Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x Clementina) Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reception and Pretreatment of Raw Material
2.1.1. Preparation Coating Solution
2.1.2. Treatment and Storage of Clementine Slices
2.2. Color Measurement
2.3. Weight Loss, Moisture, and Headspace Gas Composition
2.4. Textural Analysis
2.5. Sensory Analysis
2.6. Chemical Analysis
2.6.1. Total Soluble Solids (TSS) and Titratable Acidity (TA)
2.6.2. Total Phenolic Content (TPC)
2.6.3. Total Flavonoid Content (TFC)
2.6.4. Determination of Organic Acids
2.6.5. Total Antioxidant Activity (TAA)
2.7. Microbiological Analysis
2.8. Statistical Analysis
3. Results
3.1. Color Measurements
3.2. Moisture, Weight Loss, and Headspace Gas Composition
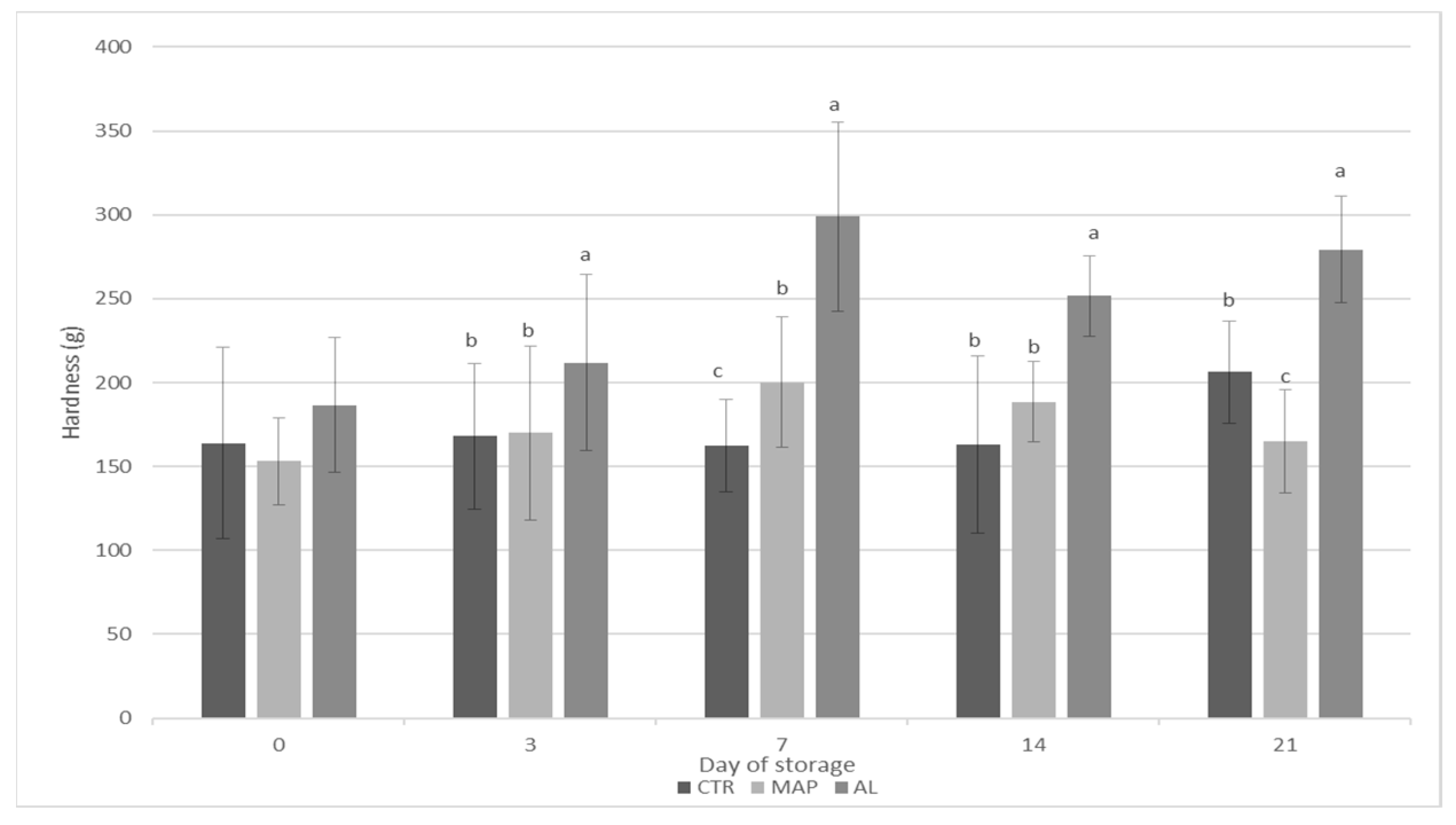
3.3. Textural and Sensory Analysis
3.4. Total Soluble Solids and Titratable Acidity
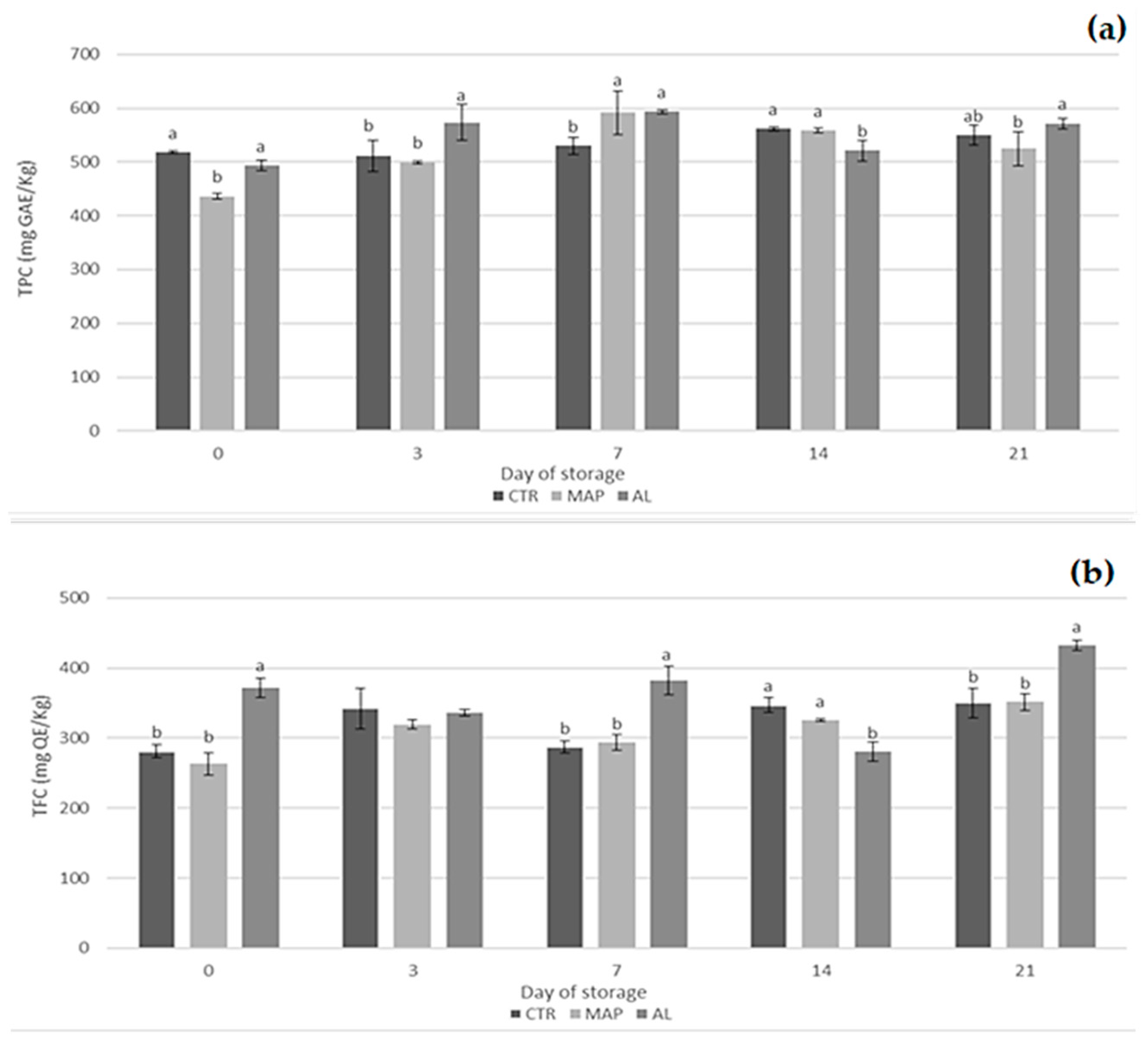
3.5. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), Organic Acid, and Total Antioxidant Activity (TAA)
3.6. Microbiological Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, T.; Burri, B.J. Potential Nutritional Benefits of Current Citrus Consumption. Agriculture 2013, 6, 170–187. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of flavonoids, vitamin C and antioxidant capacity in minimally processed citrus segments and juices during storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible Coatings for Fresh-Cut Fruits, Critical Reviews. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar]
- Zappia, A.; De Bruno, A.; Torino, R.; Piscopo, A.; Poiana, M. Influence of Light Exposure during Cold Storage of Minimally Processed Vegetables (Valeriana sp.). J. Food Qual. 2018, 2018, 4694793. [Google Scholar] [CrossRef]
- Rojas-Graru, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; Mchugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Celine, M.; Valerie, G.; Karine, G.; Sandrine, C.; Nathalie, G.; Stephane, G.; Sebastien, G. Consumer behaviour in the prediction of postharvest losses reduction for fresh strawberries packed in modified atmosphere packaging. Postharvest Biol. Technol. 2020, 163, 111–119. [Google Scholar]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer Science: New York, NY, USA, 2009; Volume 9. [Google Scholar]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Castro-Yobal, M.A. Evaluation of physicochemical properties of film-based alginate for food packing applications. e-Polymers 2021, 21, 82–95. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.; Anandakumar, S.; Hema, V.; Sinija, V. Development of active packaging film from sodium alginate/carboxymethyl cellulose containing shallot waste extracts for anti-browning of fresh-cut produce. Int. J. Biol. Macromol. 2021, 188, 790–799. [Google Scholar] [CrossRef]
- Zappia, A.; Spanti, A.; Princi, R.; Imeneo, V.; Piscopo, A. Evaluation of the Efficacy of Antioxidant Extract from Lemon By-Products on Preservation of Quality Attributes of Minimally Processed Radish (Raphanus sativus L.). Antioxidants 2023, 12, 235. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Chapter 10—Starch-Based Edible Films and Coatings: An Eco-friendly Alternative for Food Packaging. In Starches for Food Application, 1st ed.; Clerici, M.T.P.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 359–420. [Google Scholar]
- Alvarez, M.V.; Pérez-Gago, M.B.; Taberner, V.; Settier-Ramírez, L.; Martínez-Blay, V.; Palou, L. Postharvest Application of Novel Bio-Based Antifungal Composite Edible Coatings to Reduce Sour Rot and Quality Losses of ‘Valencia’ Oranges. Coatings 2023, 13, 1412. [Google Scholar] [CrossRef]
- Jurić, S.; Bureš, M.S.; Vlahoviček-Kahlina, K.; Stracenski, K.S.; Fruk, G.; Jalšenjak, N.; Bandić, L.M. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem. X 2023, 17, 100575. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Glicerina, V.; Siroli, L.; Betoret, E.; Canali, G.; Dalla Rosa, M.; Lanciotti, R.; Romani, S. Characterization and evaluation of the influence of an alginate, cocoa and a bilayer alginate–cocoa coating on the quality of fresh-cut oranges during storage. J. Sci. Food Agric. 2022, 102, 4454–4461. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1994. [Google Scholar]
- AOAC; CA. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Legua, P.; Forner, J.B.; Hernandez, F.C.A.; Forner-Giner, M.A. Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Sci. Hortic. 2014, 174, 60–64. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- Cohen, E.; Shalom, Y.; Rosenberger, I. Postharvest ethanol buildup and off-flavor in ‘Murcott’ tangerine fruits. J. Am. Soc. Hortic. Sci. 1990, 115, 775–778. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Application of pregelatinized corn starch and basil essential oil edible coating with cellulose nanofiber as Pickering emulsion agent to prevent quality-quantity loss of mandarin orange. Food Packag. Shelf Life 2023, 35, 101010. [Google Scholar] [CrossRef]
