Google Scholar] [CrossRef]
- Borah, N.; Tamuly, C. Emerging nano photo-catalysts for degradation of paracetamol and its prospective: A short review. Int. J. Environ. Anal. Chem. 2023, 1–16. [Google Scholar] [CrossRef]
- Orooji, Y.; Han, N.; Nezafat, Z.; Shafiei, N.; Shen, Z.; Nasrollahzadeh, M.; Karimi-Maleh, H.; Luque, R.; Bokhari, A.; Klemeš, J.J. Valorisation of nuts biowaste: Prospects in sustainable bio (nano) catalysts and environmental applications. J. Clean. Prod. 2022, 347, 131220. [Google Scholar] [CrossRef]
- Van Biert, L.; Visser, K. Fuel cells systems for sustainable ships. In Sustainable Energy Systems on Ships; Elsevier: Amsterdam, The Netherlands, 2022; pp. 81–121. [Google Scholar]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Erixno, O. Solid Oxide Fuel Cell-Based Polygeneration Systems in Residential Applications: A Review of Technology, Energy Planning and Guidelines for Optimizing the Design. Processes 2022, 10, 2126. [Google Scholar] [CrossRef]
- Sajid, A.; Pervaiz, E.; Ali, H.; Noor, T.; Baig, M.M. A perspective on development of fuel cell materials: Electrodes and electrolyte. Int. J. Energy Res. 2022, 46, 6953–6988. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct methanol fuel cells system—A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Al Labadidi, M.; Hamada, A.T.; Orhan, M.F. Design and Utilization of a Direct Methanol Fuel Cell. Membranes 2022, 12, 1266. [Google Scholar] [CrossRef] [PubMed]
- Chibac-Scutaru, A.L.; Coseri, S. Advances in the use of cellulose-based proton exchange membranes in fuel cell technology: A review. Int. J. Biol. Macromol. 2023, 247, 125810. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Liu, X.; Hong, H.; Zhang, H.; Cao, Y.; Qu, W.; Jin, H. Solar methanol by hybridizing natural gas chemical looping reforming with solar heat. Appl. Energy 2020, 277, 115521. [Google Scholar] [CrossRef]
- Akin, M.; Erduran, V.; Altuner, E.E.; Timuralp, C.; Isik, I.; Şen, F. Fundamentals of alcohol fuel cells. In Nanomaterials for Direct Alcohol Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–94. [Google Scholar]
- Güler, S.; Yavaş, A.; Mustafov, S.D.; Şen, F. The material development and characterization of direct alcohol fuel cells. In Nanomaterials for Direct Alcohol Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–73. [Google Scholar]
- Acres, G.J. Recent advances in fuel cell technology and its applications. J. Power Sources 2001, 100, 60–66. [Google Scholar] [CrossRef]
- Ren, X.; Wilson, M.S.; Gottesfeld, S. High performance direct methanol polymer electrolyte fuel cells. J. Electrochem. Soc. 1996, 143, L12. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the future with liquid sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef]
