Research on the Microstructure and Properties of Al Alloy/Steel CMT Welding-Brazing Joints with Al–Si Flux-Cored Welding Wires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wetting and Spreading Tests
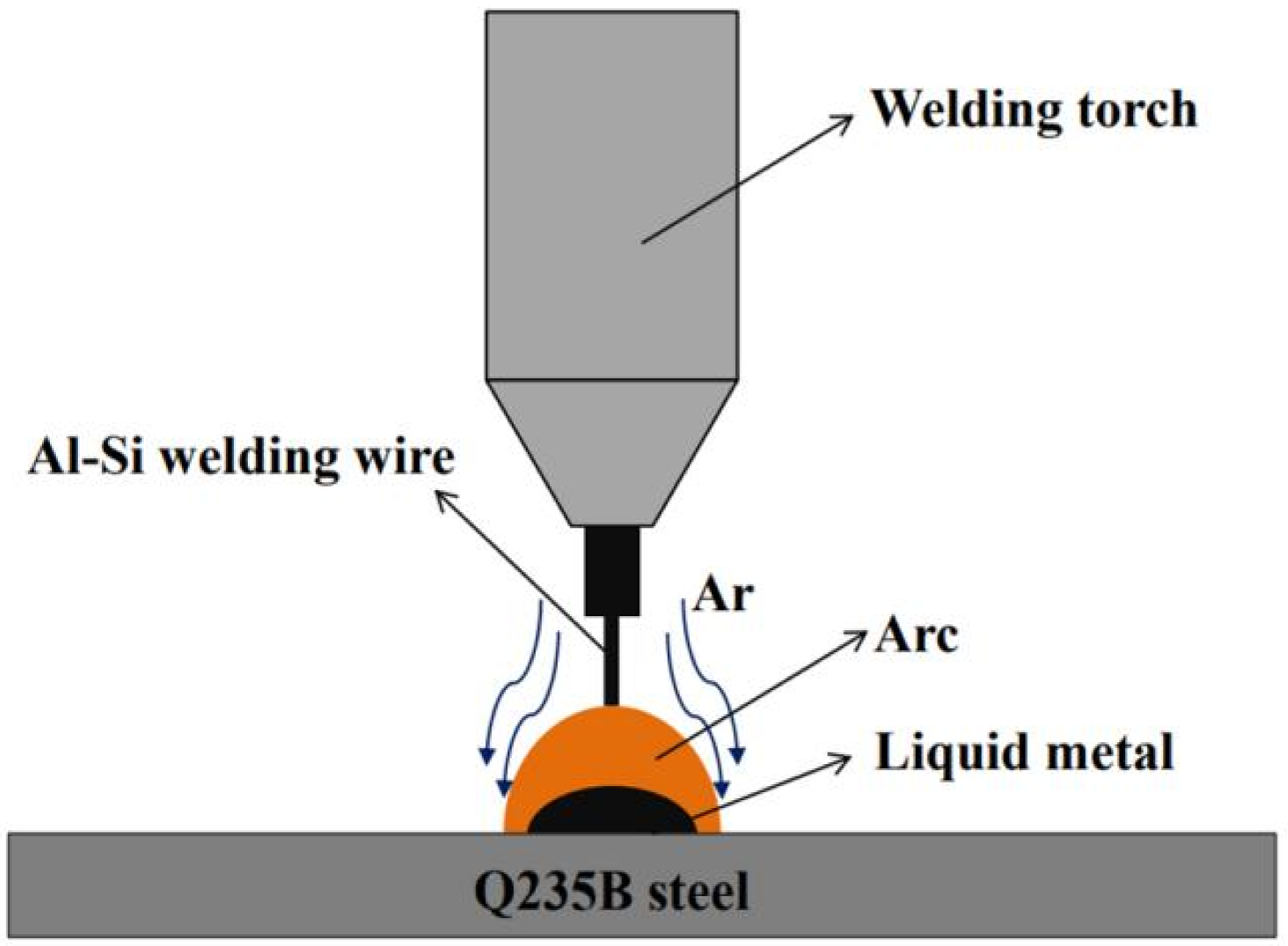
2.3. CMT Welding Brazing Tests
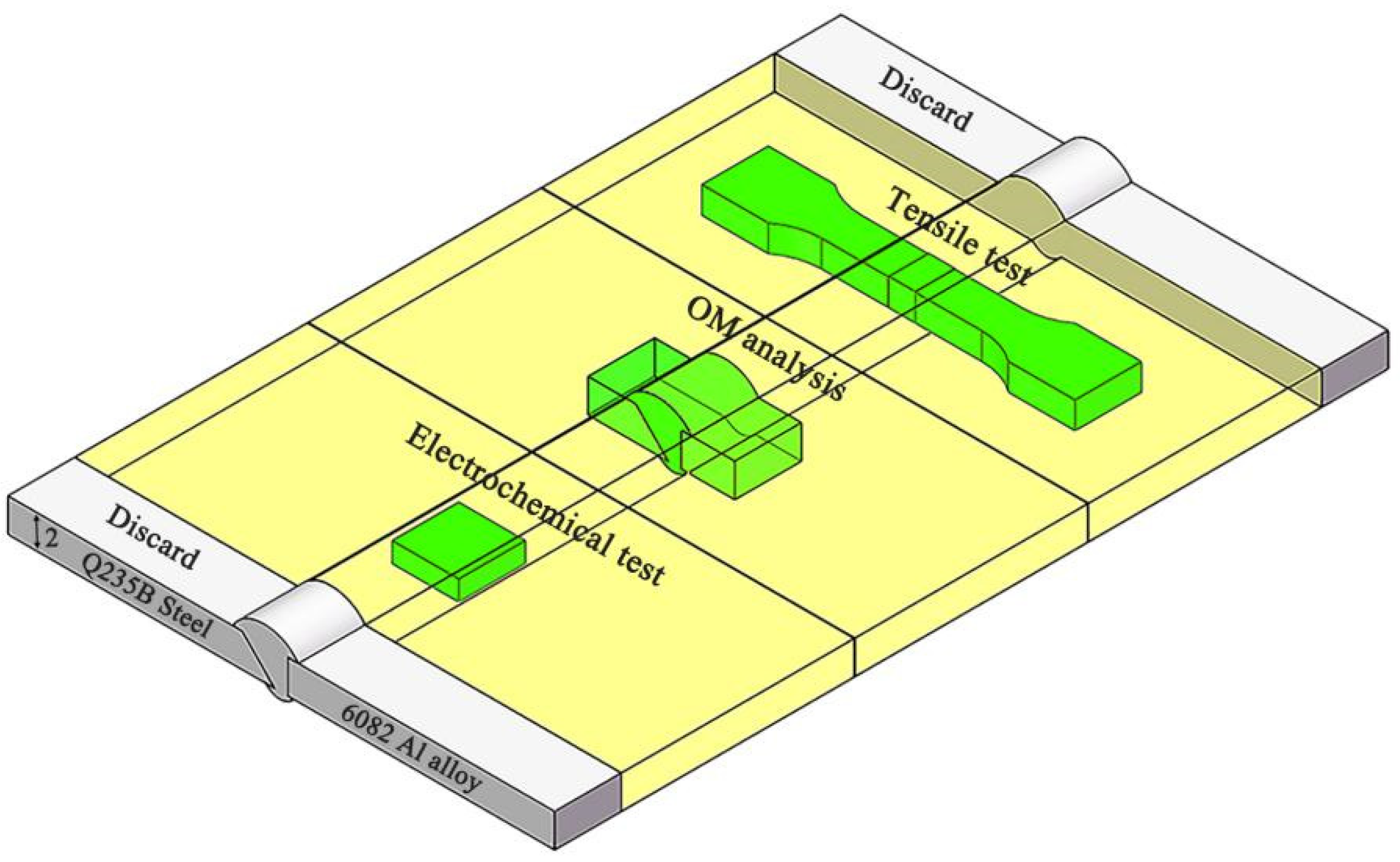
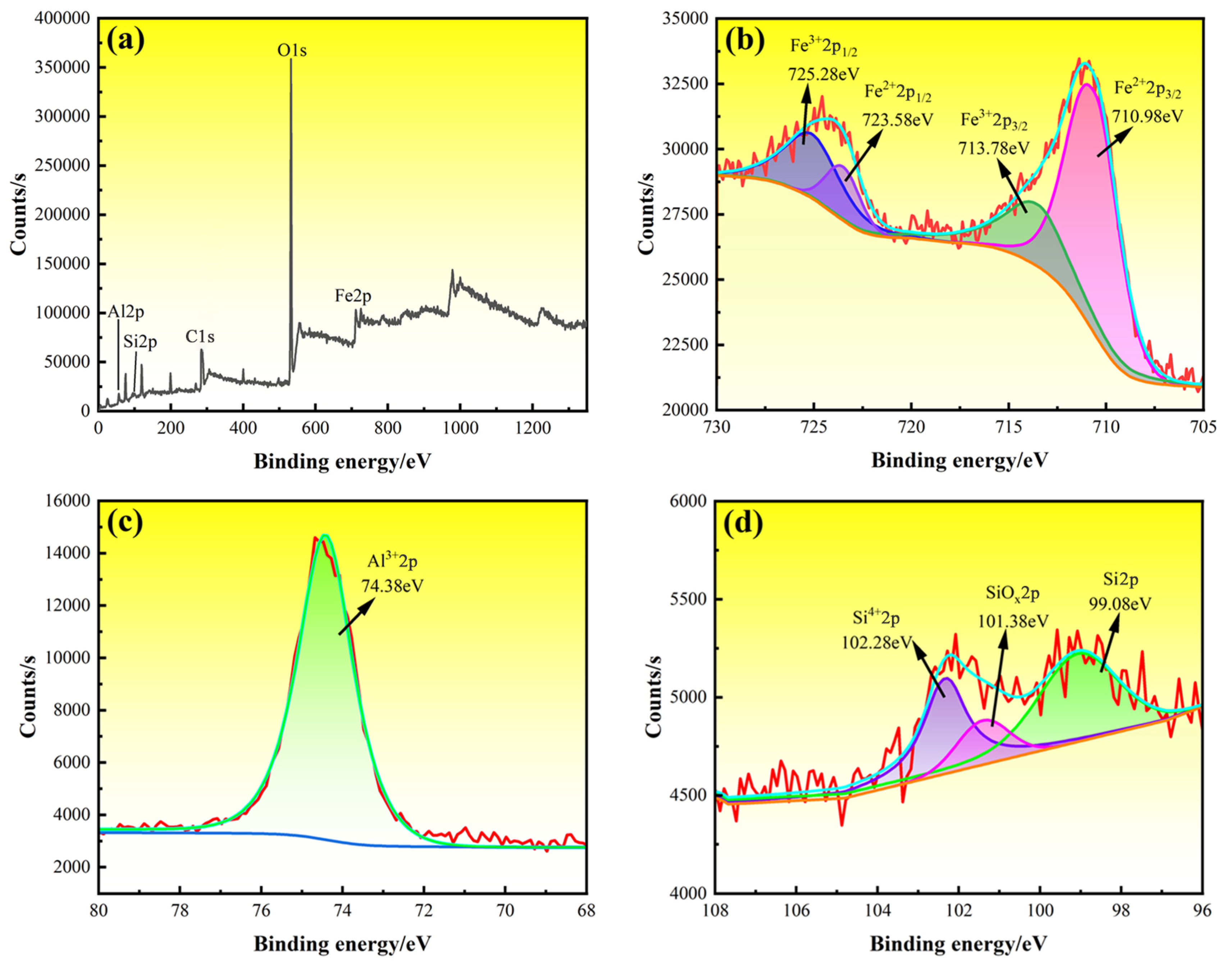
2.4. Microstructure Characterization and Properties Tests
3. Results and Discussion
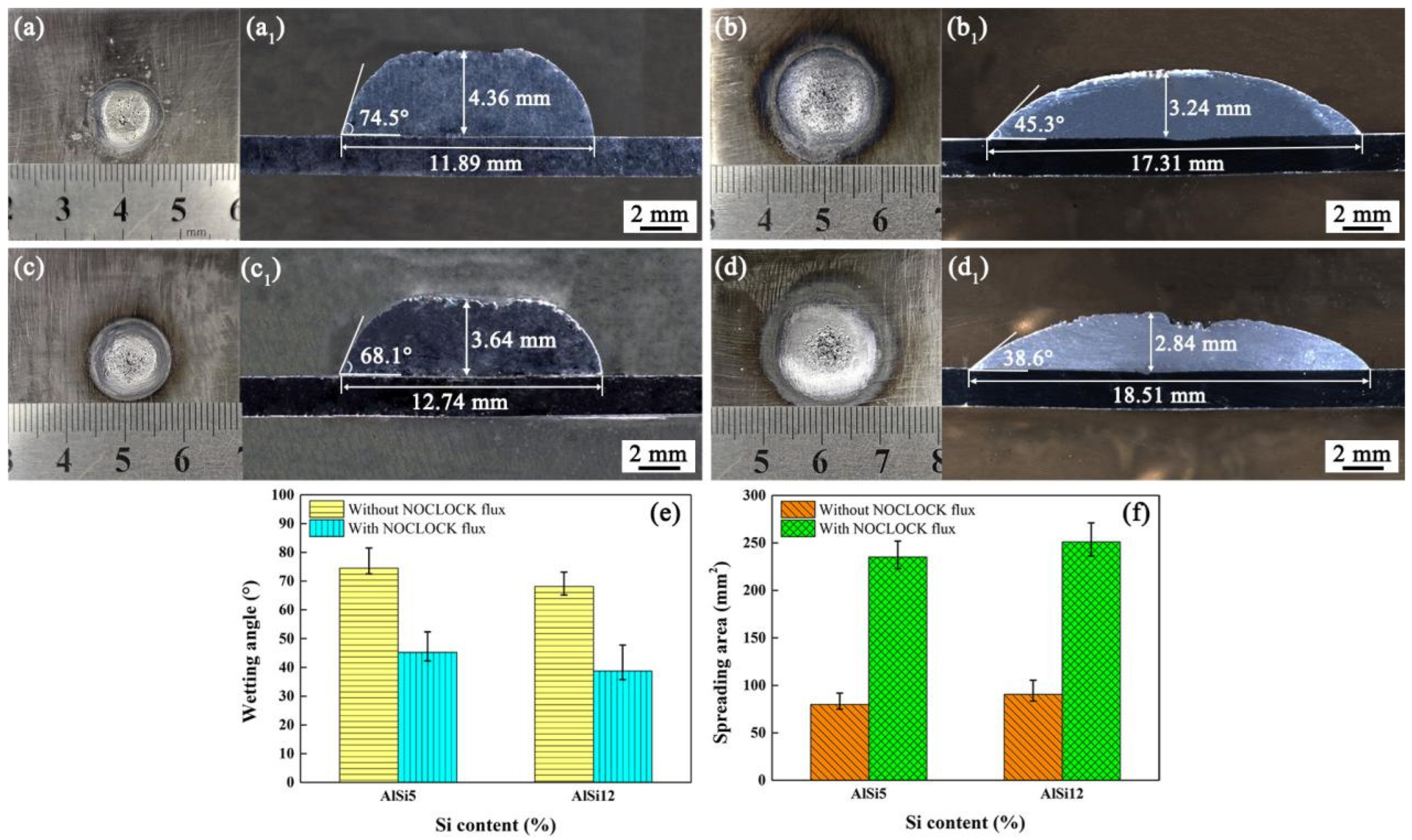
3.1. Effect of Noclock Flux on the Wettability and Spreadability of Al–Si Welding Wires
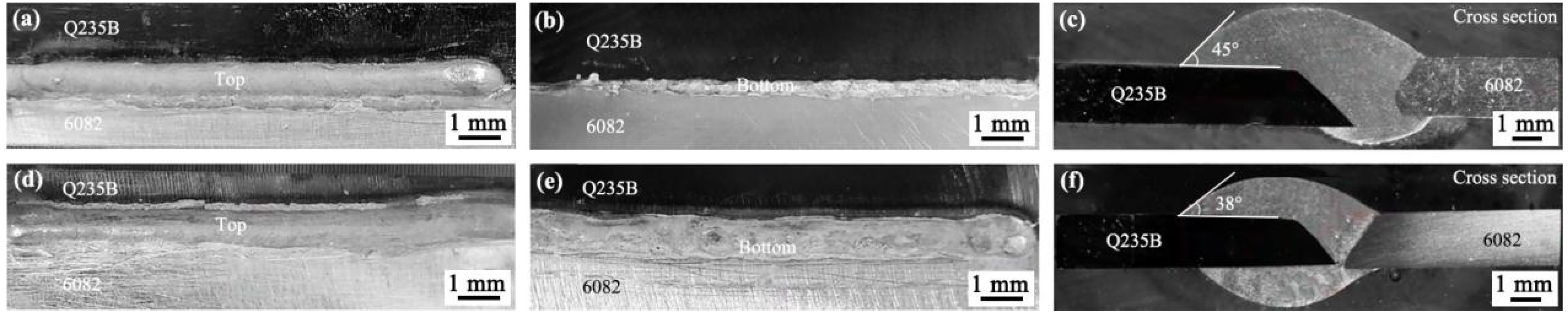
3.2. Macromorphology Analysis of Al Alloy/Steel CMT Welding-Brazing Joints with AlSi5 and AlSi12 Flux-Cored Welding Wire
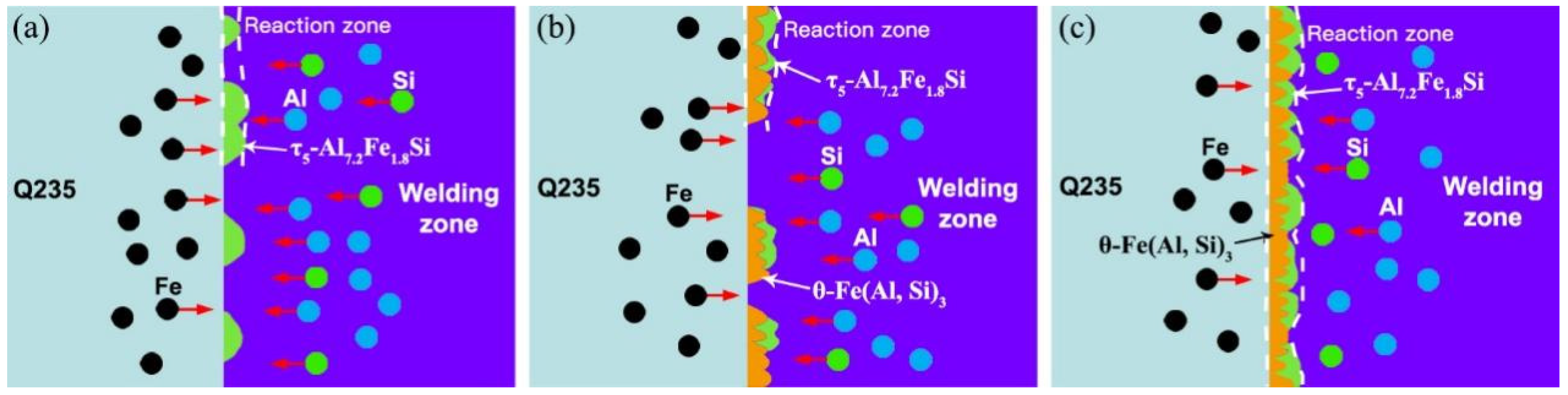
3.3. Microstructure Analysis of Al Alloy/Steel CMT Welding-Brazing Joints with AlSi5 and AlSi12 Flux-Cored Welding Wire
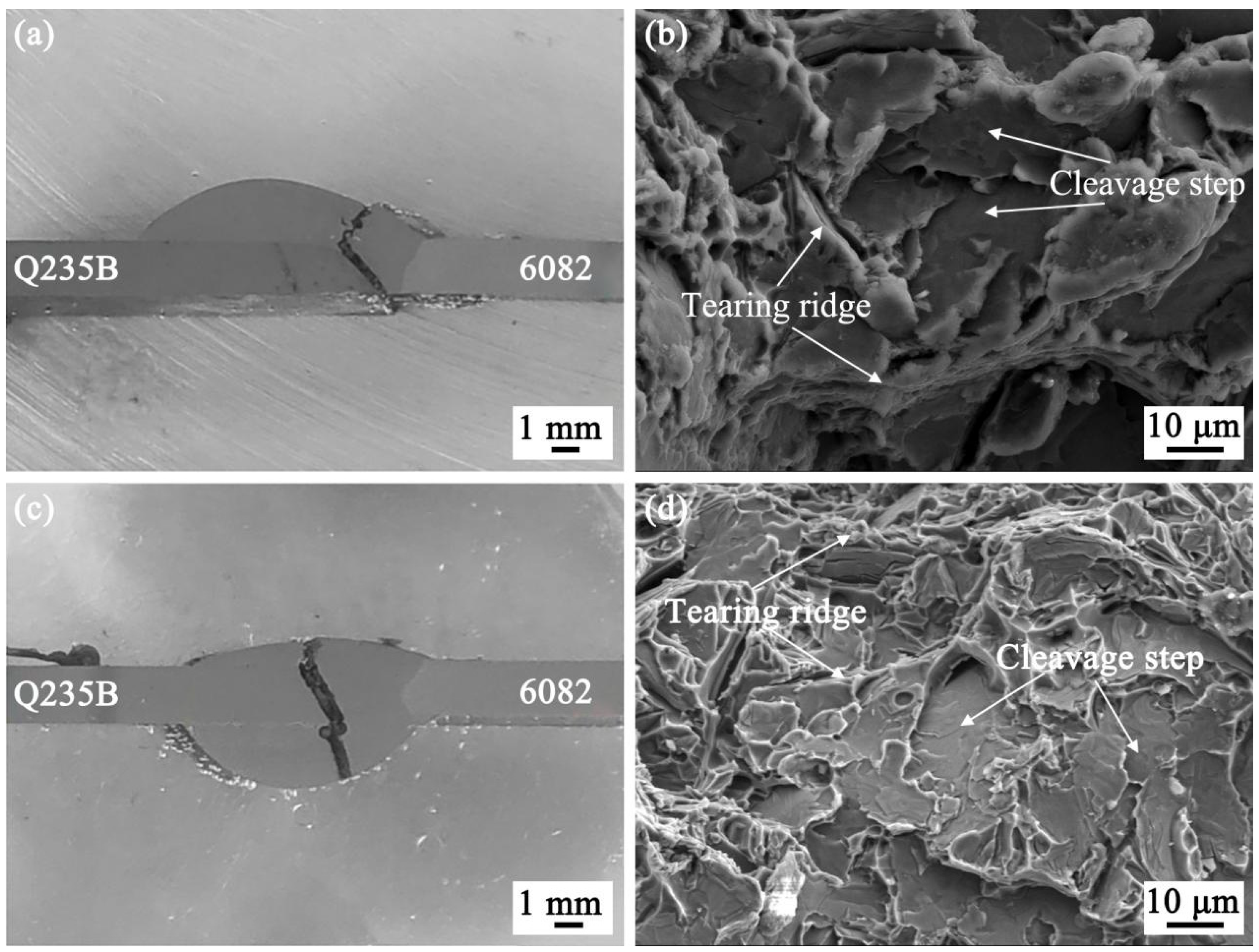
3.4. Tensile Properties Analysis of Al Alloy/Steel CMT Welding-Brazing Joints with AlSi5 and AlSi12 Flux-Cored Welding Wire
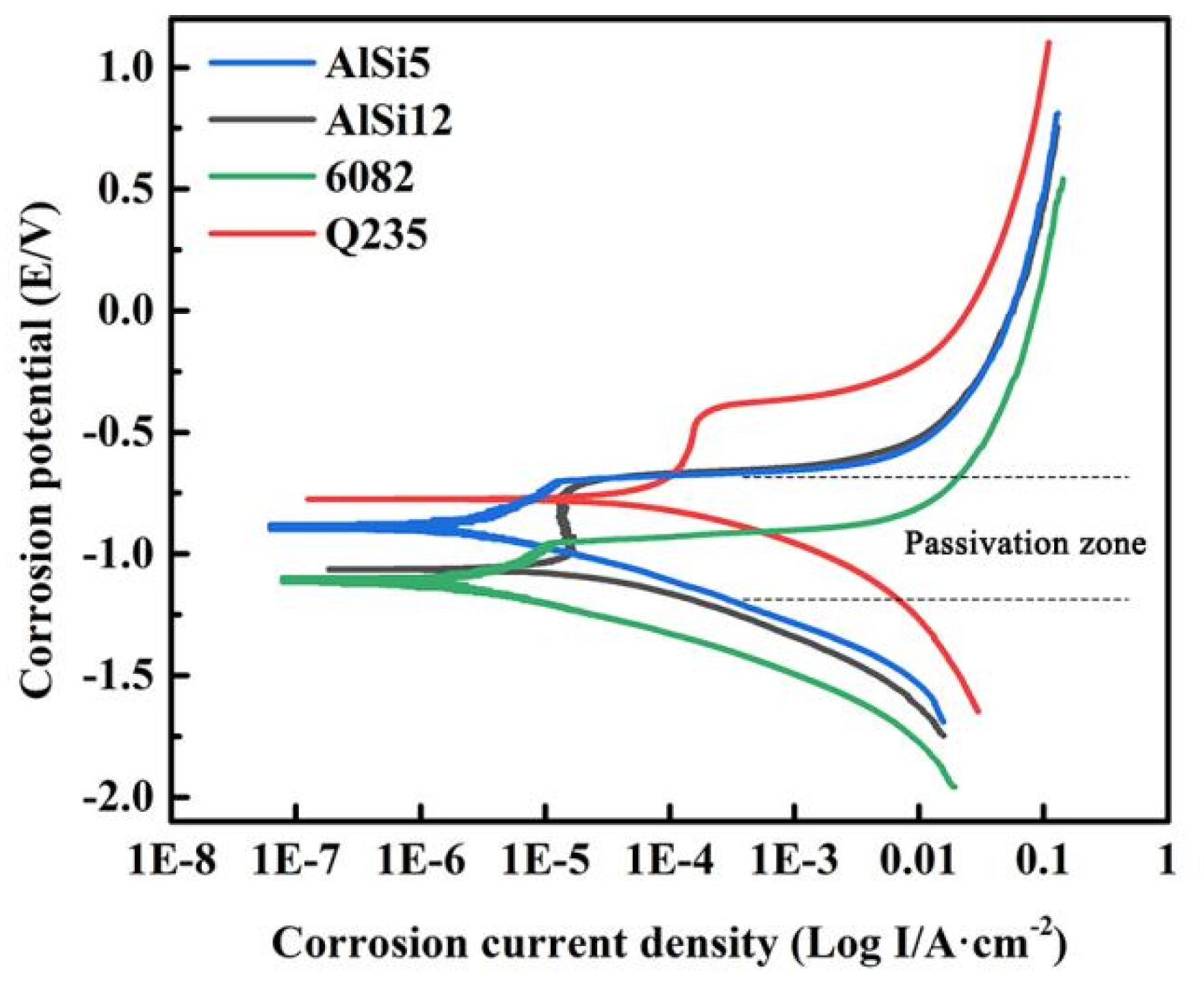
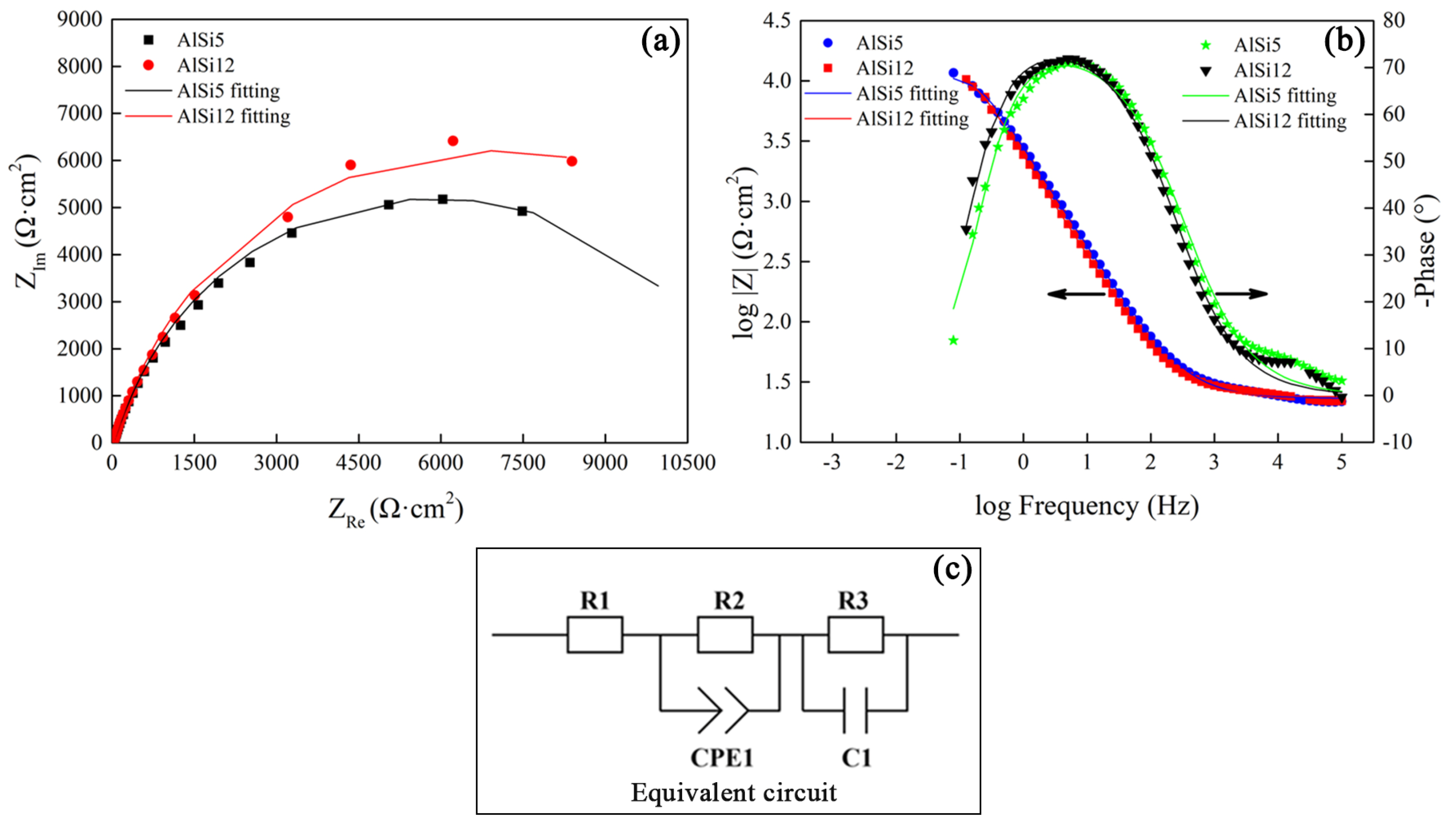
3.5. Corrosion Resistance Analysis of Al Alloy/Steel CMT Welding-Brazing Joints with AlSi5 and AlSi12 Flux-Cored Welding Wire
4. Conclusions
- (1)