- Moshonas, M.G.; Shaw, P.E. Quantification of volatile constituents in mandarin juices and its use for comparison with orange juices by multivariate analysis. J. Agric. Food Chem. 1997, 45, 3968–3972. [Google Scholar] [CrossRef]
- Perez-Lopez, A.G.; Carbonell-Barrachina, A.A. Volatile odour components and sensory quality of fresh and processed mandarin juices. J. Sci. Food Agric. 2006, 86, 2404–2411. [Google Scholar] [CrossRef]
- Barboni, T.; Luro, F.; Chiaramonti, N.; Desjobert, J.M.; Muselli, A.; Costa, J. Volatile composition of hybrids Citrus juices by headspace solid phase microextraction/gas chromatography/mass spectrometry. Food Chem. 2009, 116, 382–390. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Shaw, P.E. Changes in volatile components of stored tangerines and other specialty citrus fruits with different coatings. J. Food Sci. 2002, 67, 1742–1745. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.-Y.; Guo, L.-X.; Kamran, H.M.; Sadka, A.; Liu, Y.-Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bellomo, S.E.; Fabroni, S.; Russo, G. Juice quality of two new mandarin-like hybrids (Citrus clementina Hort. ex Tan x Citrus sinensis L. Osbeck) containing anthocyanins. J. Agric. Food Chem. 2008, 56, 2074–2078. [Google Scholar] [CrossRef]
- Tietel, Z.; Plotto, A.; Fallik, E.; Lewinsohn, E.; Porat, R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2011, 91, 14–23. [Google Scholar] [CrossRef]
- Hijaz, F.; Gmitter, F.G.; Bai, J., Jr.; Baldwin, E.; Biotteau, A.; Leclair, C.; McCollum, T.G.; Plotto, A. Effect of fruit maturity on volatiles and sensory descriptors of four mandarin hybrids. J. Food Sci. 2020, 85, 1548–1564. [Google Scholar] [CrossRef]
- Banda, K.; Caleb, O.J.; Jacobs, K.; Opara, U.L. Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biol. Tech. 2015, 109, 97–105. [Google Scholar] [CrossRef]
- Kader, A.A.; Ben-Yehoshua, S. Effects of superatmospheric oxygen levels on postharvest physiology and quality of fresh fruits and vegetables. Postharvest Biol. Tech. 2000, 20, 1–13. [Google Scholar] [CrossRef]
