A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extracting Amylose (AM) from Barley
2.3. Extracting Nanocellulose Fibers from Sugar Beet Pulp
2.4. Creating the Biocomposite Films
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Thickness and Moisture Content
2.7. Swelling Index (SI) and Water Solubility (WS)
2.8. Mechanical Properties
2.9. Scanning Electron Microscopy (SEM)
2.10. Light Transmittance and Opacity
2.11. Thermogravimetric Analysis (TGA)
2.12. Wide Angle X-ray Scattering(WAXS)
2.13. Permeability
2.14. Degradation Studies
2.15. Statistical Analysis
3. Results and Discussions
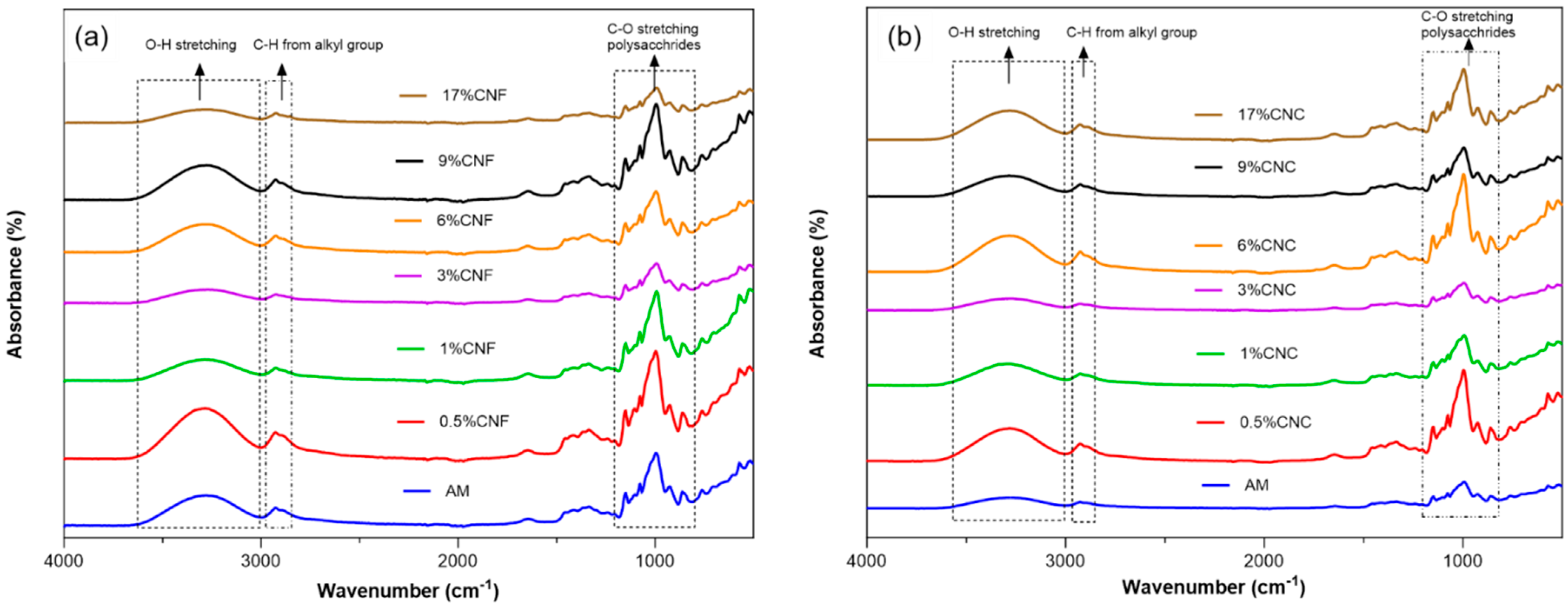
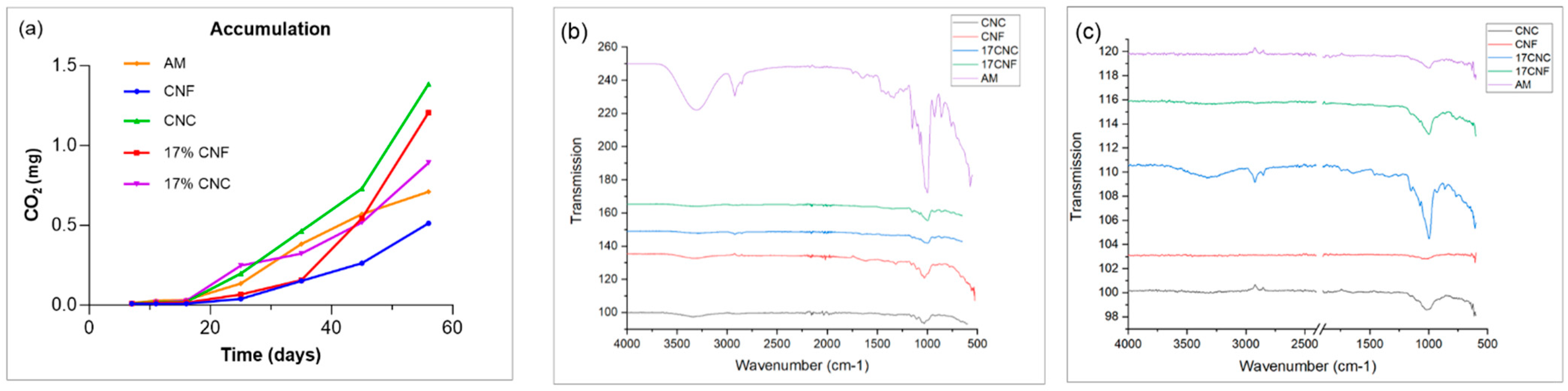
3.1. FTIR
3.2. Thickness and Moisture
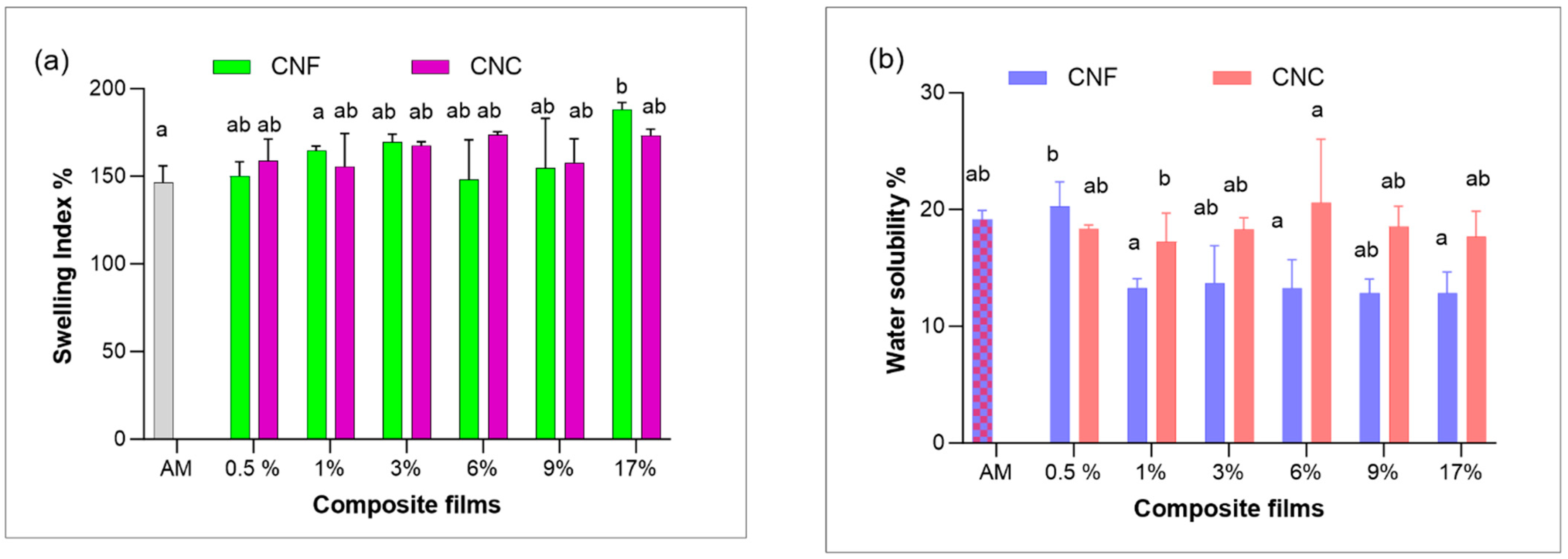
3.3. Swelling Index (SI) and Water Solubility(SW)
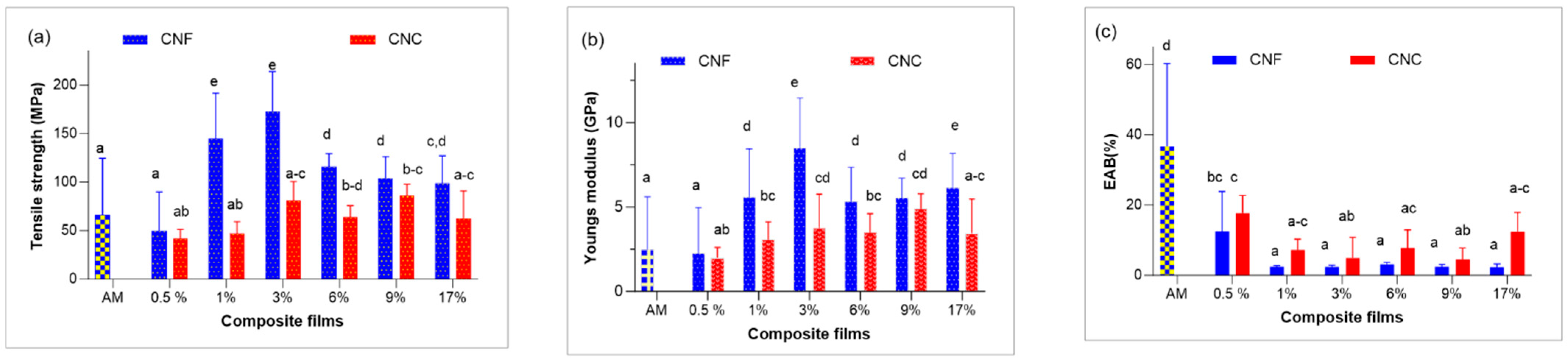
3.4. Mechanical Properties
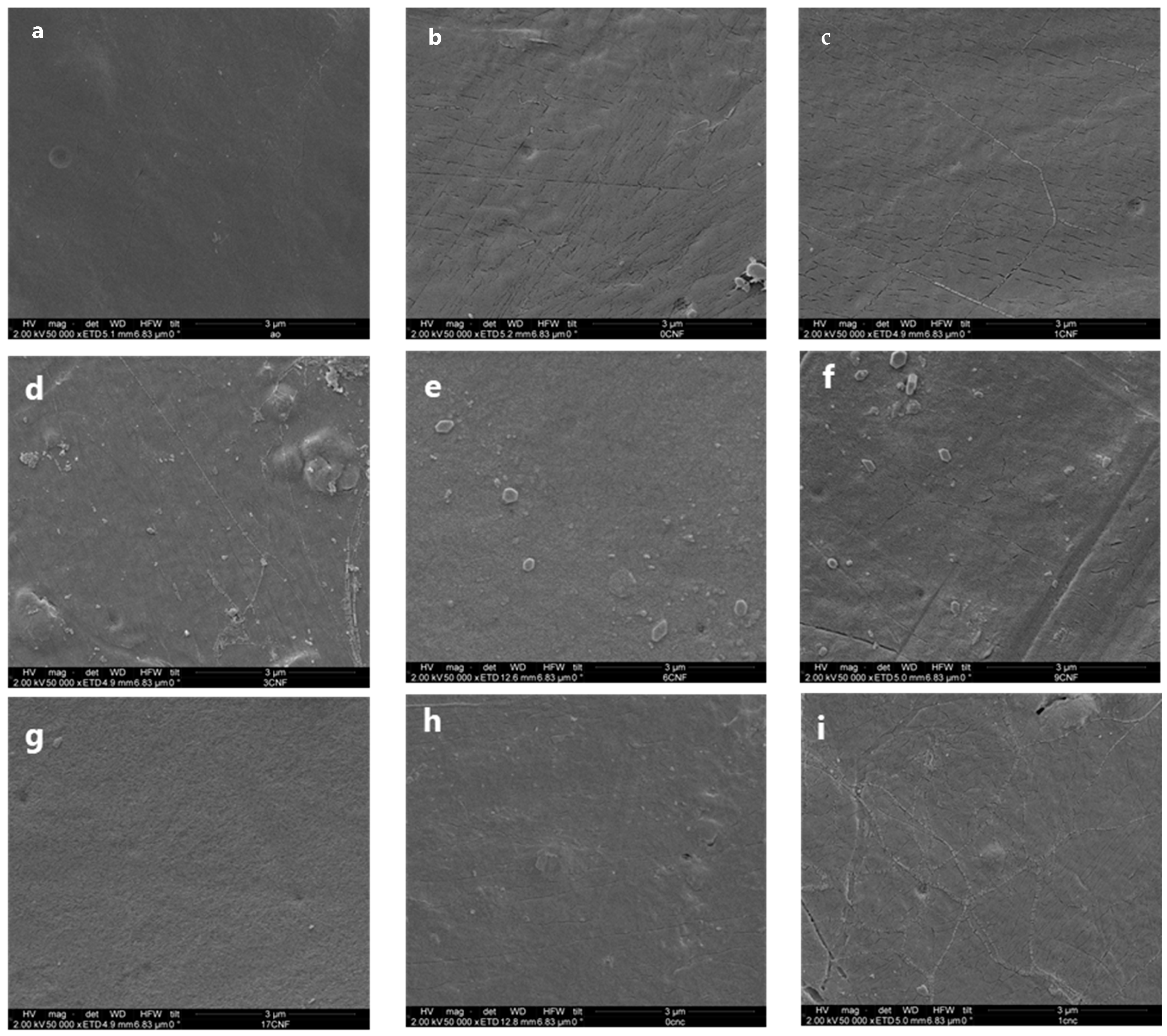
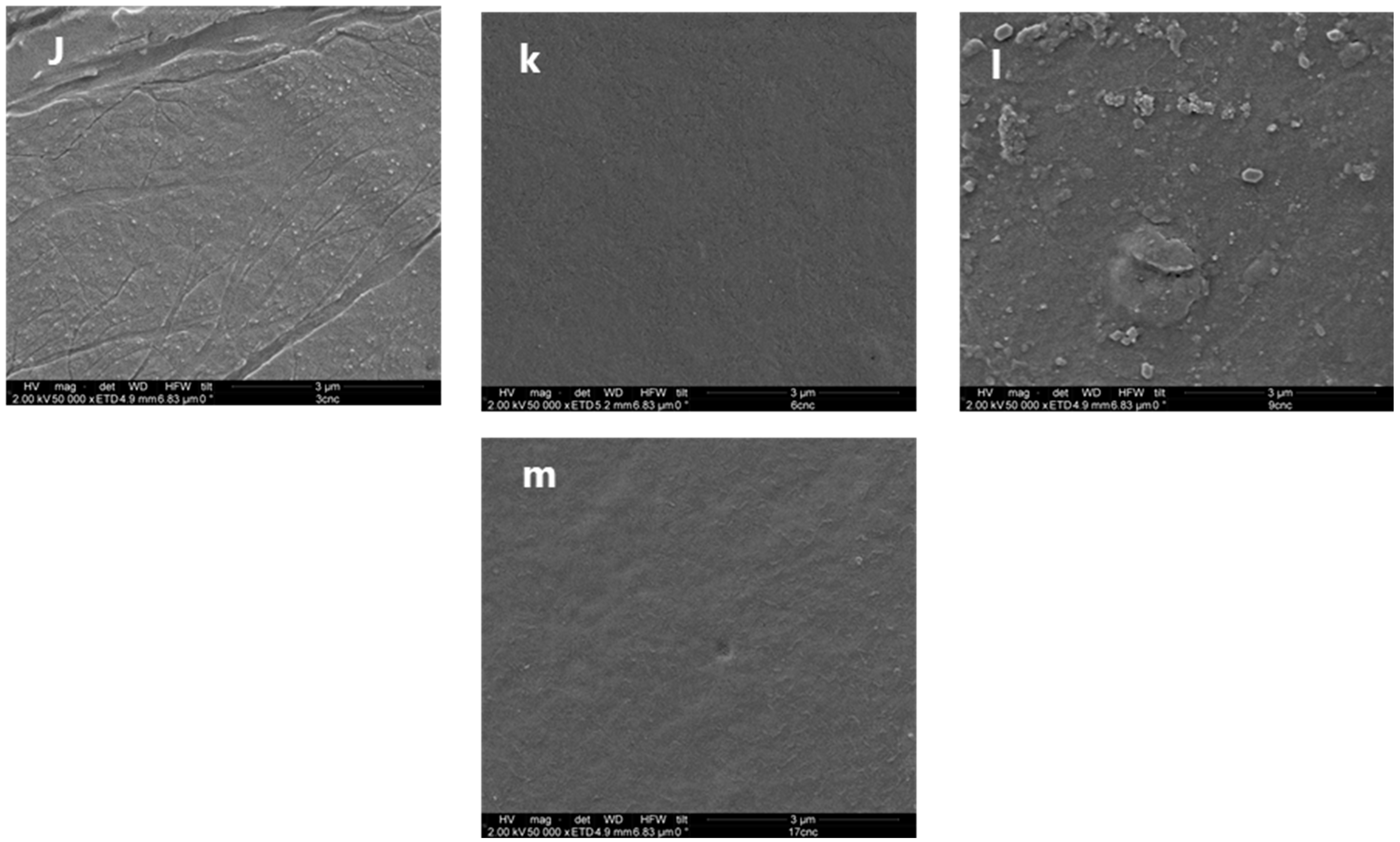
3.5. SEM
3.6. Light Transmittance and Opacity