- The addition of Noclock flux improved the wettability and spreadability of the Al–Si welding wires on the low-carbon steel surface, resulting in the good appearance of the Al alloy/steel CMT welding joints. Compared to the AlSi5 flux-cored welding wire, the AlSi12 flux-cored welding wire had better wettability and spreadability.
- (2)
- When using AlSi5 and AlSi12 flux-cored welding wires, the Al alloy/steel CMT welding-brazing joints were both composed of θ-Fe (Al, Si)3 and τ5-Al7.2Fe1.8Si formed at the interface reaction zone as well as an α-Al solid solution and Al-Si eutectic phase generated in the Al alloy fusion zone. Compared with the two joints, the IMC thickness of the joint with the AlSi12 flux-cored welding wire was thinner.
- (3)
- The tensile strength of the Al alloy/steel joint with the AlSi12 flux-cored welding wire was slightly lower than that of the joint with the AlSi5 flux-cored welding wire, and its corrosion resistance was better. This was attributed to the thinner IMC thickness at the interface of the joint.
- (4)
- The reason for the good corrosion resistance of the Al alloy/steel joint with the AlSi12 flux-cored welding wire was due to the presence of a large amount of Fe, Al, and Si oxides in the passivation film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, S.; Lee, Y.; Park, G.; Kim, B.; Moon, J.; Park, S.