- Kim, J.; Henao, C.A.; Johnson, T.A.; Dedrick, D.E.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Methanol production from CO2 using solar-thermal energy: Process development and techno-economic analysis. Energy Environ. Sci. 2011, 4, 3122–3132. [Google Scholar] [CrossRef]
- Bandason, W.; Parwada, C.; Mushunje, A. Macadamia nuts (Macadamia intergrifolia) value chain and technical efficiency among the small-scale farmers in Zimbabwe. Res. World Agric. Econ. 2022, 3, 700. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Li, H.; Shi, Z.; Kan, H. Preparation and properties of hydrochars from macadamia nut shell via hydrothermal carbonization. R. Soc. Open Sci. 2018, 5, 181126. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, R.; Zhao, J.; Fan, Z. Advanced carbon materials with different spatial dimensions for supercapacitors. Nano Mater. Sci. 2021, 3, 241–267. [Google Scholar] [CrossRef]
- Dao, T.M.; Le Luu, T. Synthesis of activated carbon from macadamia nutshells activated by H2SO4 and K2CO3 for methylene blue removal in water. Bioresour. Technol. Rep. 2020, 12, 100583. [Google Scholar] [CrossRef]
- Wongcharee, S.; Aravinthan, V.; Erdei, L. Mesoporous activated carbon-zeolite composite prepared from waste macadamia nut shell and synthetic faujasite. Chin. J. Chem. Eng. 2019, 27, 226–236. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.; Bandoch, G.F.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Chang, Y.; Ren, Y.; Zhu, L.; Li, Y.; Li, T.; Ren, B. Preparation of macadamia nut shell porous carbon and its electrochemical performance as cathode material for lithium–sulfur batteries. Electrochim. Acta 2022, 420, 140454. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, K.; Zhou, W.; Zhu, Y.; He, Y.; Chen, H. Graphene-like carbon derived from macadamia nut shells for high-performance supercapacitor. Russ. J. Electrochem. 2019, 55, 242–246. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.; Cruickshank, J. Performance and modelling of a direct methanol solid polymer electrolyte fuel cell. J. Power Sources 1997, 65, 159–171. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.M.; Kramer, S.; Argyropoulos, P.; Sundmacher, K. Limiting current behaviour of the direct methanol fuel cell. Electrochim. Acta 1999, 45, 945–957. [Google Scholar] [CrossRef]
- Queirós, C.S.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefin. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Albatrni, H.; Qiblawey, H.; Al-Marri, M.J. Walnut shell based adsorbents: A review study on preparation, mechanism, and application. J. Water Process Eng. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Gondhalekar, S.C.; Shukla, S.R. Biosorption of cadmium metal ions on raw and chemically modified walnut shells. Environ. Prog. Sustain. Energy 2015, 34, 1613–1619. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, S.X.; Wang, Q.; Tan, M.X.; Wang, D.Y.; Yu, Z.Q.; Luo, S.H.; Zhang, Y.H.; Liu, X. Walnut septum-derived hierarchical porous carbon for ultra-high-performance supercapacitors. Rare Met. 2022, 41, 2280–2291. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Atasie, V.N.; Akintokun, P.O. Chemical composition and physicochemical properties of cashew nut (Anacardium occidentale) oil and cashew nut shell liquid. J. Agric. Food Environ. Sci. 2008, 2, 1–10. [Google Scholar]
