The Synthesis and Synergistic Effect of Heterocyclic Groups Grafted on Acrylic Polymers by Ester Groups for Marine Antifouling
Abstract
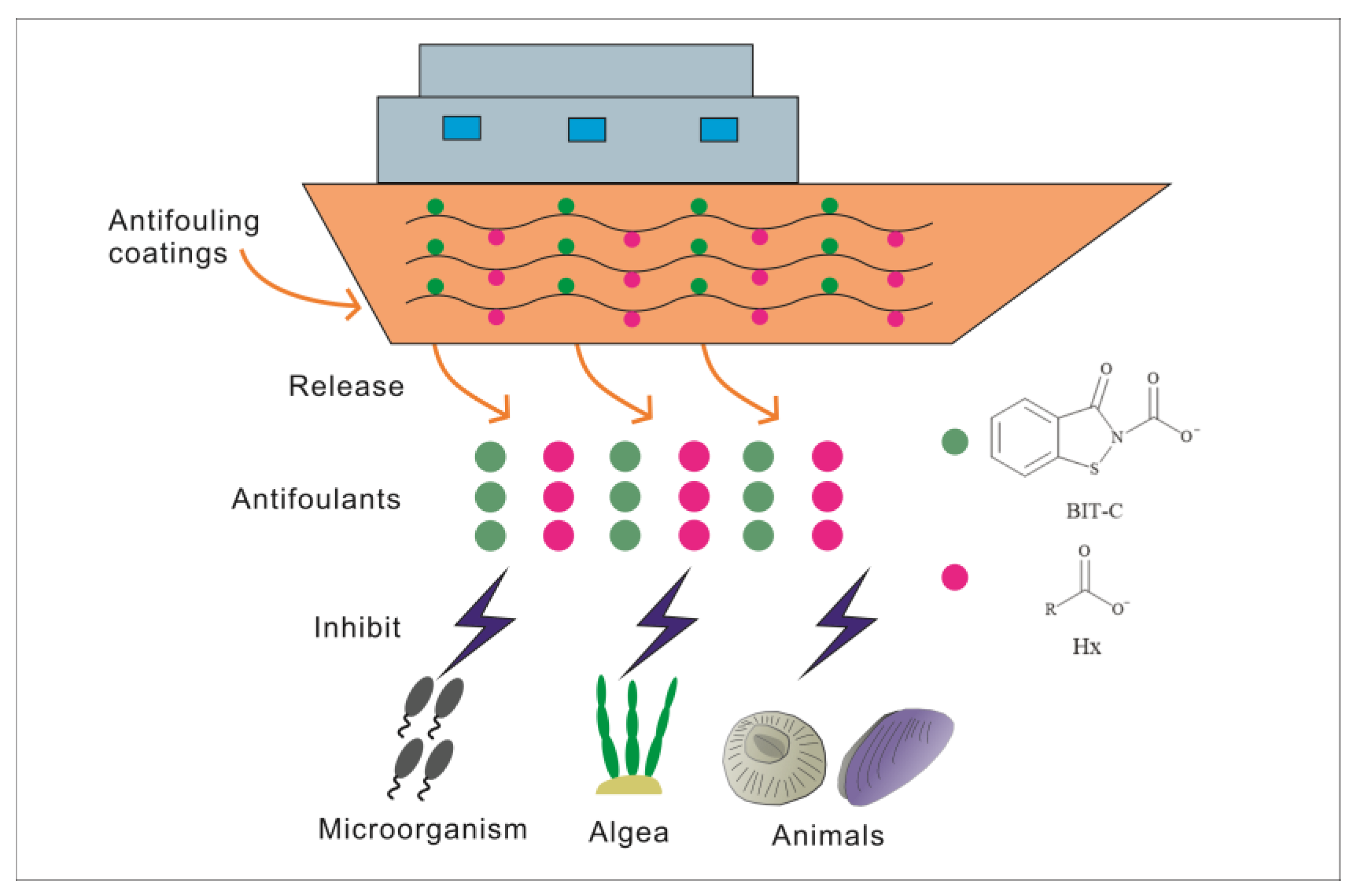
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Allyl 3-Oxobenzo[d]isothiazole-2(3H)-carboxylate (BIT-C)
2.3. Synthesis of Heterocyclic Monomers (Hx)
2.4. Synthesis of Polymer APBHx
2.5. Characterization
2.6. Thermal Analysis
2.7. Weight Change Measurement
2.8. Water Contact Angle Test
2.9. Antibacterial Test
2.10. Anti-Algae Test
2.11. Marine Field Test
3. Results and Discussion
3.1. Characterization
3.2. Thermal Analysis
3.3. Weight Change Measurements
3.4. Water Contact Angle Test
3.5. Antibacterial Test
3.6. Anti-Algae Test
3.7. Marine Field Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prakash, S.; Ramasubburayan, R.; Iyapparaj, P.; Ramaswamy Arthi, A.P.; Ahila, N.K.; Ramkumar, V.S.; Immanuel, G.; Palavesam, A. Environmentally benign antifouling potentials of triterpene-glycosides from Streptomyces fradiae: A mangrove isolate. RSC Adv. 2015, 5, 29524–29534. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Zhang, J.; Habib, S.; Lu, G.; Dai, J.; Liu, X. Bio-based polybenzoxazines coatings for efficient marine antifouling. Prog. Org. Coat. 2023, 174, 107298. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.G.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A.; et al. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Dai, G.; Xie, Q.; Ma, C.; Zhang, G. Biodegradable Poly(ester- co-acrylate) with Antifoulant Pendant Groups for Marine Anti-Biofouling. ACS Appl. Mater. Interfaces 2019, 11, 11947–11953. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Pan, J.; Ma, C.; Zhang, G. Dynamic surface antifouling: Mechanism and systems. Soft Matter 2019, 15, 1087–1107. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Duan, J.; Liu, X.; Dong, X.; Zhang, Y.; Liu, C.; Hou, B. Environmentally benign smart self-healing silicone-based coating with dual antifouling and anti-corrosion properties. Appl. Mater. Today 2022, 28, 101551. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Z.; Gao, Y.; Wang, X.; Chen, J.; Yang, J. Synergistic effect of copolymeric resin grafted 1,2-benzisothiazol-3(2H)-one and heterocyclic groups as a marine antifouling coating. RSC Adv. 2021, 11, 18787–18796. [Google Scholar] [CrossRef]