-J.; Lee, C. Phase transformation and the mechanical characteristics of heat-affected zones in austenitic Fe–Mn–Al–Cr–C lightweight steel during post-weld heat treatment. Mater. Charact. 2021, 177, 111150. [Google Scholar] [CrossRef]
- Harwarth, M.; Chen, G.; Rahimi, R.; Biermann, H.; Zargaran, A.; Duffy, M.; Zupan, M.; Mola, J. Aluminum-alloyed lightweight stainless steels strengthened by B2-(Ni, Fe)Al precipitates. Mater. Des. 2021, 206, 109813. [Google Scholar] [CrossRef]
- Adamiak, M.; Wyględacz, B.; Czupryński, A.; Górka, J. A study of susceptibility and evaluation of causes of cracks formation in braze-weld filler metal in lap joints aluminum-carbon steel made with use of CMT method and high power diode laser. Arch. Metall. Mater. 2017, 62, 2113–2123. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, M.; Pu, J.; Rao, J.; Long, W.; Shen, Y. Effect of Ni coating on microstructure and property of Al alloy/steel CMT welding-brazing joints. Coatings 2023, 13, 418. [Google Scholar] [CrossRef]
- Bergh, T.; Arbo, S.M.; Hagen, A.B.; Blindheim, J.; Friis, J.; Khalid, M.Z.; Ringdalen, I.G.; Holmestad, R.; Westermann, I.; Vullum, P.E. On intermetallic phases formed during interdiffusion between aluminium alloys and stainless steel. Intermetallics 2022, 142, 107443. [Google Scholar] [CrossRef]