- Ziogas, V.; Bravos, N.; Hussain, S.B. Preharvest foliar application of Si–Ca-based biostimulant affects postharvest quality and shelf-life of clementine mandarin (Citrus clementina Hort. Ex Tan). Horticulturae 2022, 8, 996. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Harats, D.; Chevion, S.; Nahir, M.; Norman, Y.; Sagee, O.; Berry, E.M. Citrus fruit supplementation reduces lipoprotein oxidation in young men ingesting a diet high in saturated fat: Presumptive evidence for an interaction between vitamins C and E in vivo. J. Clin. Nutr. 1998, 67, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; López-Jaén, A.B.; De La Mano-Hernández, A.; Sentandreu, E.; Simó-Jordá, R.; Valls-Bellés, V. Oxidative markers in children with severe obesity following low-calorie diets supplemented with mandarin juice. Acta Paediatr. 2010, 99, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Loaiza-Velarde, J.; Bonfanti, A.; Saltveit, M.E. Early wound- and ethyl-induced changes in pheyl propanoid metabolism in harvested lettuce. J. Am. Soc. Hortic. Sci. 1997, 122, 399–404. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; Princi, M.P.; De Bruno, A.; Araniti, F.; Lupini, A.; Abenavoli, M.R.; Poiana, M. Quality of shredded carrots minimally processed by different dipping solutions. J. Food Sci. Technol. 2019, 56, 2584–2593. [Google Scholar] [CrossRef]
- Alos, E.; Rodrigo, M.J.; Zacarías, L. Differential transcriptional regulation of L-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta 2004, 239, 1113–1128. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, S.; Xu, R.; Tu, S.; Tu, K. Interactions among chilling tolerance, sucrose degradation and organic acid metabolism in UV-C-irradiated peach fruit during postharvest cold storage. Acta Physiol. Plant. 2019, 41, 79. [Google Scholar] [CrossRef]


| Parameter | Sample | Time (Days) | Sig. | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | |||
| L* | CTRL | 50.19 ± 1.69 | 50.72 ± 1.42 | 53.25 ± 1.22 | 52.86 ± 2.65 | 51.71 ± 1.67 | n.s. |
| MAP | 48.97 ± 1.84 | 50.94 ± 1.54 | 52.31 ± 1.98 | 50.68 ± 2.03 | 52.51 ± 1.88 | n.s. | |
| AL | 54.80 ± 1.33 | 52.62 ± 1.69 | 53.84 ± 1.16 | 52.57 ± 1.54 | 53.92 ± 2.10 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| a* | CTRL | 8.30 ± 1.21 b | 8.41 ± 1.27 b | 8.74 ± 1.01 | 8.85 ± 2.14 | 8.67 ± 1.32 | n.s. |
| MAP | 9.73 ± 1.00 a | 8.35 ± 1.58 b | 8.42 ± 1.18 | 8.43 ± 1.14 | 9.25 ± 1.48 | n.s. | |
| AL | 8.18 ± 1.3 b | 9.27 + 1.01 a | 8.97 ± 1.53 | 8.60 ± 1.96 | 9.46 ± 1.56 | n.s. | |
