Production and Preliminary Characterization of Linseed Mucilage-Based Films Loaded with Cardamom (Elettaria cardamomum) and Copaiba (Copaifera officinalis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Obtaining the Extract of E. cardamomum
2.3. Linseed Mucilage Extraction
2.4. Phytochemical Tests of Plant Material
2.5. Production and Preliminary Characterization of Films Based on Linseed Mucilage Loaded with CA and CO
2.5.1. Color Determination
2.5.2. Thickness Measurement
2.5.3. Solubility Tests in Artificial Saliva
2.5.4. Antioxidant Activity (DPPH, 1,1-Diphenyl-2-picrylhydrazyl, and ABTS, 2,2’-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid) and Total Phenolic Content
2.5.5. Texture Profile Analysis (APT)
2.5.6. Field Emission Scanning Electron Microscopy (FE-SEM)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analysis of Plant Extracts
3.2. Development and Partial Characterization of Films Based on Linseed Mucilage Loaded with CA and CO
3.2.1. Film Production
3.2.2. Physicochemical Properties
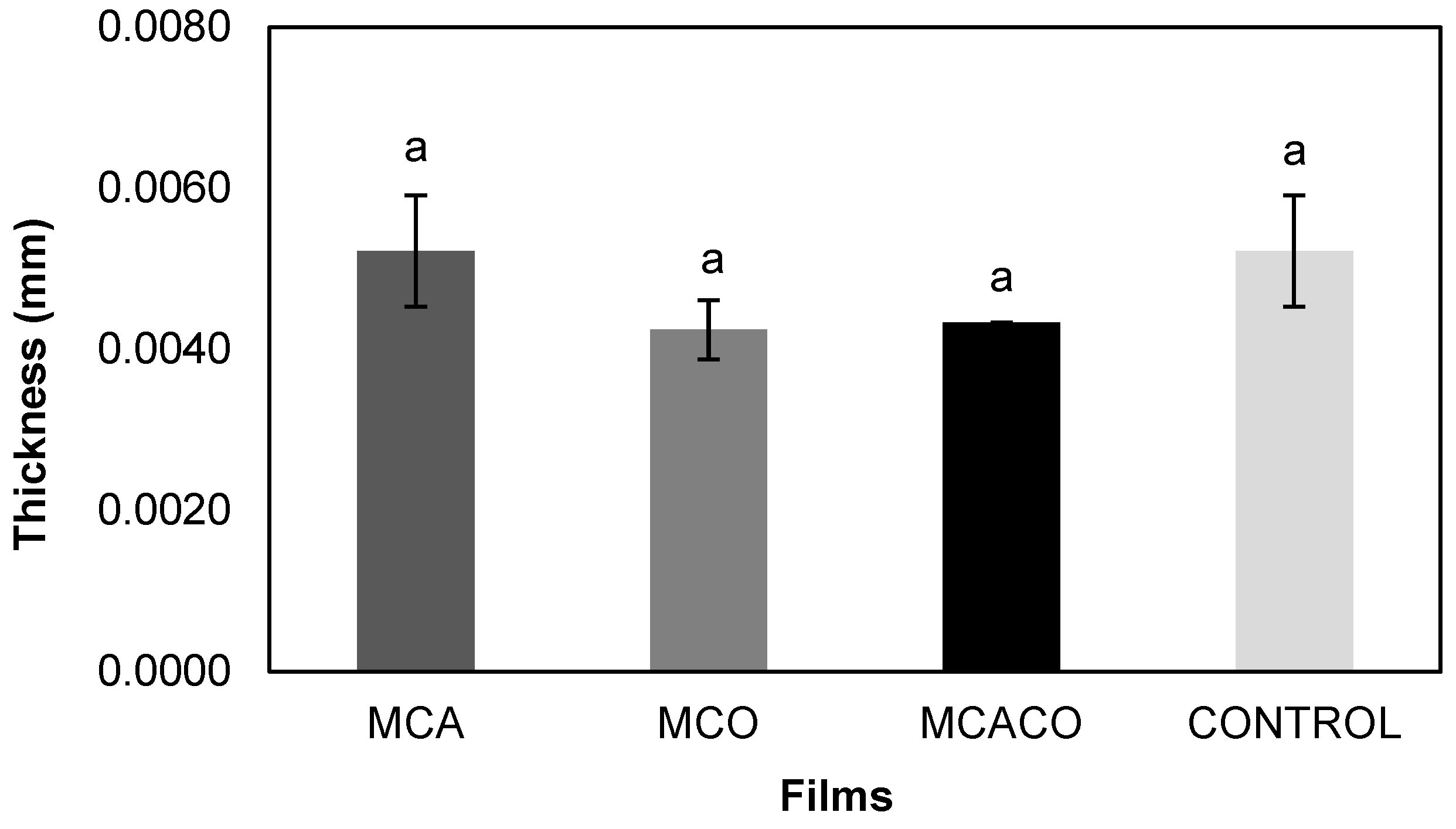
Color and Thickness
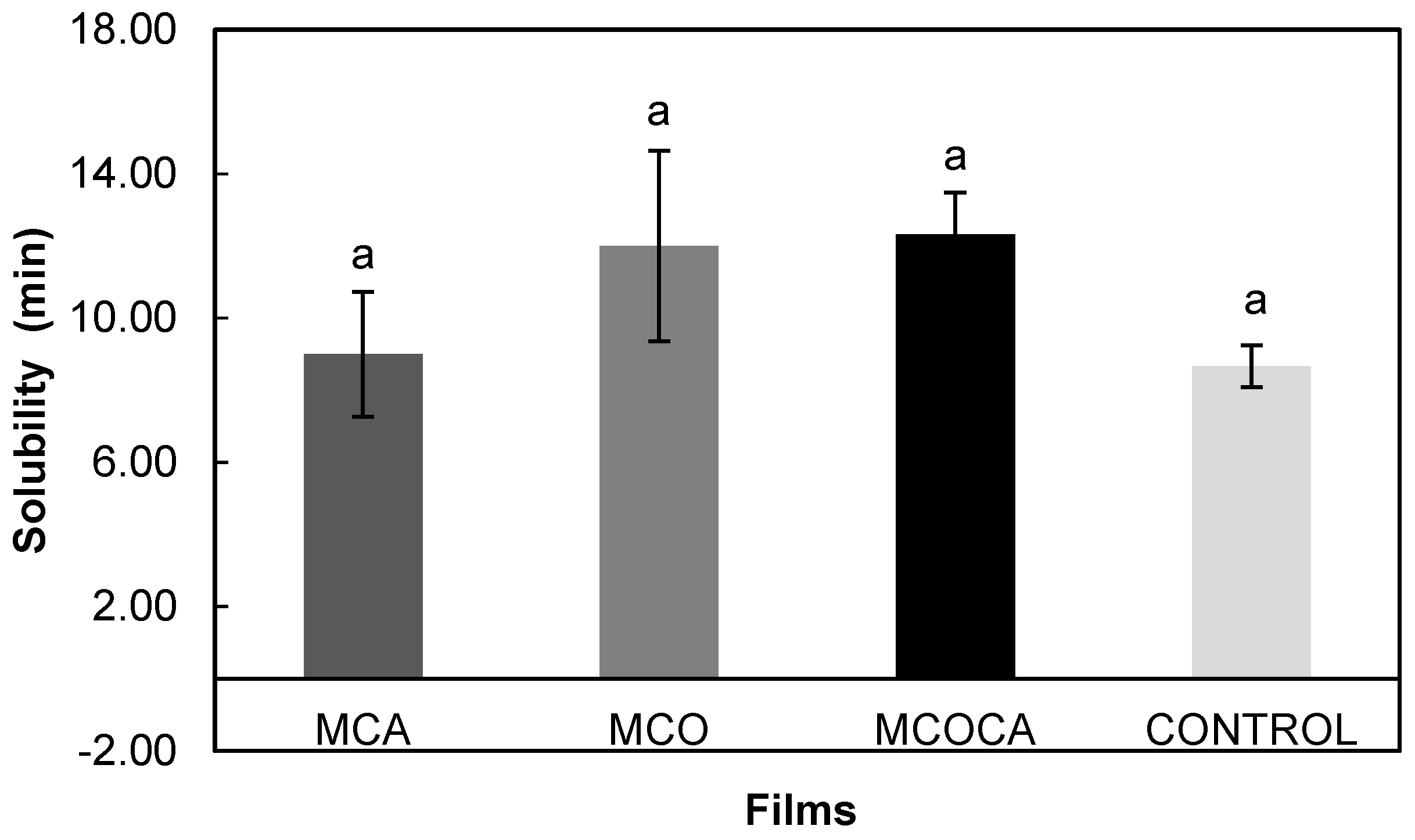
Solubility Tests in Artificial Saliva
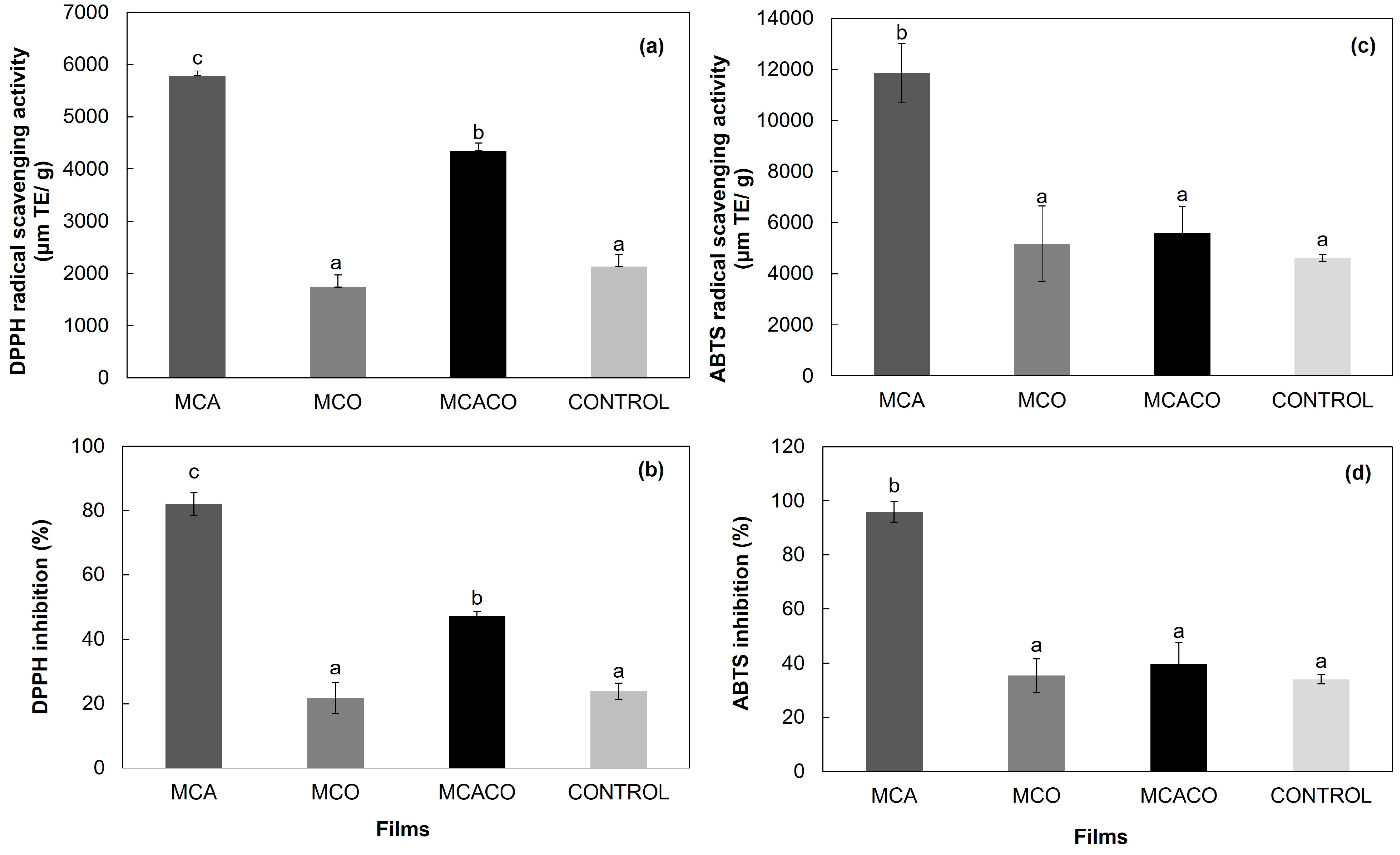
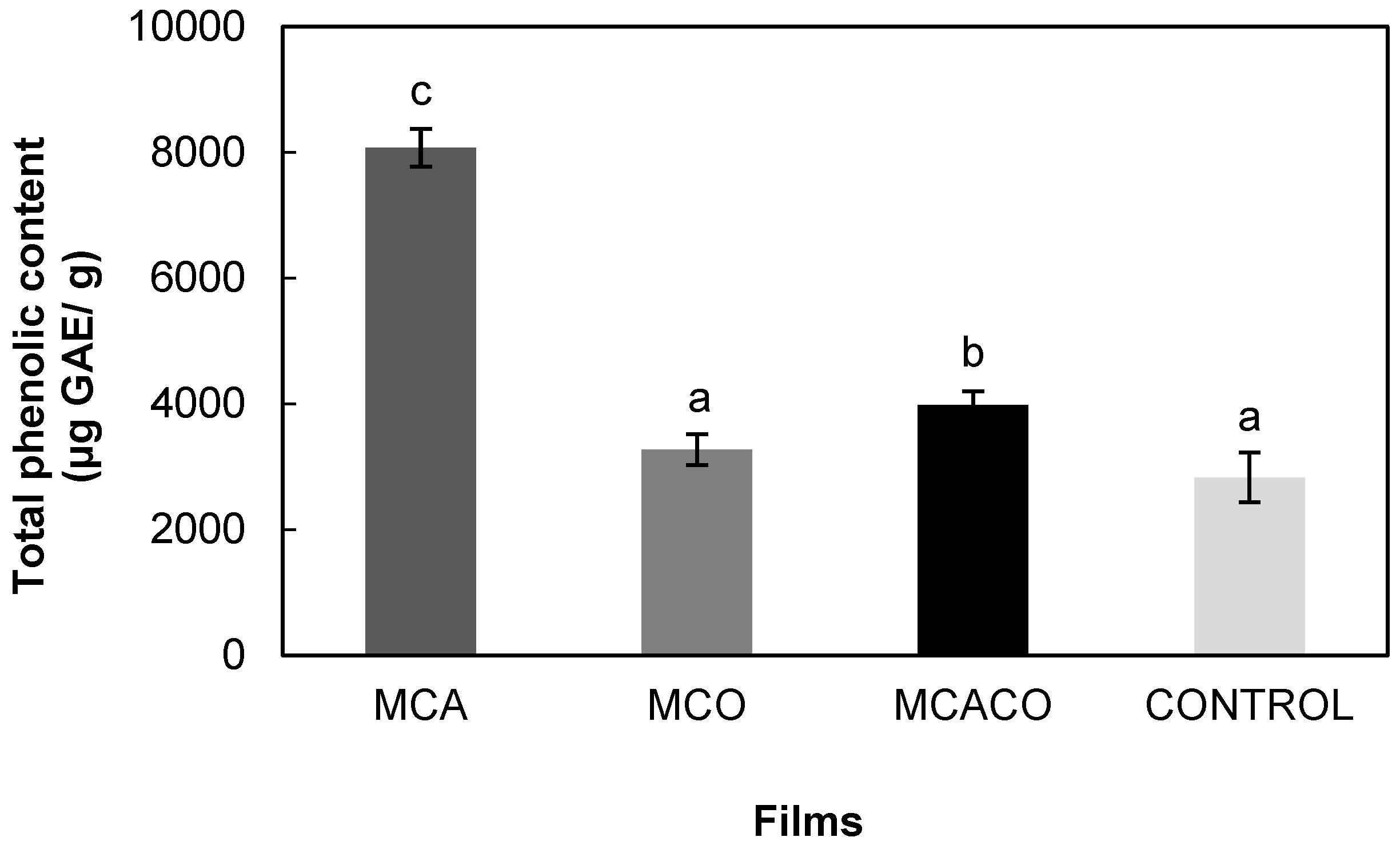
Antioxidant Activity (DPPH and ABTS) and Total Phenolic Content
3.3. Texture Profile Analysis (TPA)
Hardness, Deformation, Adhesiveness and Fracturability
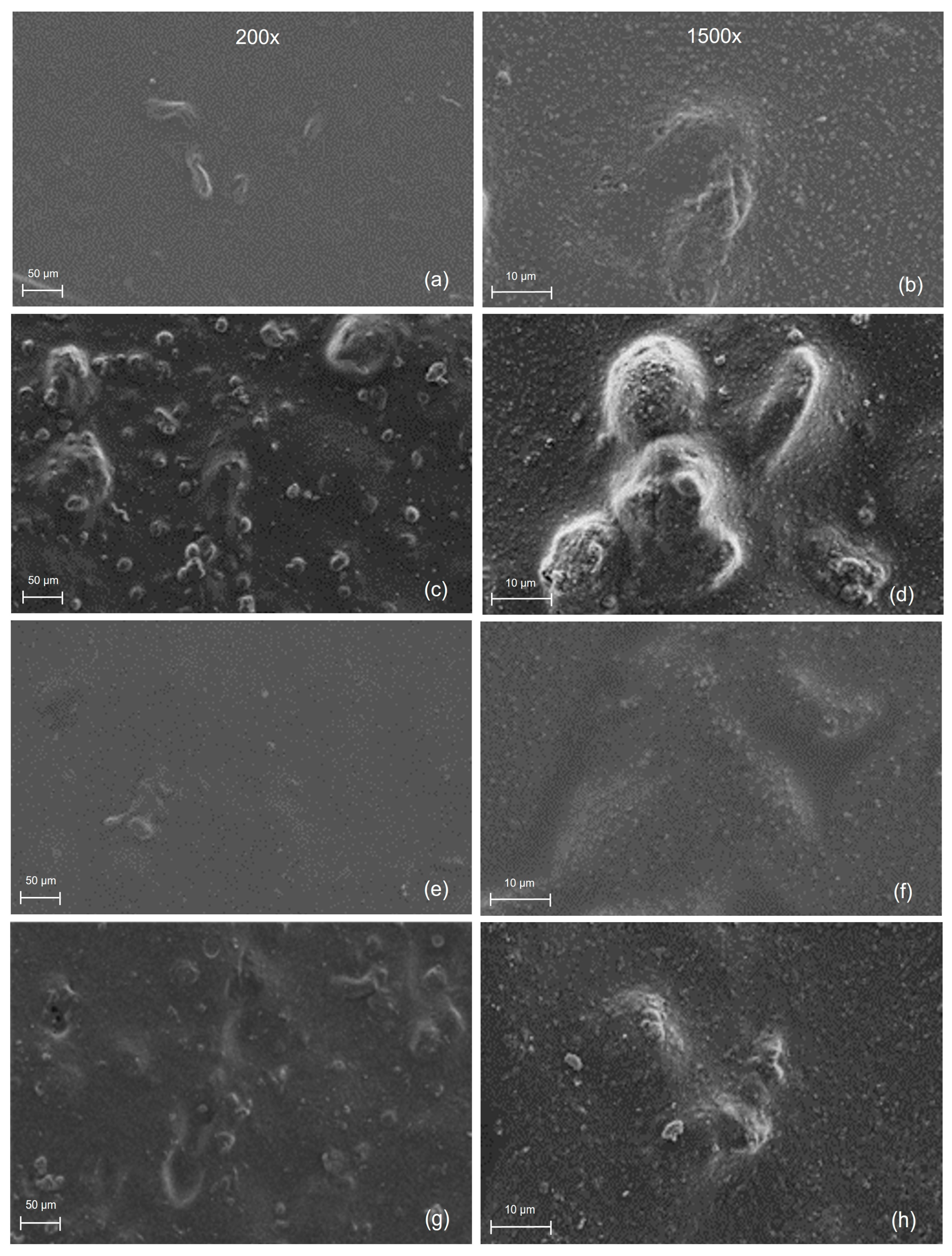
3.4. Microscopic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Trindade, R.; Da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Garza, G.R.; Elizondo-Luévano, J.H.; Bazaldúa-Rodríguez, A.F.; Chávez-Montes, A.; Pérez-Hernández, R.A.; Martínez-Delgado, A.J.; López-Villarreal, S.M.; Rodríguez-Rodríguez, J.; Sánchez-Casas, R.M.; Castillo-Velázquez, U.; et al. Benefits of cardamom (Elettaria cardamomum (L.) Maton) and turmeric (Curcuma longa L.) extracts for their applications as natural anti-inflammatory adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef]
- De Medeiros, M.L.; Xavier-Júnior, F.H.; Araújo-Filho, I.; Rêgo, A.C.; Veiga-Júnior, V.F.; Maciel, M.A. Copaiba oil for nano-pharmaceutics and drug delivery. Encycl. Nanosci. Nanotechnol. 2019, 27, 165–189. [Google Scholar]
- Diefenbach, A.L.; Muniz, F.W.M.G.; Oballe, H.J.R.; Rösing, C.K. Antimicrobial activity of copaiba oil (Copaifera ssp.) on oral pathogens: Systematic review. Phytother. Res. 2018, 32, 586–596. [Google Scholar] [CrossRef]