- Dang, Z.; Qin, G.; Wang, J. Bonding mechanism and fracture behavior of inertia friction welded joint of 2219 aluminum alloy to 304 stainless steel. Mater. Sci. Eng. A 2023, 866, 144641. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, K.; Wang, Z.; Hu, X.; Liu, H.; Nie, Z. Effect of intermetallic compounds on interfacial bonding of Al/Fe composites. Mater. Lett. 2023, 333, 133597. [Google Scholar] [CrossRef]
- Wloka, J.; Laukant, H.; Glatzel, U.; Virtanen, S. Corrosion properties of laser beam joints of aluminium with zinc-coated steel. Corros. Sci. 2007, 49, 4243–4258. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, X.; Xu, C.; Chen, Z.; Feng, X.; Zhou, Q.; Liu, J.; Li, L. Equivalent characterization of pre-strained material properties and mechanical behavior prediction of steel/aluminum self-piercing riveted joints. Thin. Wall. Struct. 2023, 182, 110243. [Google Scholar] [CrossRef]
- Chen, W.; Deng, H.; Dong, S.; Zhu, Z. Numerical modelling of lockbolted lap connections for aluminium alloy plates. Thin. Wall. Struct. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Sundaraselvan, S.; Senthilkumar, N.; Balamurugan, T.; Kaviarasu, C.; Sathishkumar, G.B.; Rajesh, M. Optimization of friction welding process parameters for joining Al6082 and mild steel using RSM. Mater. Today Proc. 2023, 74, 91–96. [Google Scholar] [CrossRef]