| Sign. | * | * | n.s. | n.s. | n.s. | ||
| b* | CTRL | 19.48 ± 1.07 | 20.90 ± 1.54 | 19.78 ± 1.43 | 21.71 ± 97 | 20.21 ± 1.82 | n.s. |
| MAP | 19.66 ± 1.21 | 19.92 ± 1.36 | 21.23 ± 1.61 | 21.90 ± 2.15 | 21.83 ± 1.59 | n.s. | |
| AL | 20.27 ± 1.5 | 20.48 ± 1.41 | 21.63 ± 1.53 | 20.60 ± 2.63 | 21.39 ± 1.58 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Parameter | Sample | Time (Days) | Sig. | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | |||
| Moisture (g/100 g) | CTRL | 85.02 ± 0.55 B | 88.46 ± 2.00 B | 87.60 ± 1.90 AB | 83.2 ± 2.16 A | 85.59 ± 1.85 AB | * |
| MAP | 85.65 ± 1.50 | 86.10 ± 1.50 | 82.93 ± 1.16 | 83.3 ± 1.66 | 86.69 ± 0.98 | n.s. | |
| AL | 85.38 ± 2.00 | 85.56 ± 0.30 | 86.74 ± 1.97 | 85.8 ± 1.35 | 86.77 ± 1.23 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Weight Loss (g/100 g) | CTRL | - | 0.03 ± 0.01 A | 0.02 ± 0.01 A | 0.07 ± 0.02 B | 0.10 ± 0.03 B | ** |
| MAP | - | 0.03 ± 0.01 A | 0.02 ± 0.01 A | 0.08 ± 0.02 B | 0.10 ± 0.03 B | ** | |
| AL | - | 0.05 ± 0.02 A | 0.05 ± 0.01 A | 0.07 ± 0.01 AB | 0.10 ± 0.04 B | ** | |
| Sign. | n.s. | n.s. | n.s. | n.s. | |||
| O2 (%) | CTRL | 21.00 ± 0.00 aA | 14.5 ± 1.11 aBC | 14.3 ± 1.90 aBC | 7.1 ± 0.99 bB | 5.6 ± 0.71 bC | ** |
| MAP | 5.00 ± 0.00 bA | 3.4 ± 0.98 bAB | 1.8 ± 0.40 bA | 1.2 ± 0.43 cA | 1.1 ± 0.05 cA | ** | |
| AL | 21.00 ± 0.00 aA | 16.4 ± 0.43 aB | 13.3 ± 0.63 aBC | 8.25 ± 0.57 aC | 8.3 ± 0.68 aC | ** | |
| Sign. | ** | ** | ** | ** | ** | ||
| CO2 (%) | CTRL | 0.02 bC | 9.4 ± 1.37 B | 10.0 ± 2.30 bB | 13.0 ± 1.13 bB | 19.9 ± 0.14 aA | ** |
| MAP | 5.00 aC | 9.9 ± 0.76 B | 13.5 ± 1.56 aBC | 17.2 ± 2.3 aA | 15.8 ± 1.79 bBC | ** | |
| AL | 0.02 bC | 9.2 ± 0.43 B | 9.8 ± 0.81 bB | 14.9 ± 0.10 abA | 14.3 ± 0.49 bA | ** | |
| Sign. | ** | n.s. | * | ** | * | ||
| Parameter | Sample | Time (Days) | Sig. | |
|---|---|---|---|---|
| 0 | 21 | |||
| Sweet | CTRL | 6.80 ± 1.30 a | 4.00 ± 0.08 b | ** |
| MAP | 6.70 ± 1.20 a | 5.00 ± 0.60 a | ** | |
| AL | 5.50 ± 1.00 b | 5.00 ± 0.80 a | * | |
| Sign. | * | * | ||
| Bitter | CTRL | 5.70 ± 0.90 ab | 6.00 ± 1.20 ab | n.s. |
| MAP | 4.50 ± 1.00 b | 4.00 ± 1.30 a | n.s. | |
| AL | 6.20 ± 0.70 a | 7.00 ± 1.20 b | n.s. | |
| Sign. | * | ** | ||
| Fruity | CTRL | 6.70 ± 0.70 a | 6.00 ± 1.00 | n.s. |
| MAP | 6.70 ± 0.70 a | 6.00 ± 1.50 | n.s. | |
| AL | 5.00 ± 0.80 b | 6.00 ± 0.80 | * | |