- Ilyas, H.; Iqbal, M.J. Importance of some therapeutic plants and their antimicrobial activity to maintain oral health. Biochem. Pharmacol. An. Open Access J. 2021, 10, 08. [Google Scholar]
- Pinto, M.F.; Quevedo, B.V.; Asami, J.; Komatsu, D.; Hausen, M.D.A.; Duek, E.A.D.R. Electrospun membrane based on poly(L-co-D,L lactic acid) and natural rubber containing copaiba oil designed as a dressing with antimicrobial properties. Antibiotics 2023, 12, 898. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.D.; Saptami, K.; Caputo, L.; De Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E.; et al. Chemical and biological evaluation of essential oils from cardamom species. Molecules 2018, 23, 2818. [Google Scholar] [CrossRef] [PubMed]
- Moulai-Hacene, F.; Boufadi, M.Y.; Keddari, S.; Homrani, A. chemical composition and antimicrobial properties of Elettaria cardamomum extract. Pharmacogn. J. 2020, 12, 1058–1063. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; de Oliveira, E.R.; Kato, M.M.; Mitra, S.K.; Rosa, D.D.S. Chemical stabilization behind cardamom pickering emulsion using nanocellulose. Polysaccharides 2022, 3, 200–216. [Google Scholar] [CrossRef]
- Meenakshi, M.; Muralidharan, N.P. The role of cardamom oil in oral health. IJLSR 2015, 1, 322–325. [Google Scholar]
- Aneja, K.R.; Joshi, R. Antimicrobial activity of Amomum subulatum and Elettaria cardamomum against dental caries causing microorganisms. Ethnobotanical Leaflets 2009, 7, 3. [Google Scholar]
- Ibrahim, G.A.; Al–Obaidi, W.A. Effect of small cardamom extracts on Mutans streptococci in comparison to chlorhexidine gluconate and de-ionized water (in vitro study). J. Bagh Coll. Dent. 2013, 25, 104–108. [Google Scholar]
- Dahigaonkar, K.; Yelpure, S.C.; Syed, F.N.; Wajid, A.F. Use of spices in treatment of dental infections. World J. Pharm. Res. 2018, 7, 360–371. [Google Scholar] [CrossRef]
- Karimi, N.; Jabbari, V.; Nazemi, A.; Ganbarov, K.; Karimi, N.; Tanomand, A.; Karimi, S.; Abbasi, A.; Yousefi, B.; Khodadadi, E.; et al. Thymol, cardamom and Lactobacillus plantarum nanoparticles as a functional candy with high protection against Streptococcus mutans and tooth decay. Microb. Pathog. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Binimeliz, M.F.; Martins, M.L.; Filho, J.C.C.F.; Cabral, L.M.; Da Cruz, A.G.; Maia, L.C.; Fonseca-Gonçalves, A. Antimicrobial effect of a cardamom ethanolic extract on oral biofilm: An ex vivo study. Nat. Oral. Care Dent. Ther. 2020, 121–131. [Google Scholar] [CrossRef]