- Sun, H.; Yu, G.; Chen, S.; Huang, J.; Yang, J. Effect of ZnAl filler metals on the characteristics of the joint made by the high-frequency induction brazing of 304 stainless steel and 6A02 aluminum. J. Manuf. Process. 2021, 68, 961–972. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, M.; Pu, J.; Shan, Q.; Sun, Y.; Wang, S.Q.; Hermann, S.K.U.G. Effect of Cu Coating on microstructure and properties of Al/steel welding-brazing joints obtained by cold metal transfer (CMT). Coatings 2022, 12, 1123. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Huang, J.K. Corrosion properties of aluminum-steel dissimilar metals MIG welding-brazing joint. Hot Work. Technol. 2018, 47, 214–217+222. [Google Scholar]
- Qin, G.; Ji, Y.; Ma, H.; Ao, Z. Effect of modified flux on MIG arc brazing-fusion welding of aluminum alloy to steel butt joint. J. Mater. Process. Technol. 2017, 245, 115–121. [Google Scholar] [CrossRef]
- Ye, Z.; Huang, J.; Gao, W.; Zhang, Y.; Cheng, Z.; Chen, S.; Yang, J. Microstructure and mechanical properties of 5052 aluminum alloy/mild steel butt joint achieved by MIG-TIG double-sided arc welding-brazing. Mater. Des. 2017, 123, 69–79. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Yazdian, N.; Wang, H.-P.; Carlson, B.; Kovacevic, R. Effect of filler wire composition on performance of Al/Galvanized steel joints by twin spot laser welding-brazing method. J. Manuf. Process. 2018, 31, 20–34. [Google Scholar] [CrossRef]