- Ragupathy, S.; Raghu, K.; Prabu, P. Synthesis and characterization of TiO2 loaded cashew nut shell activated carbon and photocatalytic activity on BG and MB dyes under sunlight radiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 314–320. [Google Scholar] [CrossRef]
- Grosso, N.R.; Guzman, C.A. Chemical composition of aboriginal peanut (Arachis hypogaea L.) seeds from Peru. J. Agric. Food Chem. 1995, 43, 102–105. [Google Scholar] [CrossRef]
- Cai, N.; Cheng, H.; Jin, H.; Liu, H.; Zhang, P.; Wang, M. Porous carbon derived from cashew nut husk biomass waste for high-performance supercapacitors. J. Electroanal. Chem. 2020, 861, 113933. [Google Scholar] [CrossRef]
- Aigbodion, V.S. Explicit microstructure and electrical conductivity of epoxy/carbon nanotube and green silver nanoparticle enhanced hybrid dielectric composites. Nanocomposites 2021, 7, 35–43. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Wang, N. Recent Progress in Piezoelectric-Triboelectric Effects Coupled Nanogenerators. Nanomaterials 2023, 13, 385. [Google Scholar] [CrossRef]
- Jonnala, R.S.; Dunford, N.T.; Chenault, K. Nutritional composition of genetically modified peanut varieties. J. Food Sci. 2005, 70, S254–S256. [Google Scholar] [CrossRef]
- Khalil, J.K.; Chughtai, M.I.D. Chemical composition and nutritional quality of five peanut cultivars grown in Pakistan. Plant Foods Hum. Nutr. 1983, 33, 63–70. [Google Scholar] [CrossRef]
- Hoffpauir, C.L. Peanut composition, relation to processing and utilization. J. Agric. Food Chem. 1953, 1, 668–671. [Google Scholar] [CrossRef]
- Childs, E.; Abajian, A. Physico-chemical characterization of peanut hull as a potential fiber additive. J. Food Sci. 1976, 41, 1235–1236. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A Gen. 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, J.; Zhang, H.; Shao, Y.; Li, Y. Fabrication of magnetic carbon composites from peanut shells and its application as a heterogeneous Fenton catalyst in removal of methylene blue. Appl. Surf. Sci. 2015, 324, 490–498. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Zhuang, L.; Zhang, L.; Wang, K.; Yao, X. Defective carbons derived from macadamia nut shell biomass for efficient oxygen reduction and supercapacitors. ChemElectroChem 2018, 5, 1874–1879. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Kimura, H.; Wu, D.; Xie, X.; Yang, X.; Hou, C.; Sun, X.; Du, W. Facile synthesis, microstructure and electrochemical performance of peanut shell derived porous activated carbon/Co3O4 composite for hybrid supercapacitors. Ceram. Int. 2022, 48, 34576–34583. [Google Scholar] [CrossRef]