| Sign. | * | n.s. | ||
| Citrusy | CTRL | 6.70 ± 0.70 a | 6.00 ± 1.20 ab | n.s. |
| MAP | 6.5 ± 0.50 a | 5.00 ± 0.80 a | ** | |
| AL | 5.30 ± 1.20 b | 7.00 ± 0.60 b | * | |
| Sign. | * | ** | ||
| Color | CTRL | 7.00 ± 0.06 b | 6.50 ± 1.30 b | n.s. |
| MAP | 6.50 ± 0.05 b | 6.00 ± 0.60 b | n.s. | |
| AL | 9.00 ±0.05 a | 8.00 ± 0.60 a | n.s. | |
| Sign. | ** | * | ||
| Turgidity | CTRL | 6.8 ± 1.1 a | 6.2 ± 0.9 a | n.s. |
| MAP | 7.2 ± 1.1 ab | 6 ± 1.00 a | n.s. | |
| AL | 8.0 ± 0.6 b | 7 ± 1.2 b | n.s. | |
| * | * | |||
| Overallacceptability | CTRL | 8.00 ± 0.50 a | 5.00 ± 0.50 b | ** |
| MAP | 8.00 ± 1.00 a | 6.00 ± 0.50 a | ** | |
| AL | 7.2 ± 0.50 b | 6.2 ± 0.50 a | ** | |
| Sign. | * | * | ||
| Parameter | Sample | Time (Days) | Sig. | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | |||
| SSC (°Bx) | CTRL | 12.1 ± 0.05 AB | 12.2 ± 0.17 aA | 10.0 ± 0.72 bC | 11.6 ± 0.1 aAB | 11.5 ± 0.38 B | ** |
| MAP | 11.9 ± 0.4 A | 12.8 ± 0.1 aA | 11.5 ± 0.71 aA | 11.8 ± 0.07 bA | 12.3 ± 0.98 A | n.s. | |
| AL | 11.9 ±0.13 A | 12.2 ± 0.23 aA | 12.6 ± 0.78 aA | 12.2 ± 0.07 cA | 11.9 ± 0.06 A | n.s. | |
| Sig. | n.s. | n.s. | * | ** | n.s. | ||
| Titratable Acidity (%) | CTRL | 0.59 ± 0.03 aA | 0.54 ± 0.02 aA | 0.53 ± 0.04 aA | 0.50 ± 0.05 aB | 0.51 ±0.06 aAB | * |
| MAP | 0.57 ± 0.1 a | 0.57 ± 0.08 abA | 0.54 ± 0.08 aA | 0.52 ± 0.01 aA | 0.52 ± 0.05 aA | n.s. | |
| AL | 0.54 ± 0.03 aA | 0.54 ± 0.01 bA | 0.56 ± 0.05 aA | 0.54 ±0.03 aA | 0.54 ±0.01 aA | n.s. | |
| Sign. | n.s. | * | n.s. | n.s. | n.s. | ||
| Parameter | Sample | Time (Days) | Sig. | ||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | |||
| Oxalic Acid (mg L−1) | CTRL | 190.24 ± 5.70 A | 150.59 ± 2.70 B | 161.88 ± 2.70 B | 152.86 ± 1.60 B | 204.30 ± 2.80 A | ** |
| MAP | 227.37 ± 8.20 A | 152.11 ± 9.40 B | 247.45 ± 7.20 A | 118.81 ± 1.80 B | 208.89 ± 2.80 AB | ** | |
| AL | 150.58 ± 6.30 | 147.59 ± 5.30 | 186.93 ± 5.80 | 186.24 ± 90 | 211.57 ± 12.40 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Citric Acid (mg L−1) | CTRL | 4818.34 ± 83.40 | 4478.34 ± 28.70 | 4925.01 ± 96.70 | 4430.29 ± 43.40 | 4489.26 ± 41.20 | n.s. |
| MAP | 5502.17 ± 39.70 | 5061.70 ± 47.00 | 4367.87 ± 48.90 | 4996.61 ± 72.80 | 4444.42 ± 76.30 | n.s. | |
| AL | 5005.19 ± 20.70 | 4235.22 ± 59.40 | 4997.44 ± 43.40 | 4632.13 ± 72.00 | 4860.58 ± 64.50 | n.s. | |