- Wallerstein, D.; Solla, E.L.; Lusquiños, F.; Comesaña, R.; del Val, J.; Riveiro, A.; Pou, J. Advanced characterization of intermetallic compounds in dissimilar aluminum-steel joints obtained by laser welding-brazing with AlSi filler metals. Mater. Charact. 2021, 179, 111345. [Google Scholar] [CrossRef]
- Yuan, R.; Deng, S.; Cui, H.; Chen, Y.; Lu, F. Interface characterization and mechanical properties of dual beam laser welding-brazing Al/steel dissimilar metals. J. Manuf. Process. 2019, 40, 37–45. [Google Scholar] [CrossRef]
- Peng, M.; Liu, H.; Liang, Y.; Xu, W.; Zhao, Y.; Chen, S.; Weng, J.; Yang, J. CMT welding-brazing of Al/steel dissimilar materials using cycle-step mode. J. Mater. Res. Technol. 2022, 18, 1267–1280. [Google Scholar] [CrossRef]
- Yu, G.; Chen, S.; Zhao, Z.; Wen, Z.; Huang, J.; Yang, J.; Chen, S. Comparative study of laser swelding-brazing of aluminum alloy to galvanized steel butted joints using five different filler wires. Opt. Laser Technol. 2022, 147, 107618. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, H.; Li, P.; Yang, J.; Wu, B.; Hao, X.; Xia, Y.; Qi, G. A novel corrosion transformation process in aluminum alloy/galvanized steel welded joint. Corros. Sci. 2022, 194, 109936. [Google Scholar] [CrossRef]