- Guo, P.Z.; Ji, Q.Q.; Zhang, L.L.; Zhao, S.Y.; Zhao, X.S. Preparation and characterization of peanut shell-based microporous carbons as electrode materials for supercapacitors. Acta Phys.-Chim. Sin. 2011, 27, 2836–2840. [Google Scholar]
- Ahsaine, H.A.; Zbair, M.; Anfar, Z.; Naciri, Y.; El Alem, N.; Ezahri, M.J.M.T.C. Cationic dyes adsorption onto high surface area ‘almond shell’activated carbon: Kinetics, equilibrium isotherms and surface statistical modeling. Mater. Today Chem. 2018, 8, 121–132. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Z.; Zhang, G.; Zhao, P. Excellent CO2 adsorption performance of nitrogen-doped waste biocarbon prepared with different activators. J. Clean. Prod. 2020, 264, 121645. [Google Scholar] [CrossRef]
- Kaya, N.; Arslan, F.; Yildiz Uzun, Z. Production and characterization of carbon-based adsorbents from waste lignocellulosic biomass: Their effectiveness in heavy metal removal. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 769–780. [Google Scholar] [CrossRef]
- Benítez, A.; Morales, J.; Caballero, Á. Pistachio shell-derived carbon activated with phosphoric acid: A more efficient procedure to improve the performance of Li–S batteries. Nanomaterials 2020, 10, 840. [Google Scholar] [CrossRef]
- Akçakal, Ö.; Şahin, M.; Erdem, M. Synthesis and characterization of high-quality activated carbons from hard-shelled agricultural wastes mixture by zinc chloride activation. Chem. Eng. Commun. 2019, 206, 888–897. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, K.; Zhou, W.; Zhu, Y.; Chen, H. Biomass carbon materials derived from macadamia nut shells for high-performance supercapacitors. Bull. Mater. Sci. 2018, 41, 138. [Google Scholar] [CrossRef]
- Xu, X.; Matsumura, Y.; Stenberg, J.; Antal, M.J. Carbon-catalyzed gasification of organic feedstocks in supercritical water. Ind. Eng. Chem. Res. 1996, 35, 2522–2530. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Hou, J. High-Efficiency Oxygen Reduction Reaction Revived from Walnut Shell. Molecules 2023, 28, 2072. [Google Scholar] [CrossRef]
- Sciarria, T.P.; de Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Goldfarb, J.L.; Adani, F. Metal-free activated biochar as an oxygen reduction reaction catalyst in single chamber microbial fuel cells. J. Power Sources 2020, 462, 228183. [Google Scholar] [CrossRef]