- Wang, X.; Dong, M.; Meng, Z.; Chen, J.; Yang, J.; Wang, X. Synthesis and Biological Activity of Acrylate Copolymers Containing 3-Oxo-N-allyl-1,2-benzisothiazole-3(2H)-carboxamide Monomer as a Marine Antifouling Coating. ChemistryOpen 2021, 10, 523–533. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef] [PubMed]
- Labriere, C.; Elumalai, V.; Staffansson, J.; Cervin, G.; Le Norcy, T.; Denardou, H.; Rehel, K.; Moodie, L.W.K.; Hellio, C.; Pavia, H.; et al. Phidianidine A and Synthetic Analogues as Naturally Inspired Marine Antifoulants. J. Nat. Prod. 2020, 83, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Soliman, Y.A.A.; Brahim, A.M.; Moustafa, A.H.; Hamed, M.A.F. Antifouling evaluation of extracts from Red Sea soft corals against primary biofilm and biofouling. Asian Pac. J. Trop. Biomed. 2017, 7, 991–997. [Google Scholar] [CrossRef]
- Melrose, J. Mucin-like glycopolymer gels in electrosensory tissues generate cues which direct electrolocation in amphibians and neuronal activation in mammals. Neural Regen. Res. 2019, 14, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, Q.; Deng, X.; Cui, J. Earthworm-Inspired Rough Polymer Coatings with Self-Replenishing Lubrication for Adaptive Friction-Reduction and Antifouling Surfaces. Adv. Mater. 2018, 30, e1802141. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, D.; Lu, Z. Slippery liquid-infused porous surface bio-inspired by pitcher plant for marine anti-biofouling application. Colloids Surf. B Biointerfaces 2015, 136, 240–247. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.R.; Hellum, A.M. Modeling how shark and dolphin skin patterns control transitional wall-turbulence vorticity patterns using spatiotemporal phase reset mechanisms. Sci. Rep. 2014, 4, 6650. [Google Scholar] [CrossRef]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Takahashi, K. Release Rate of Biocides from Antifouling Paints. In Ecotoxicology of Antifouling Biocides; Springer: Tokyo, Japan, 2009; pp. 3–22. [Google Scholar]
- Ding, W.; Ma, C.; Zhang, W.; Chiang, H.; Tam, C.; Xu, Y.; Zhang, G.; Qian, P.Y. Anti-biofilm effect of a butenolide/polymer coating and metatranscriptomic analyses. Biofouling 2018, 34, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hao, X.; Qiu, S.; Lu, G.; Liu, W.; Wang, L.; Wei, Y.; Chen, B.; Lan, X.; Zhao, H. Efficient capture and release of carboxylatedbenzisothiazolinone from UiO-66-NH2 for antibacterial and antifouling applications. J. Colloid. Interf. Sci. 2022, 623, 710–722. [Google Scholar] [CrossRef]