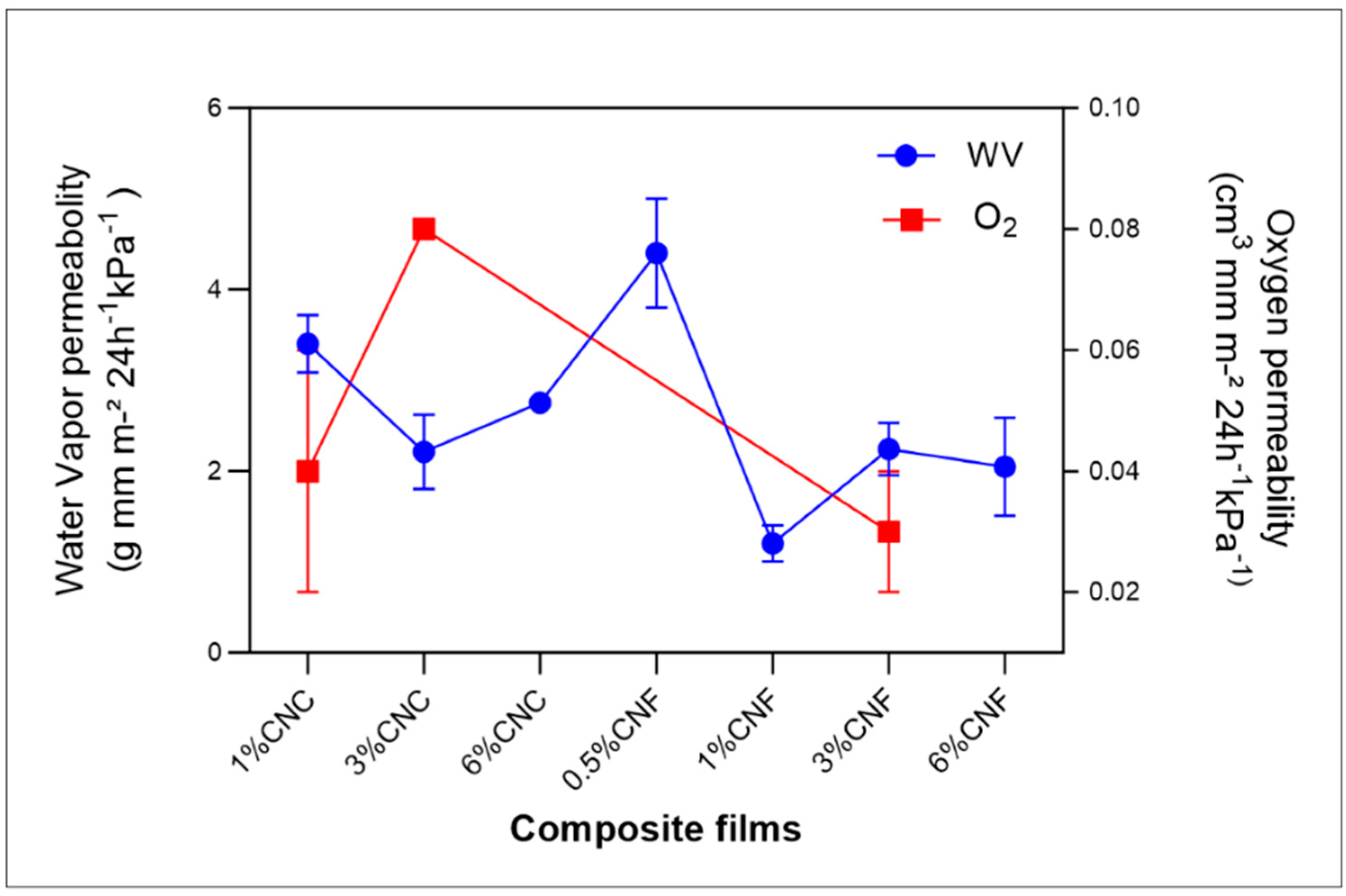
3.7. Water Vapor (WV) and Gas Permeability
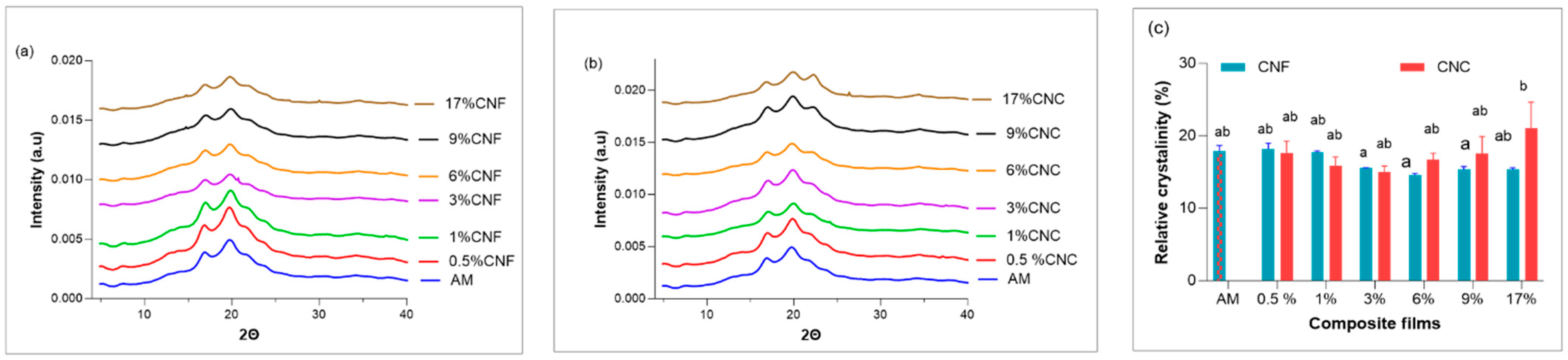
3.8. Wide Angle X-ray Scattering (WAXS)
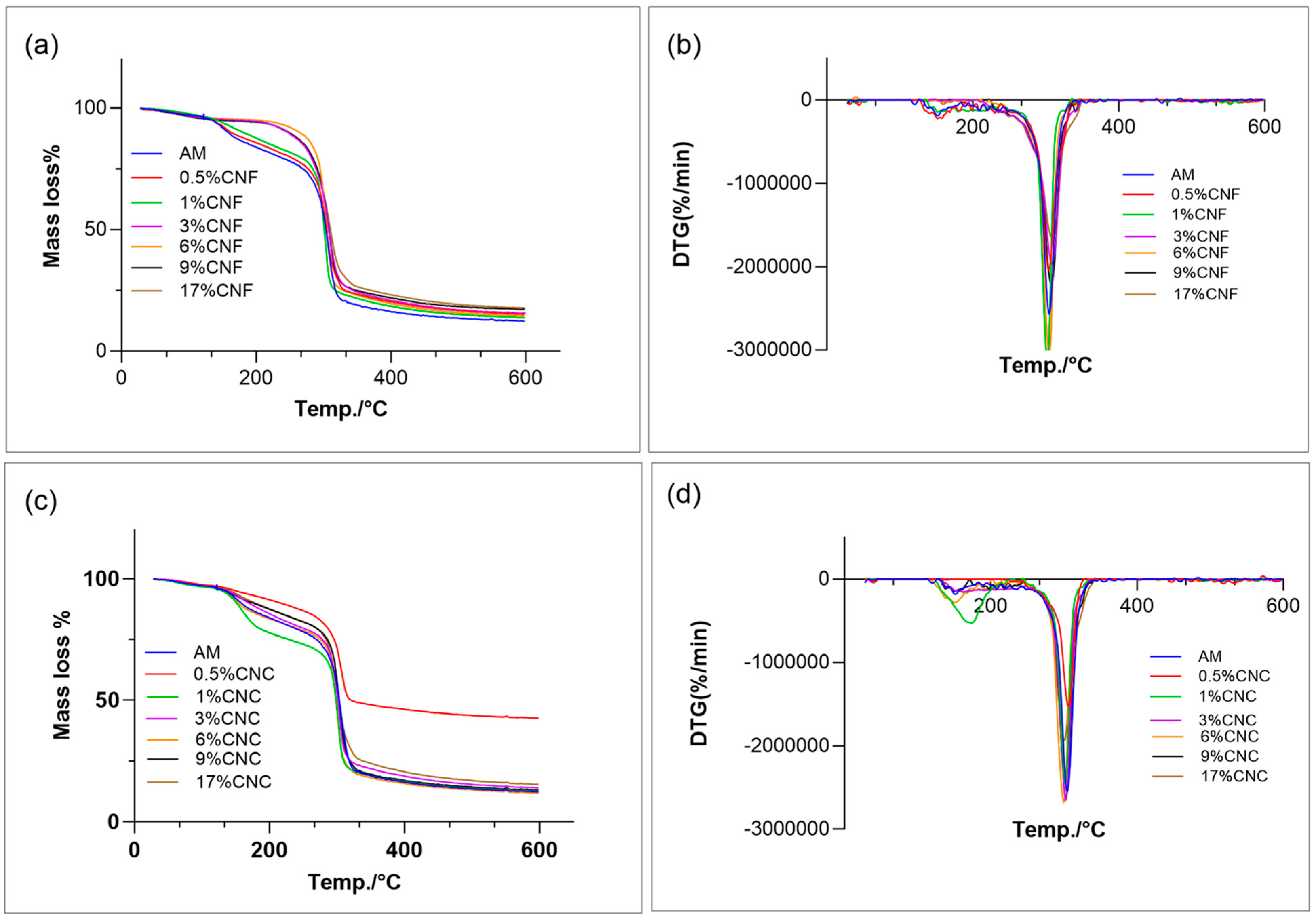
3.9. Thermogravimetric Analysis (TGA)
3.10. Compost Biodegradation
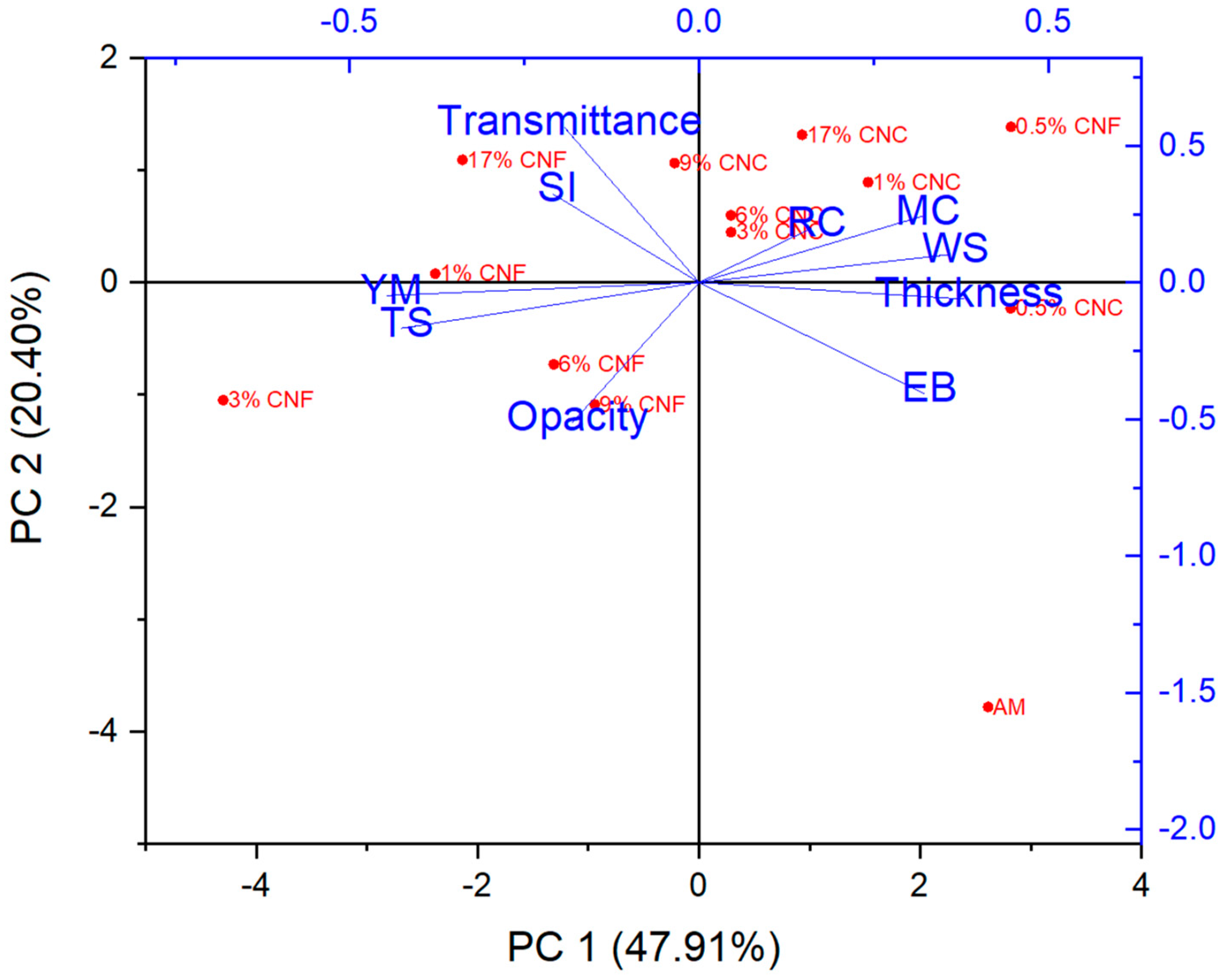
3.11. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousa, M.H.; Dong, Y.; Davies, I.J. Recent advances in bionanocomposites Preparation, Properties, and Applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 225–254. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Ingole, P.G. Nanocomposite and Bio-Nanocomposite Polymeric Materials/Membranes Development in Energy and Medical Sector: A Review. Int. J. Biol. Macromol. 2021, 193, 2121–2139. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Sultana, S.; Sultana, T.; Tabasum, S.; Zia, K.M.; Muzammil, Z.; Sultana, S. Chapter 3-Natural polymers as constituents of bionanocomposites. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 55–85. [Google Scholar]