- Makky, E.A.; Ali, M.J.; Yusoff, M.M. Oral Hygiene Improvement using Combined Mouthwash with Plant extracts. MATEC Web Conf. 2018, 150, 06034. [Google Scholar] [CrossRef][Green Version]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 2019, 61, 102089. [Google Scholar] [CrossRef]
- Leandro, L.M.; De Sousa, V.F.; Barbosa, P.C.S.; Neves, J.K.O.; Da Silva, J.A.; Da Veiga-Junior, V.F. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) Oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef]
- Bandeira, M.F.C.L.; Freitas, A.L.; Menezes, M.D.S.C.; Silva, J.D.S.; Sombra, G.A.D.; Araújo, E.A.M.; Toda, C.; Moreschi, A.R.C.; Conde, N.C.O. Adhesive resistance of a copaiba oil-based dentin biomodifier. Braz. Oral. Res. 2020, 34, e001. [Google Scholar] [CrossRef]
- Genesi, B.P.; De Melo Barbosa, R.; Severino, P.; Rodas, A.C.; Yoshida, C.M.; Mathor, M.B.; Lopes, P.S.; Viseras, C.; Souto, E.B.; Da Silva, C.F. Aloe vera and copaiba oleoresin-loaded chitosan films for wound dressings: Microbial permeation, cytotoxicity, and in vivo proof of concept. Int. J. Pharm. 2023, 634, 122648. [Google Scholar] [CrossRef]
- Simões, C.; Conde, N.; Venâncio, G.; Milério, P.; Fulgência, M.; Veiga, V. Antibacterial activity of copaiba oil gel on dental biofilm. Open Dent. J. 2016, 10, 188–195. [Google Scholar] [CrossRef]
- Valadas, L.A.R.; Gurgel, M.F.; Mororó, J.M.; Da Cruz Fonseca, S.G.; Fonteles, C.S.R.; De Carvalho, C.B.M.; Fechine, F.V.; Neto, E.M.R.; De França Fonteles, M.M.; Chagas, F.O.; et al. Dose-response evaluation of a copaiba-containing varnish against Streptococcus mutans in vivo. Saudi Pharm. J. 2019, 27, 363–367. [Google Scholar] [CrossRef]
- Valadas, L.A.R.; Lobo, P.L.D.; Fonseca, S.G.D.C.; Fechine, F.V.; Rodrigues Neto, E.M.; Fonteles, M.M.D.F.; Aguiar Trévia, L.R.D.; Vasconcelos, H.L.P.; Lima, S.M.D.S.; Lotif, M.A.L.; et al. Clinical and antimicrobial evaluation of Copaifera langsdorffii Desf. dental varnish in children: A clinical study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6647849. [Google Scholar] [CrossRef]