- Matsuda, T.; Ogaki, T.; Hayashi, K.; Iwamoto, C.; Nozawa, T.; Ohata, M.; Hirose, A. Fracture dominant in friction stir spot welded joint between 6061 aluminum alloy and galvannealed steel based on microscale tensile testing. Mater. Des. 2022, 213, 110344. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, S.Q.; Li, Y.; Huang, J.H.; Chen, S.J.; Yang, J. Butt welding-brazing of steel to aluminum by hybrid laser-CMT. J. Mater. Process. Technol. 2019, 272, 163–169. [Google Scholar] [CrossRef]
- Mei, S.W.; Gao, M.; Yan, J.; Zhang, C.; Li, G.; Zeng, X.Y. Interface properties and thermodynamic analysis of laser–arc hybrid welded Al/steel joint. Sci. Technol. Weld. Join. 2013, 18, 293–300. [Google Scholar] [CrossRef]
- Singh, J.; Arora, K.S.; Shukla, D.K. Lap weld-brazing of aluminum to steel using novel cold metal transfer process. J. Mater. Process. Technol. 2020, 283, 116728. [Google Scholar] [CrossRef]
- GB/T 11364-2008; Test Method of Wettability for Brazing Filler Metals. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- GB/T 2651-2008; Tensile Test Method on Welded Joints. Ministry of Chemical Industry of the People Republic of China: Beijing, China, 2008.