- Sagnelli, D.; Hebelstrup, K.H.; Leroy, E.; Rolland-Sabaté, A.; Guilois, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Lourdin, D.; Blennow, A. Plant-Crafted Starches for Bioplastics Production. Carbohydr. Polym. 2016, 152, 398–408. [Google Scholar] [CrossRef]
- Tetlow, I.J.; Bertoft, E. A Review of Starch Biosynthesis in Relation to the Building Block-Backbone Model. Int. J. Mol. Sci. 2020, 21, 7011. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef]
- Carciofi, M.; Blennow, A.; Nielsen, M.M.; Holm, P.B.; Hebelstrup, K.H. Barley Callus: A Model System for Bioengineering of Starch in Cereals. Plant Methods 2012, 8, 36. [Google Scholar] [CrossRef]
- Frost, B.A.; Johan Foster, E. Isolation of Thermally Stable Cellulose Nanocrystals from Spent Coffee Grounds via Phosphoric Acid Hydrolysis. J. Renew. Mater. 2020, 8, 187–203. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. ChemSusChem 2009, 2, 1096–1107. [Google Scholar] [CrossRef]
- Funahashi, R.; Ono, Y.; Tanaka, R.; Yokoi, M.; Daido, K.; Inamochi, T.; Saito, T.; Horikawa, Y.; Isogai, A. Changes in the Degree of Polymerization of Wood Celluloses during Dilute Acid Hydrolysis and TEMPO-Mediated Oxidation: Formation Mechanism of Disordered Regions along Each Cellulose Microfibril. Int. J. Biol. Macromol. 2018, 109, 914–920. [Google Scholar] [CrossRef]
- Kontturi, E.; Meriluoto, A.; Penttilä, P.A.; Baccile, N.; Malho, J.M.; Potthast, A.; Rosenau, T.; Ruokolainen, J.; Serimaa, R.; Laine, J.; et al. Degradation and Crystallization of Cellulose in Hydrogen Chloride Vapor for High-Yield Isolation of Cellulose Nanocrystals. Angew. Chemie-Int. Ed. 2016, 55, 14455–14458. [Google Scholar] [CrossRef]
- Spiliopoulos, P.; Spirk, S.; PPaakkonen, T.; Viljanen, M.; Svedstrom, K.; Pitkanen, L.; Awais, M.; Kontturi, E. Visualizing Degradation of Cellulose Nanofibers by Acid Hydrolysis. Biomacromolecules 2021, 22, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous Preparation of Cellulose Nanocrystals and Micron-Sized Porous Colloidal Particles of Cellulose by TEMPO-Mediated Oxidation. Green Chem. 2015, 17, 808–811. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Wang, X.; Tian, W.; Dong, Y.; Jiang, S. Finite Element Analysis of Strengthening Mechanism of Ultrastrong and Tough Cellulosic Materials. Polymers 2022, 14, 4490. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Wang, J.; Ding, L.; Zhang, K.; Han, J.; Jiang, S. Micro- and Nano-Fibrils of Manau Rattan and Solvent-Exchange-Induced High-Haze Transparent Holocellulose Nanofibril Film. Carbohydr. Polym. 2022, 298, 120075. [Google Scholar] [CrossRef] [PubMed]
- Babaee, M.; Jonoobi, M.; Hamzeh, Y.; Ashori, A. Biodegradability and Mechanical Properties of Reinforced Starch Nanocomposites Using Cellulose Nanofibers. Carbohydr. Polym. 2015, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Xu, J.; Sagnelli, D.; Faisal, M.; Perzon, A.; Taresco, V.; Mais, M.; Giosafatto, C.V.L.; Hebelstrup, K.H.; Ulvskov, P.; Jørgensen, B.; et al. Amylose/Cellulose Nanofiber Composites for All-Natural, Fully Biodegradable and Flexible Bioplastics. Carbohydr. Polym. 2021, 253, 117277. [Google Scholar] [CrossRef]
- Perzon, A.; Jørgensen, B.; Ulvskov, P. Sustainable Production of Cellulose Nanofiber Gels and Paper from Sugar Beet Waste Using Enzymatic Pre-Treatment. Carbohydr. Polym. 2020, 230, 115581. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kawasaki, S.; Takeda, Y. Influence of Physicochemical Properties of Starch on Crispness of Tempura Fried Batter. Cereal Chem. 2003, 80, 339–345. [Google Scholar] [CrossRef]
- Holland, C.; Perzon, A.; Cassland, P.R.C.; Jensen, J.P.; Langebeck, B.; Sørensen, O.B.; Whale, E.; Hepworth, D.; Plaice-Inglis, R.; Moestrup, Ø.; et al. Nanofibers Produced from Agro-Industrial Plant Waste Using Entirely Enzymatic Pretreatments. Biomacromolecules 2019, 20, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.E.F.; de Almeida Rebechi, I.; Fontaneli, R.S. Digital Micrometer Used in Thickness Measurement of Plastic Film Compared to Standardized Instrument. Mater. Sci. Appl. 2017, 8, 577–583. [Google Scholar] [CrossRef]
- Faisal, M.; Bevilacqua, M.; Bro, R.; Bordallo, H.N.; Judas, J.; Kirkensgaard, K.; Hebelstrup, K.H.; Blennow, A. International Journal of Biological Macromolecules Colorimetric PH Indicators Based on Well-Defined Amylose and Amylopectin Matrices Enriched with Anthocyanins from Red Cabbage. Int. J. Biol. Macromol. 2023, 250, 126250. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, E.; Moradi, M.; Tajik, H.; Molaei, R.; Ezati, P. Preparation of On-Package Halochromic Freshness/Spoilage Nanocellulose Label for the Visual Shelf Life Estimation of Meat. Int. J. Biol. Macromol. 2020, 164, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002.
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and Characterization of Active and Intelligent Packaging Films Based on Cassava Starch and Anthocyanins from Lycium Ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- ASTM F1249-13; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Colorimetric Sensor. ASTM International: West Conshohocken, PA, USA, 2010.