| Sign. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Malic Acid (mg L−1) | CTRL | 861.98 ± 42.80 A | 825.72 ± 62.80 A | 662.36 ± 54.00 abAB | 538.78 ± 42.90 B | 681.32 ± 44.70 AB | ** |
| MAP | 615.39 ± 38.10 | 625.48 ± 58.00 | 504.96 ± 46.10 b | 481.06 ± 51.90 | 498.81 ± 58.70 | n.s. | |
| AL | 624.55 ± 54.30 AB | 713.00 ± 49.00 | 716.35 ± 68.70 aA | 483.06 ± 61.40 B | 625.54 ± 67.10 AB | * | |
| Sign. | n.s. | n.s. | * | n.s. | n.s. | ||
| Ascorbic Acid (mg L−1) | CTRL | 153.64 ± 19.25 A | 101.23 ± 25.30 bAB | 89.04 ± 5.88 cB | 97.19 ± 1.65 bAB | 90.46 ± 1.00 bB | ** |
| MAP | 118.74 ± 6.10 | 110.71 ± 4.30 ab | 133.20 ± 0.50 b | 121.99 ± 22.30 ab | 127.93 ± 23.60 ab | n.s. | |
| AL | 124.49 ± 18.80 B | 147.18 ± 35.50 aAB | 169.82 ± 14.10 aA | 168.56 ± 4.70 aA | 167.85 ± 8.40 aA | ** | |
| Sign. | n.s. | * | ** | * | * | ||
| ABTS (mM Trolox kg−1) | CTRL | 1.33 ±0.20 aB | 1.80 ±0.05 aA | 1.31 ± 0.23 aB | 1.24 ± 0.05 aB | 1.42 ± 0.06 bB | ** |
| MAP | 1.05 ±0.08 bA | 1.21 ±0.25 bA | 1.46 ± 0.35 aA | 1.30 ± 0.04 aA | 1.39 ±0.12 bA | n.s. | |
| AL | 1.26 ±0.12 abB | 1.97 ± 0.15 aA | 1.27 ± 0.17 aB | 1.32 ± 0.08 aB | 1.76 ±0.17 aA | ** | |
| Sign. | * | ** | n.s. | n.s. | ** | ||
| DPPH (mM Trolox kg−1) | CTRL | 0.43 ± 0.01 aA | 0.39 ± 0.02 cAB | 0.39 ± 0.02 abAB | 0.32 ± 0.01 cB | 0.40 ± 0.08 aAB | * |
| MAP | 0.44 ± 0.05 aAB | 0.47 ± 0.01 aA | 0.31 ± 0.07 bB | 0.36 ± 0.02 bAB | 0.43 ± 0.1 aAB | * | |
| AL | 0.43 ± 0.04 aB | 0.44 ± 0.01 bB | 0.41 ± 0.01 aB | 0.41 ± 0.01 aB | 0.50 ± 0.03 aA | ** | |
| Sign. | n.s. | ** | * | ** | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boninsegna, M.A.; De Bruno, A.; Piscopo, A. Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x Clementina) Fruits. Coatings 2023, 13, 1562. https://doi.org/10.3390/coatings13091562
Boninsegna MA, De Bruno A, Piscopo A. Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x Clementina) Fruits. Coatings. 2023; 13(9):1562. https://doi.org/10.3390/coatings13091562
Chicago/Turabian StyleBoninsegna, Miriam Arianna, Alessandra De Bruno, and Amalia Piscopo. 2023. "Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x Clementina) Fruits" Coatings 13, no. 9: 1562. https://doi.org/10.3390/coatings13091562
APA StyleBoninsegna, M. A., De Bruno, A., & Piscopo, A. (2023). Quality Evaluation of Ready-to-Eat Coated Clementine (Citrus x Clementina) Fruits. Coatings, 13(9), 1562. https://doi.org/10.3390/coatings13091562