- Fan, L.; Chen, J.; Guo, J.; Jiang, X.; Jiang, W. Influence of manganese, iron and pyrolusite blending on the physiochemical properties and desulfurization activities of activated carbons from walnut shell. J. Anal. Appl. Pyrolysis 2013, 104, 353–360. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R. Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour. Technol. 2012, 119, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Bai, Q.; Ding, C. NiMoO4 nanoparticles embedded in nanoporous carbon nanosheets derived from peanut shells: Efficient electrocatalysts for urea oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125276. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M.; Bennour, F. Industrial application of photocatalysts prepared by hydrothermal and sol–gel methods. J. Ind. Eng. Chem. 2015, 21, 356–362. [Google Scholar] [CrossRef]
- Rechnia, P.; Malaika, A.; Krzyżyńska, B.; Kozłowski, M. Decomposition of methane in the presence of ethanol over activated carbon catalyst. Int. J. Hydrogen Energy 2012, 37, 14178–14186. [Google Scholar] [CrossRef]
- Ai, J.; Wu, X.; Wang, Y.; Zhang, D.; Zhang, H. Treatment of landfill leachate with combined biological and chemical processes: Changes in the dissolved organic matter and functional groups. Environ. Technol. 2019, 40, 2225–2231. [Google Scholar] [CrossRef]
- Guo, F.; Liang, S.; Jia, X.; Peng, K.; Jiang, X.; Qian, L. One-step synthesis of biochar-supported potassium-iron catalyst for catalytic cracking of biomass pyrolysis tar. Int. J. Hydrogen Energy 2020, 45, 16398–16408. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Zhou, W.; Ma, X.; Xu, J.; Cao, Y.; Chai, H. Anchoring CoFe2O4 nanospheres on two-dimensional microporous carbon from walnut shell as efficient multifunctional electrocatalyst. J. Solid State Chem. 2021, 299, 122106. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, Y.; Wu, H.R.; Lu, B.A.; Zhang, J.N. Highly Stable Pt-Based Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Materials 2023, 16, 2590. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Cheng, L.; Hu, W.; Xu, P.; Yang, Y.; Li, J.; Gao, F.; Yang, K.; Liu, S.; et al. Tuning the p-Orbital Electron Structure of s-Block Metal Ca Enables a High-Performance Electrocatalyst for Oxygen Reduction. Adv. Mater. 2021, 33, 2107103. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Minelli, S.; Vertova, A.; Minguzzi, A. Nanostructured Pt-based catalysts for oxygen reduction reaction in alkaline media. Curr. Opin. Electrochem. 2022, 36, 101166. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. 2021, 133, 17976–17996. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, 2006805. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xiang, J.; Guan, G.; Zhang, H.; Zhang, Y.; Zhang, K. Tunable high-performance microwave absorption of cobalt nanoparticles wrapped in N-self-doped carbon nanofibers at ultralow filler loadings. J. Alloys Compd. 2023, 933, 167808. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ma, Y.; Wu, H.; Cheng, L. Single-atom catalysts for CO oxidation, CO2 reduction, and O2 electrochemistry. J. Energy Chem. 2022, 65, 254–279. [Google Scholar] [CrossRef]
- Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Metal-based electrocatalysts for methanol electro-oxidation: Progress, opportunities, and challenges. Small 2021, 17, 1904126. [Google Scholar] [CrossRef]
- Gong, L.; Yang, Z.; Li, K.; Xing, W.; Liu, C.; Ge, J. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell. J. Energy Chem. 2018, 27, 1618–1628. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Javed, R.M.N.; Al-Othman, A.; Almomani, F. The novel contribution of non-noble metal catalysts for intensified carbon dioxide hydrogenation: Recent challenges and opportunities. Energy Convers. Manag. 2023, 279, 116755. [Google Scholar] [CrossRef]
- Rauf, M.; Wang, J.; Handschuh-Wang, S.; Zhou, Z.; Iqbal, W.; Khan, S.A.; Zhuang, L.; Ren, X.; Li, Y.; Sun, S. Highly stable N-containing polymer-based Fe/Nx/C electrocatalyst for alkaline anion exchange membrane fuel cell applications. Prog. Nat. Sci. Mater. Int. 2022, 32, 27–33. [Google Scholar] [CrossRef]
- Guo, W.; Wang, J.; Yan, H. Rhombic dodecahedral PtCo nanocrystals as a highly active electrocatalyst for methanol oxidation reaction. Funct. Mater. Lett. 2022, 15, 2251018. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-based catalysts on various carbon supports and conducting polymers for direct methanol fuel cell applications: A review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.S.; Natter, H.; Hempelmann, R.; Wippermann, K. Preparation and characterisation of Pt–Ru model electrodes for the direct methanol fuel cell. Electrochim. Acta 2003, 48, 3047–3051. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Shaari, N.; Saharuddin, T.S.T. Progress and major BARRIERS of nanocatalyst development in direct methanol fuel cell: A review. Int. J. Hydrogen Energy 2022, 47, 22114–22146. [Google Scholar] [CrossRef]
- Stevanović, S.I.; Jovanović, V.M. Graphene as Catalyst Support for the Reactions in Fuel Cells. Handb. Graphene Energy Healthc. Environ. Appl. 2019, 5, 339. [Google Scholar]
- Zhao, L.; Wang, Z.B.; Li, J.L.; Zhang, J.J.; Sui, X.L.; Zhang, L.M. Hybrid of carbon-supported Pt nanoparticles and three dimensional graphene aerogel as high stable electrocatalyst for methanol electrooxidation. Electrochim. Acta 2016, 189, 175–183. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Gou, L.; Wei, S.; Hou, X.; Wu, L. High-performance methanol electrolysis towards energy-saving hydrogen production: Using Cu2O-Cu decorated Ni2P nanoarray as bifunctional monolithic catalyst. Chem. Eng. J. 2023, 454, 140292. [Google Scholar] [CrossRef]
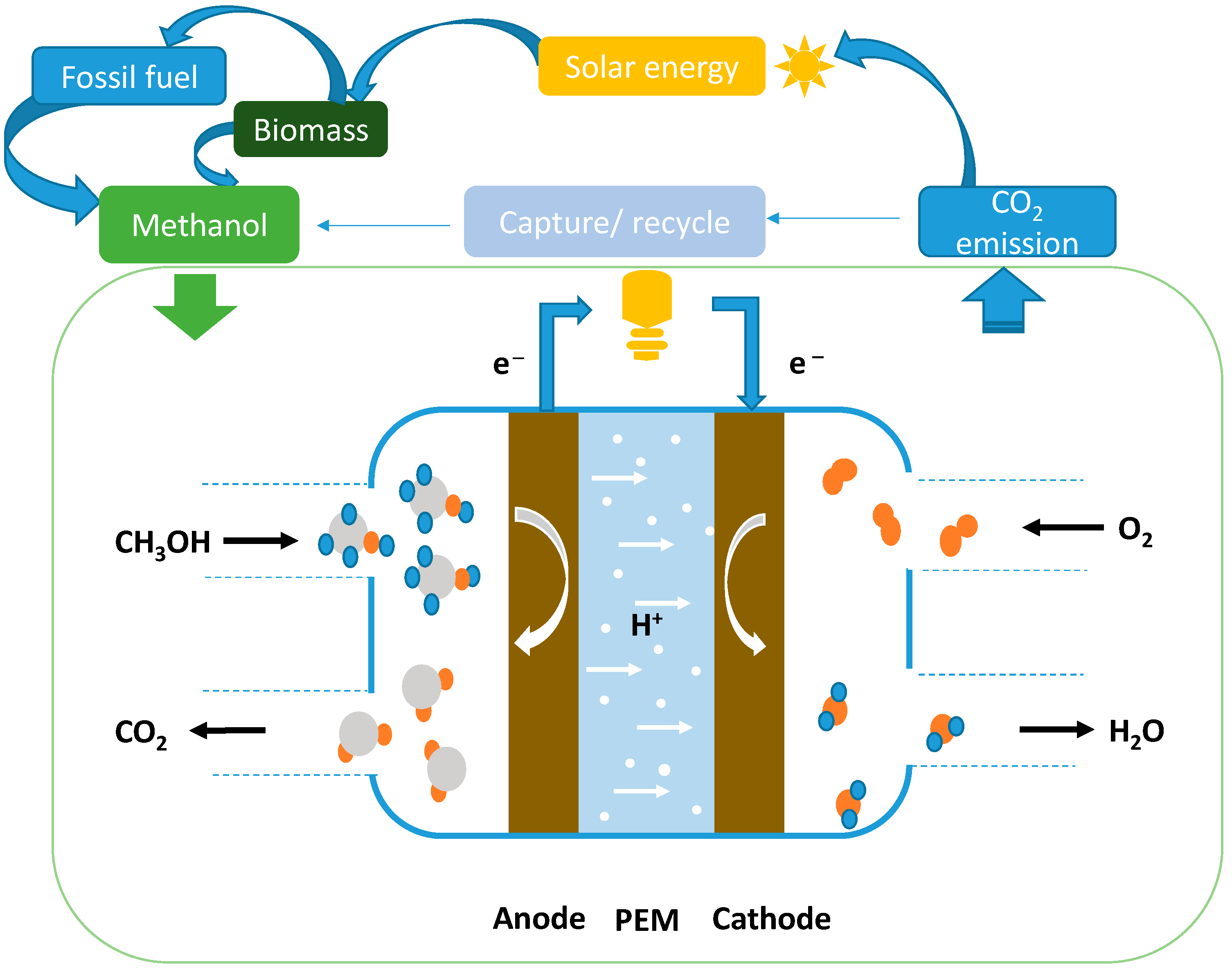


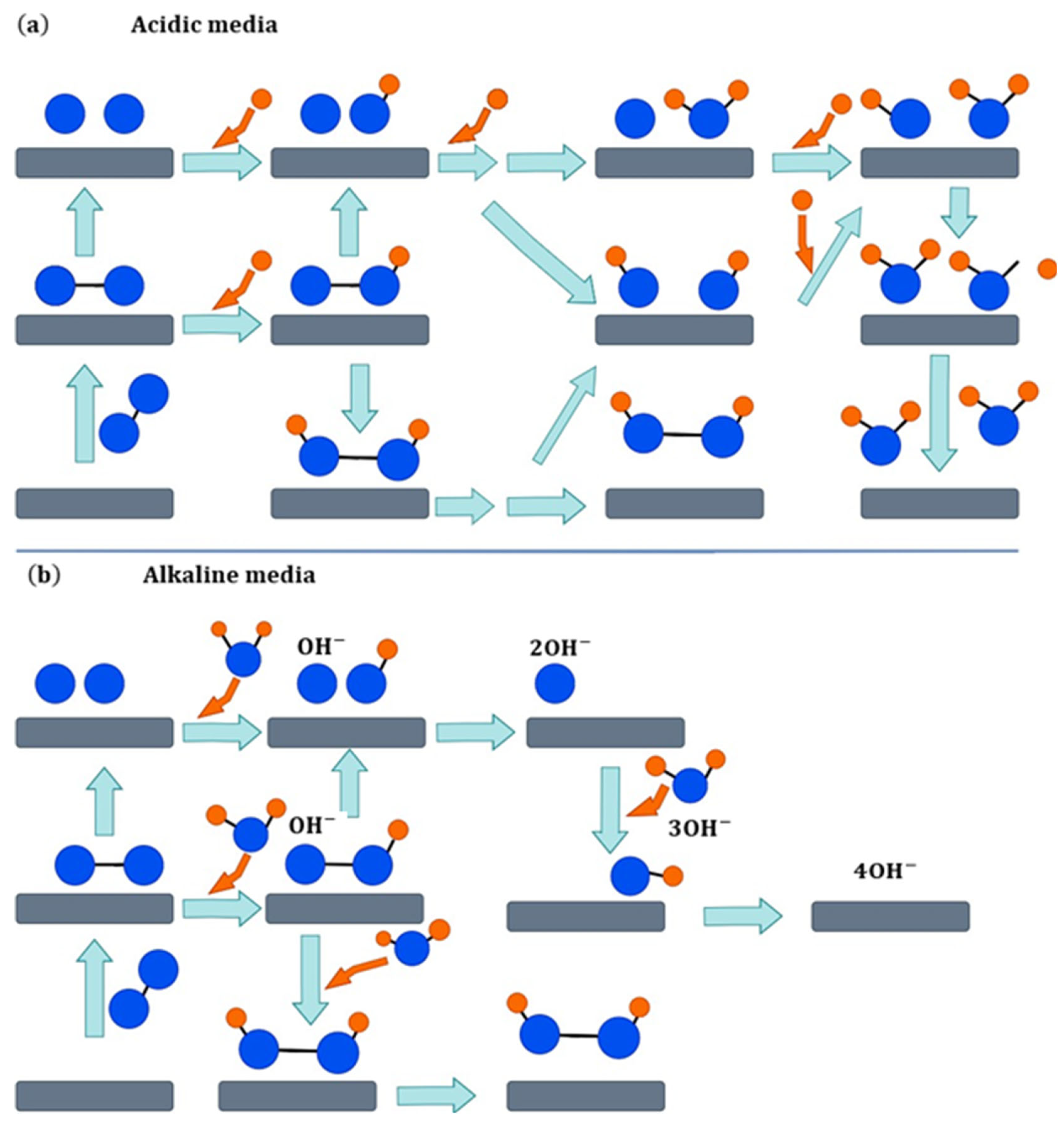
| No. | Origin | Derivative | Specific Surface Area (m2/g) | Pore Volumes (cm3/g) | Pore Size (nm) | Utilization | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Almond shell | Activated carbon (AC) | 1250 | 0.3885 | 2.47 | Treatment of methylene blue (MB) | [56] |