- Zhang, J.; Xue, S.; Xue, P.; Yang, J.; Lv, Z. Effect of Ga2O3 on the wettability and spreadability of CsF-RbF-AlF3 Flux/Zn-Al filler metal on aluminum and steel. Rare Metal Mater. Eng. 2017, 46, 1900–1904. [Google Scholar]
- Zhu, H.; Xue, S.B.; Sheng, Z. Mechanism of CsF-AlF3 and KF-AlF3 fluxes reacting with oxide films of 6063 aluminum alloy. Trans. China Weld. Inst. 2009, 30, 13–16+20+113. [Google Scholar]
- Wang, T.; Chen, C.; Ma, J.; Wei, S.; Xiong, M.; Mao, F.; Zhang, P.; Zhang, G. Influence of Si on the intermetallic compound formation in the hot-dipped aluminide medium carbon steel. Mater. Charact. 2023, 197, 112700. [Google Scholar] [CrossRef]
- Marker, M.C.J.; Skolyszewska-Kühberger, B.; Effenberger, H.S.; Schmetterer, C.; Richter, K.W. Phase equilibria and structural investigations in the system Al–Fe–Si. Intermetallics 2011, 19, 1919–1929. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Khond, A.A.; Palikundwar, U.A. Prediction of glass forming compositions in Al-Fe-Si alloy system by thermodynamic approach. J. Non-Cryst. Solids 2017, 477, 50–57. [Google Scholar] [CrossRef]
- Du, Y.; Schuster, J.C.; Liu, Z.-K.; Hu, R.; Nash, P.; Sun, W.; Zhang, W.; Wang, J.; Zhang, L.; Tang, C.; et al. A thermodynamic description of the Al–Fe–Si system over the whole composition and temperature ranges via a hybrid approach of CALPHAD and key experiments. Intermetallics 2008, 16, 554–570. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Chen, H.; Xu, H.; Hu, M.; Ji, Z. Anisotropic elasticity, electronic structure and thermodynamic properties of Al-Fe-Si intermetallic compounds from first principles calculations. Solid State Commun. 2019, 298, 113643. [Google Scholar] [CrossRef]
- Yang, J.; Hu, A.; Li, Y.; Zhang, P.; Chandra Saha, D.; Yu, Z. Heat input, intermetallic compounds and mechanical properties of Al/steel cold metal transfer joints. J. Mater. Process. Technol. 2019, 272, 40–46. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, H.W. Study of the correlation between pitting corrosion and the component ratio of the dual phase in duplex stainless steel welds. Corros. Sci. 2013, 74, 396–407. [Google Scholar] [CrossRef]
- Gu, Y.F.; Li, J.; Shi, Y.; Huang, J.K.; Fan, D. Corrosion property of arc welding brazed joint between aluminum and steel. Chin. J. Nonferr. Met. 2016, 26, 758–765. [Google Scholar]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys: An experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef]




| Materials | Chemical Composition | Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Fe | Mn | Mg | Cu | Zn | Ti | Cr | C | Melting Point/°C | Heat Conductivity W/(m·K) | Tensile Strength/MPa | |
| 6082 | Bal. | 1.0 | 0.5 | 0.6 | 0.9 | 0.1 | 0.2 | 0.15 | — | — | 649 | 125 | 240 |
| Q235 | — | 0.2 | Bal. | 0.2 | — | — | — | — | 0.7 | 0.2 | 1500 | 77.5 | 375 |
| AlSi5 | Bal. | 5.7 | 0.2 | 0.05 | 0.0048 | 0.007 | 0.002 | 0.2 | <0.01 | — | — | — | — |
| AlSi12 | Bal. | 12.2 | 0.2 | 0.07 | 0.0002 | 0.0057 | 0.0048 | 0.2 | <0.01 | — | — | — | — |
| Noclock Flux | KAlF4 | CsAlF4 | |||||||||||
| 97 | 3 | ||||||||||||
| Parameters | Value |
|---|---|
| Shielding gas flow, L/min | 18 |
| Wire feeding speed, m/min | 5.5 |
| Welding speed, mm/min | 400 |
| Welding voltage, V | 12.0 |
| Welding current, A | 105 |
| Test Points | Element Ration (at. %) | Possible Phases | ||
|---|---|---|---|---|
| Al | Si | Fe | ||
| A | 71.3 | 10.4 | 18.3 | θ-Fe (Al, Si)3 |
| B | 76.5 | 5.0 | 18.5 | τ5-Al7.2Fe1.8Si |
| C | 97.23 | 0.92 | 1.85 | α-Al solid solution |
| D | 80.3 | 19.5 | 0.2 | Al-Si eutectic |
| E | 70.1 | 15.6 | 14.3 | θ-Fe (Al, Si)3 |
| F | 78.4 | 5.3 | 16.3 | τ5-Al7.2Fe1.8Si |
| G | 98.01 | 0.85 | 1.14 | α-Al solid solution |
| H | 1.81 | 98.19 | — | primary Si phase |
| J | 85.6 | 13.1 | 1.3 | Al-Si eutectic |
| Materials | Self-Corrosion Potential/V | Corrosion Current Density/(A·cm−2) |
|---|---|---|
| Al alloy | −0.592 | 3.35 × 10−6 |
| Steel | −1.190 | 5.20 × 10−5 |
| Joint with AlSi5 | −0.713 | 4.95 × 10−6 |
| Joint with AlSi12 | −0.702 | 4.82 × 10−6 |
| R1 (Ω/cm2) | CPE1-T (Scm−2s−1) | CPE1-P (Scm−2s−1) | R2 (Ω/cm2) | C1 (μFcm−1) | R3 (Ω/cm2) | |
|---|---|---|---|---|---|---|
| AlSi5 | 17.39 | 1.87 × 10−4 | 0.72 | 1540 | 7.23 × 10−4 | 818.7 |
| AlSi12 | 15.56 | 3.09 × 10−4 | 0.70 | 2551 | 2.29 × 10−4 | 725.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Pu, J.; Wu, M.; Zhang, C.; Rao, J.; Long, W.; Shen, Y. Research on the Microstructure and Properties of Al Alloy/Steel CMT Welding-Brazing Joints with Al–Si Flux-Cored Welding Wires. Coatings 2023, 13, 1590. https://doi.org/10.3390/coatings13091590
Liu H, Pu J, Wu M, Zhang C, Rao J, Long W, Shen Y. Research on the Microstructure and Properties of Al Alloy/Steel CMT Welding-Brazing Joints with Al–Si Flux-Cored Welding Wires. Coatings. 2023; 13(9):1590. https://doi.org/10.3390/coatings13091590
Chicago/Turabian StyleLiu, Haodong, Juan Pu, Mingfang Wu, Chao Zhang, Jiawei Rao, Weimin Long, and Yuanxun Shen. 2023. "Research on the Microstructure and Properties of Al Alloy/Steel CMT Welding-Brazing Joints with Al–Si Flux-Cored Welding Wires" Coatings 13, no. 9: 1590. https://doi.org/10.3390/coatings13091590