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability Standards for Carrier Bags and Plastic Films in Aquatic Environments: A Critical Review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef]
- Razzaq, W.; Javaid, A. Synthesis & Characterization of Cotton Fiber Reinforced Starch/PVA Biodegradable Composite Films. J. Fac. Eng. Technol. 2015, 22, 27–35. [Google Scholar]
- de Almeida, V.S.; Barretti, B.R.V.; Ito, V.C.; Malucelli, L.; da Silva Carvalho Filho, M.A.; Demiate, I.M.; Pinheiro, L.A.; Lacerda, L.G. Thermal, Morphological, and Mechanical Properties of Regular and Waxy Maize Starch Films Reinforced with Cellulose Nanofibers (CNF). Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Behzad, T.; Bagheri, R. Preparation and Characterization of Cellulose Nanofiber Reinforced Thermoplastic Starch Composites. Fibers Polym. 2014, 15, 347–354. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant Starch/Pectin Free-Standing Films Reinforced with Nanocellulose Intended for Colonic Methotrexate Release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-Cellulose Reinforced Starch Bio Composite Films—A Review on Green Composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; Henkes, A.K.; Bruni, G.P.; Viana, L.A.N.; de Moura, C.M.; Flores, W.H.; Galio, A.F. Fabrication and Characterization of Native and Oxidized Potato Starch Biodegradable Films. Food Biophys. 2018, 13, 163–174. [Google Scholar] [CrossRef]
- Csiszár, E.; Kun, D.; Fekete, E. The Role of Structure and Interactions in Thermoplastic Starch–Nanocellulose Composites. Polymers 2021, 13, 3186. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic Reinforcing Effect of TiO2 and Montmorillonite on Potato Starch Nanocomposite Films: Thermal, Mechanical and Barrier Properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhou, M.; Wei, Y.J.; Cheng, F.; Zhu, P.X. Comparison of Mechanical Reinforcement Effects of Cellulose Nanocrystal, Cellulose Nanofiber, and Microfibrillated Cellulose in Starch Composites. Polym. Compos. 2019, 40, E365–E372. [Google Scholar] [CrossRef]
- Famiglietti, M.; Zannini, D.; Turco, R.; Mariniello, L. Mechanical, Barrier and Thermal Properties of Amylose-Argan Proteins-Based Bioplastics in the Presence of Transglutaminase. Int. J. Mol. Sci. 2023, 24, 3405. [Google Scholar] [CrossRef]
- Arifin, H.R.; Djali, M.; Nurhadi, B.; Hasim, S.A.; Hilmi, A.; Puspitasari, A.V. Improved Properties of Corn Starch-Based Bio-Nanocomposite Film with Different Types of Plasticizers Reinforced by Nanocrystalline Cellulose. Int. J. Food Prop. 2022, 25, 509–521. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in Packaging: Advances in Barrier Layer Technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Mariniello, L.; D’Agostino, A.; D’Agostino, M.; Cammarota, M.; Schiraldi, C.; Porta, R. Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. Int. J. Mol. Sci. 2022, 23, 8478. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a Natural Source for Groundbreaking Applications in Materials Science: Today’s State. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zhang, Y.; Li, F.; Jiao, X.; Li, Q. The Effects of Cellulose Nanocrystal and Cellulose Nanofiber on the Properties of Pumpkin Starch-Based Composite Films. Int. J. Biol. Macromol. 2021, 192, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Yang, Y.; Wang, C. Physical and Degradable Properties of Mulching Films Prepared from Natural Fibers and Biodegradable Polymers. Appl. Sci. 2016, 6, 147. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Assessment of Aliphatic-Aromatic Copolyester Biodegradable Mulch Films. Part I: Field Study. Chemosphere 2008, 71, 942–953. [Google Scholar] [CrossRef] [PubMed]

| Composition | Opacity | Transmittance |
|---|---|---|
| AM | 6.4 ± 1.0 d | 41 ± 15 a |
| 0.5% CNC | 2.7 ± 1 abc | 60 ± 19 ab |
| 1% CNC | 2.3 ± 1 a | 69 ± 7 ab |
| 3% CNC | 3 ± 0.0 abc | 67 ± 8 ab |
| 6% CNC | 4 ± 0.0 c | 70 ± 2 ab |
| 9% CNC | 3.1 ± 1.0 abc | 79± 1 b |
| 17% CNC | 3 ± 0.0 abc | 71 ± 3 ab |
| 0.5% CNF | 2.5 ± 1.0 ab | 74 ± 1 b |
| 1% CNF | 3.8 ± 0.0 bc | 71 ± 4 ab |
| 3% CNF | 3.8 nd | 76 ± 12 b |
| 6% CNF | 3.4 ± 0.0 abc | 66 ± 1 ab |
| 9% CNF | 3.2 ± 0.0 abc | 66 ± 5 ab |
| 17% CNF | 3.7 ± 0.0 abc | 68 ± 14 ab |
| Composition | Ti (°C) | DTG at Tmax(°C) | Mass Residue (%) |
|---|---|---|---|
| AM | 38 ± 7 ab | 305 ± 2 abc | 43 ± 1 ab |
| 0.5% CNC | 47 ± 6 b | 307 ± 0 bc | 60 nd |
| 1% CNC | 28 ± 2 a | 301 ± 0 ab | 38 ± 4 a |
| 3% CNC | 44 ± 2 b | 299 ± 0 abc | 46 ± 1 ab |
| 6% CNC | 50 ± 2 b | 302 ± 1 a | 45 ± 3 ab |
| 9% CNC | 47 ± 0 b | 299 ± 2 abc | 46 ± 1 ab |
| 17% CNC | 47 ± 4 b | 301 ± 1 a | 52 ± 1 a |
| 0.5% CNF | 35 ND | 307 ND | 53 ND |
| 1% CNF | 47 ± 3 b | 301 ± 0 abc | 37 ± 11 a |
| 3%CNF | 45 ± 3 b | 305 ± 3 abc | 57 ± 2 b |
| 6%CNF | 49 ± 0 b | 303 ± 1 abc | 50 ± 1 ab |
| 9% CNF | 37 ± 4 ab | 308 ± 0.1 c | 45 ± 6 ab |
| 17% CNF | 48 ± 4 b | 308 ± 4 c | 51 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Žmirić, M.; Kim, N.Q.N.; Bruun, S.; Mariniello, L.; Famiglietti, M.; Bordallo, H.N.; Kirkensgaard, J.J.K.; Jørgensen, B.; Ulvskov, P.; et al. A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics. Coatings 2023, 13, 1573. https://doi.org/10.3390/coatings13091573
Faisal M, Žmirić M, Kim NQN, Bruun S, Mariniello L, Famiglietti M, Bordallo HN, Kirkensgaard JJK, Jørgensen B, Ulvskov P, et al. A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics. Coatings. 2023; 13(9):1573. https://doi.org/10.3390/coatings13091573
Chicago/Turabian StyleFaisal, Marwa, Marija Žmirić, Ngoc Quynh Nhu Kim, Sander Bruun, Loredana Mariniello, Michela Famiglietti, Heloisa N. Bordallo, Jacob Judas Kain Kirkensgaard, Bodil Jørgensen, Peter Ulvskov, and et al. 2023. "A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics" Coatings 13, no. 9: 1573. https://doi.org/10.3390/coatings13091573