- Alvarenga, M.O.P.; Bittencourt, L.O.; Mendes, P.F.S.; Ribeiro, J.T.; Lameira, O.A.; Monteiro, M.C.; Barboza, C.A.G.; Martins, M.D.; Lima, R.R. Safety and effectiveness of copaiba oleoresin (C. reticulata Ducke) on inflammation and tissue repair of oral wounds in rats. Int. J. Mol. Sci. 2020, 21, 3568. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Al-Isamil, K.M.; Al-Ghizzawi, H.A.M.; Holley, R.A. Stability of cardamom (Elettaria Cardamomum) essential oil in microcapsules made of whey protein isolate, guar gum, and carrageenan. J. Food Sci. 2014, 79, C1939–C1949. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Debone, H.; Lopes, P.; Severino, P.; Yoshida, C.; Souto, E.B.; Silva, C. Chitosan/copaiba oleoresin films for would dressing application. Int. J. Phar. 2019, 555, 146–152. [Google Scholar] [CrossRef]
- Dehghani, S.; Noshad, M.; Rastegarzadeh, S.; Hojjati, M.; Fazlara, A. Electrospun chia seed mucilage/PVA encapsulated with green cardamonmum essential oils: Antioxidant and antibacterial property. Int. J. Biol. Macromol. 2020, 161, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bonan, R.F.; Bonan, P.R.; Batista, A.U.; Perez, D.E.; Castellano, L.R.; Oliveira, J.E.; Medeiros, E.S. Poly (lactic acid)/poly (vinyl pyrrolidone) membranes produced by solution blow spinning: Structure, thermal, spectroscopic, and microbial barrier properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Paranhos, S.B.; Ferreira, E.D.; Canelas, C.A.; Da Paz, S.P.; Passos, M.F.; Da Costa, C.E.; Da Silva, A.C.; Monteiro, S.N.; Candido, V.S. Chitosan membrane containing copaiba oil (Copaifera spp.) for skin wound treatment. Polymers 2021, 14, 35. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; Yañez-Echeverría, S.; García, S.; Mora-Zúñiga, A.; Arévalo, N.K. Physico-mechanical, barrier and antimicrobial properties of linseed mucilage films incorporated with H. virginiana extract. Rev. Mex. Ing. Quim. 2019, 19, 983–996. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. A Review of extraction techniques and food applications of flaxseed mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Tee, Y.B.; Wong, J.; Tan, M.C.; Talib, R.A. Development of edible film from flaxseed mucilage. BioResources 2016, 11, 10286–10295. [Google Scholar] [CrossRef]
- Salamanca, L.E.L.; Cabreta, L.E.P.; Narveaz, G.C.D.; de Alba, L.R.B. Linseed mucilage and chitosan composite films: Preparation, physical, mechanical, and microstructure properties. In Proceedings of the 11th International Congress on Engineering and Food, Athens, Greece, 22–26 May 2011. [Google Scholar]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Effects of active chitosan-flaxseed mucilage-based films on the preservation of minced trout fillets: A comparison among aerobic, vacuum, and modified atmosphere packaging. Pack. Technol. Sci. 2020, 33, 469–484. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Natarelli, C.V.L.; Manrich, A.; Oliveira, J.E.; Mattoso, L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Bangar, S.P.; Singh, A.; Trif, M.; Kumar, M.; Kumar, P.; Kaur, R.; Kaur, N. Process parameter optimization and characterization for an edible film: Flaxseed concern. Coatings 2021, 11, 1106. [Google Scholar] [CrossRef]
- De Paiva, P.H.; Correa, L.G.; Paulo, A.F.; Balan, G.C.; Ida, E.I.; Shirai, M.A. Film production with flaxseed mucilage and polyvinyl alcohol mixtures and evaluation of their properties. J. Food Sci. Technol. 2021, 58, 3030–3038. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Qiu, W.; Mei, J.; Xie, J. Effect of sonication on the properties of flaxseed gum films incorporated with carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.P.; Menezes, R.P.; Tavares, W.D.S.; Ferreira, A.M.; de Sousa, F.F.O.; Da Silva, G.A.; Zamora, R.R.; Araújo, R.S.; Souza, T.M. Copaiba essential oil loaded-nanocapsules film as a potential candidate for treating skin disorders: Preparation, characterization, and antibacterial properties. Int. J. Pharm. 2023, 633, 122608. [Google Scholar] [CrossRef]
- Sani, K.I.; Alizadeh, M. Isolated mung bean protein-pectin nanocomposite film containing true cardamom extract microencapsulation/CeO2 nanoparticles/graphite carbon quantum dots: Investigating fluorescence, photocatalytic and antimicrobial properties. Food Packag. Shelf Life 2022, 33, 100912. [Google Scholar] [CrossRef]
- Hajirostamloo, B.; Molaveisi, M.; Jafarian Asl, P.; Rahman, M.M. Novel soy protein isolate film containing cardamom essential oil microcapsules: Study of physicochemical properties and its application in Iranian white cheese packaging. J. Food Meas. Charact. 2023, 17, 324–336. [Google Scholar] [CrossRef]
- Castillo, N.E.T.; Teresa-Martínez, G.D.; Alonzo-Macías, M.; Téllez-Pérez, C.; Rodríguez-Rodríguez, J.; Sosa-Hernández, J.E.; Parra-Saldívar, R.; Melchor-Martínez, E.M.; Cardador-Martínez, A. Antioxidant activity and GC-MS profile of cardamom (Elettaria cardamomum) essential oil obtained by a combined extraction method-instant controlled pressure drop technology coupled with sonication. Molecules 2023, 28, 1093. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Meléndez, G.A.; Villa-Cedillo, S.A.; Pérez-Hernández, R.A.; Castillo-Velázquez, U.; Salas-Treviño, D.; Saucedo-Cárdenas, O.; Montes-de-Oca-Luna, R.; Gómez-Tristán, C.A.; Garza-Arredondo, A.J.; Zamora-Ávila, D.E.; et al. Cytotoxic effect in vitro of Acalypha monostachya extracts over human tumor cell lines. Plants 2021, 10, 2326. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In vitro biological activity and lymphoma cell growth inhibition by selected mexican medicinal plants. Life 2023, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Guzmán-Díaz, D.A.; Treviño-Garza, M.Z.; Rodríguez-Romero, B.A.; Gallardo-Rivera, C.T.; Amaya-Guerra, C.A.; Báez-González, J.G. Development and characterization of gelled double emulsions based on chia (Salvia hispanica L.) mucilage mixed with different biopolymers and loaded with green tea extract (Camellia sinensis). Foods 2019, 8, 677. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M.A. Comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification by products. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Hanaa, M.H.; Ismail, H.A.; Mahmoud, M.E.; Ibrahim, H.M. Antioxidant activity and phytochemical analysis of flaxseeds (Linum usitatisimum L). Minia J. Agric. Res. Develop. 2017, 37, 129–140. [Google Scholar]
- Yasmeen, M.; Nisar, S.; Tavallali, V.; Khalid, T. A review of phytochemicals and uses of flaxseed. Int. J. Chem. Biochem. Sci. 2018, 13, 70–75. [Google Scholar]
- Arroyo, J.; Almora, Y.; Quino, M.; Martínez, J.; Condorhuamán, M.; Flores, M.; Bonilla, P. Copaifera officinalis oil cytoprotector and antisecretory effects in induced gastric lesions in rats. An. Fac. Med. 2009, 70, 89–96. [Google Scholar] [CrossRef]
- Bano, S.; Ahmad, N.; Sharma, A.K. Phytochemical screening and evaluation of anti-microbial and anti-oxidant activity of Elettaria cardamom (Cardamom). J. Appl. Nat. Sci. 2016, 8, 1966–1970. [Google Scholar] [CrossRef]
- Khatri, P.; Rani, A.; Hameed, S.; Chandra, S.; Chang, C.-M.; Pandey, R.P. Current understanding of the molecular basis of spices for the development of potential antimicrobial medicine. Antibiotics 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.A.C.; Alves, L.D.B.; Goldemberg, D.C.; De Melo, A.C.; Antunes, H.S. Anti-inflammatory and wound healing effect of Copaiba oleoresin on the oral cavity: A systematic review. Heliyon 2022, 8, e08993. [Google Scholar] [CrossRef]