| 2 | Walnut shell | Biocarbon waste enriched with nitrogen. | 2353 | 1.25 | 2.11 | Adsorption of carbon dioxide | [57] |
| 3 | Hazelnut shell (HNs) | HNs char | 124.345 | - | - | Adsorption of HM | [58] |
| 4 | Pistachio shells | Activated carbon | 1345 | 0.67 | - | Lithium-Sulfur batteries | [59] |
| 5 | Apricot and peach stones, and almond shell | Activated carbon | 1125.73–2073.04 | 0.5498–1.0918 | - | Removal of MB | [56] |
| No. | Type of Nuts | Function of Nuts | Catalyst | Particle Size (nm) | Utilization | Reference |
|---|---|---|---|---|---|---|
| 1 | Macadamia nutshell | Provides charcoal | Activated carbon | - | Gasification of organic compounds in supercritical water | [61] |
| 2 | Walnut shell | Provides activated carbon | Activated carbon infused with manganese, iron oxides and pyrolusite. | - | Removal of sulfur | [62] |
| 3 | Pistachio hull | Provides biochar | Biochar | - | The use of ozone to treat resistant contaminants in water | [63] |
| 4 | Peanut shells | Origin of nanoporous carbon nanosheets as support | NiMoO4 NPs@nanoporous carbon nanosheets | 10 | Urea oxidation | [64] |
| 5 | Almond shell | Provides activated carbon as support | TiO2/ASAC | 18–24 | Degradation of total organic carbon | [65] |
| 6 | Hazelnut shells | Provides activated carbon with microporous | Activated carbon | - | Decomposition of methane | [66] |
| 7 | Cashew nutshell (CNS) | Provides activated carbon supports | TiO2/CNSAC | - | Photocatalyst for brilliant green and methylene blue removal | [67] |
| 8 | Pistachio shell | Provides activated carbon | Activated carbon | - | Removal of methyl orange and methylene blue | [68] |
| 9 | Peanut | Provides biochar as support | Biochar-supported Fe-K | - | Conversion of tar into syngas | [69] |
| 10 | Walnut shell | Provides activated carbon | CoFe2O4 supported on AC | - | Oxygen reduction reaction, oxygen evolution reaction, and hydrogen evolution reduction | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojapelo, N.A.; Seroka, N.S.; Khotseng, L. Macadamia Nut Bio-Waste: An Agricultural Waste with Potential to Be Used as Carbon Support Material in Fuel Cell Applications. Coatings 2023, 13, 1545. https://doi.org/10.3390/coatings13091545
Mojapelo NA, Seroka NS, Khotseng L. Macadamia Nut Bio-Waste: An Agricultural Waste with Potential to Be Used as Carbon Support Material in Fuel Cell Applications. Coatings. 2023; 13(9):1545. https://doi.org/10.3390/coatings13091545
Chicago/Turabian StyleMojapelo, Nakedi Albert, Ntalane Sello Seroka, and Lindiwe Khotseng. 2023. "Macadamia Nut Bio-Waste: An Agricultural Waste with Potential to Be Used as Carbon Support Material in Fuel Cell Applications" Coatings 13, no. 9: 1545. https://doi.org/10.3390/coatings13091545