- Rodrigues, G.M.; Filgueiras, C.T.; Garcia, V.A.D.S.; Carvalho, R.A.; Velasco, J.I.; Fakhouri, F.M. Antimicrobial activity and GC-MS profile of copaiba oil for incorporation into Xanthosoma mafaffa Schott starch-based films. Polymers 2020, 12, 2883. [Google Scholar] [CrossRef]
- Brandelero, R.P.H.; Brandelero, E.M.; Almeida, F.M.D. Biodegradable films of starch/PVOH/alginate in packaging systems for minimally processed lettuce (Lactuca sativa L.). Cien Agrotec. 2016, 40, 510–521. [Google Scholar] [CrossRef]
- Monteschio, J.D.O.; De Vargas Junior, F.M.; Alves Da Silva, A.L.; Das Chagas, R.A.; Fernandes, T.; Leonardo, A.P.; Kaneko, I.N.; De Moraes Pinto, L.A.; Guerrero, A.; De Melo Filho, A.A.; et al. Effect of copaíba essential oil (Copaifera officinalis L.) as a natural preservative on the oxidation and shelf life of sheep burgers. PLoS ONE 2021, 16, e0248499. [Google Scholar] [CrossRef]
- Kasote, D.M. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27–34. [Google Scholar]
- Bouaziz, F.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Chaabouni, S.E. Antioxidant properties of water-soluble gum from flaxseed hulls. Antioxidants 2016, 5, 26. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. IFSET 2022, 77, 102974. [Google Scholar] [CrossRef]
- González, A.; Alvis, A.; Arrázola, G. Effect of edible coating on the properties of sweet potato slices (Ipomoea Batatas Lam) cooked by deep-fat frying: Part 1: Texture. Inf. Technol. 2015, 26, 95–102. [Google Scholar] [CrossRef]
- Hashim, R.H.R.; Gunny, A.A.N.; Sam, S.T.; Kamaludin, N.H.I.; Shamsuddin, M.R. Effects of incorporation of essential oil into film for fruit packaging application. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012020. [Google Scholar] [CrossRef]


| Formulation | Linseed Mucilage (%) | Glycerol (%) | Tween 20 (%) | E. cardamomum (%) | C. officinalis (%) |
|---|---|---|---|---|---|
| M | 3.0 | 0.5 | 1.0 | - | - |
| MCA | 3.0 | 0.5 | 1.0 | 2.0 | 0.0 |
| MCO | 3.0 | 0.5 | 1.0 | - | 2.0 |
| MCACO | 3.0 | 0.5 | 1.0 | 1.0 | 1.0 |
| Tests | Phytoconstituents | Reaction | CA | CO | M |
|---|---|---|---|---|---|
| Liebermann-Burchard | Sterols, triterpenes | Reddish-brown ring | + | + | + |
| Shinoda | Flavonoids | Reep red | + | + | + |
| Baljet | Sesquiterpene-Lactones | Orange color | + | − | + |
| Sulfuric acid (H2SO4) | Quinones | Red color | − | − | − |
| Ferric chloride (FeCl3) | Phenols, tannins | Green color | + | − | + |
| Potassium permanganate (KMnO4) | Unsaturations | Brown precipitate | + | + | + |
| DNPH (2,4-dinitrophenylhydrazine) | Carbonyl group | Orange color | + | + | + |
| Dragendorff | Alkaloids | Orange precipitate | + | + | + |
| Sodium hydroxide (NaOH) | Coumarins, lactones | Yellow color | − | − | − |
| Molish | Carbohydrates | Purple ring formation | + | + | + |
| Foam | Saponins | Presence of stable foam | − | − | − |
| Formulation | Representative Image | Homogeneity | Appearance |
|---|---|---|---|
| MCA |  | Intermediate | Opaque, dark brown, and slightly flexible |
| MCO |  | Intermediate | Opaque, slightly brown, and slightly flexible |
| MCACO |  | Low | Opaque, light brown, and slightly flexible |
| Control |  | Intermediate | Opaque, slightly brown, and slightly flexible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treviño-Garza, M.Z.; Saldívar-Vázquez, A.K.; López-Villarreal, S.M.; Lara-Banda, M.d.R.; Elizondo-Luevano, J.H.; Chávez-Montes, A.; Báez-González, J.G.; Rodríguez-Luis, O.E. Production and Preliminary Characterization of Linseed Mucilage-Based Films Loaded with Cardamom (Elettaria cardamomum) and Copaiba (Copaifera officinalis). Coatings 2023, 13, 1574. https://doi.org/10.3390/coatings13091574
Treviño-Garza MZ, Saldívar-Vázquez AK, López-Villarreal SM, Lara-Banda MdR, Elizondo-Luevano JH, Chávez-Montes A, Báez-González JG, Rodríguez-Luis OE. Production and Preliminary Characterization of Linseed Mucilage-Based Films Loaded with Cardamom (Elettaria cardamomum) and Copaiba (Copaifera officinalis). Coatings. 2023; 13(9):1574. https://doi.org/10.3390/coatings13091574
Chicago/Turabian StyleTreviño-Garza, Mayra Z., Ana Karen Saldívar-Vázquez, Sonia Martha López-Villarreal, María del Refugio Lara-Banda, Joel Horacio Elizondo-Luevano, Abelardo Chávez-Montes, Juan Gabriel Báez-González, and Osvelia Esmeralda Rodríguez-Luis. 2023. "Production and Preliminary Characterization of Linseed Mucilage-Based Films Loaded with Cardamom (Elettaria cardamomum) and Copaiba (Copaifera officinalis)" Coatings 13, no. 9: 1574. https://doi.org/10.3390/coatings13091574
APA StyleTreviño-Garza, M. Z., Saldívar-Vázquez, A. K., López-Villarreal, S. M., Lara-Banda, M. d. R., Elizondo-Luevano, J. H., Chávez-Montes, A., Báez-González, J. G., & Rodríguez-Luis, O. E. (2023). Production and Preliminary Characterization of Linseed Mucilage-Based Films Loaded with Cardamom (Elettaria cardamomum) and Copaiba (Copaifera officinalis). Coatings, 13(9), 1574. https://doi.org/10.3390/coatings13091574