- Qian, P.Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Wang, K.-L.; Qian, P.-Y.; Xu, Y.; Chen, M.; Zheng, J.-J.; Liu, M.; Shao, C.-L.; Wang, C.-Y. Antifouling phenyl ethers and other compounds from the invertebrates and their symbiotic fungi collected from the South China Sea. AMB Express 2016, 6, 102. [Google Scholar] [CrossRef]
- Ge, H.; Liu, G.; Yin, R.; Sun, Z.; Chen, H.; Yu, L.; Su, P.; Sun, M.; Alamry, K.A.; Marwani, H.M.; et al. An aldimine condensation reaction based fluorescence enhancement probe for detection of gaseous formaldehyde. Microchem. J. 2020, 156, 104793. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Dong, M.; Li, Z.; Liu, Z.; Lu, J.; Lin, Q.; Yang, J. Synthesis and biological activities of 1H-indole-1-carboxylic acid aryl esters as a marine antifouling coating. J. Coat. Technol. Res. 2020, 17, 553–561. [Google Scholar] [CrossRef]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Saleh, S.S.; Al-Salihi, S.S.; Mohammed, I.A. Biological activity Study for some heterocyclic compounds and their impact on the gram positive and negative bacteria. Energy Procedia 2019, 157, 296–306. [Google Scholar] [CrossRef]
- Moradi, M.; Duan, J.; Du, X. Investigation of the effect of 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one inhibition on the corrosion of carbon steel in Bacillus sp. inoculated artificial seawater. Corros. Sci. 2013, 69, 338–345. [Google Scholar] [CrossRef]
- Peng, K.; Dai, X.; Mao, H.; Zou, H.; Yang, Z.; Tu, W.; Hu, J. Development of direct contact-killing non-leaching antimicrobial polyurethanes through click chemistry. J. Coat. Technol. RES 2018, 15, 1239–1250. [Google Scholar] [CrossRef]
- Jo, Y.W.; Im, W.B.; Rhee, J.K.; Shim, M.J.; Kim, W.B.; Choi, E.C. Synthesis and antibacterial activity of oxazolidinones containing pyridine substituted with heteroaromatic ring. Bioorg Med. Chem. Lett. 2004, 12, 5909–5915. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A.; Ammar, Y.A. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg Chem. 2021, 110, 104803. [Google Scholar] [CrossRef]
- Ai, X.; Mei, L.; Ma, C.; Zhang, G. Degradable hyperbranched polymer with fouling resistance for antifouling coatings. Prog. Org. Coat. 2021, 153, 106141. [Google Scholar] [CrossRef]
- Li, Y.; Chen, R.; Feng, Y.; Liu, L.; Sun, X.; Tang, L.; Takahashi, K.; Wang, J. Antifouling behavior of self-renewal acrylate boron polymers with pyridine-diphenylborane side chains. New J. Chem. 2018, 42, 19908–19916. [Google Scholar] [CrossRef]
- Feng, K.; Ni, C.; Yu, L.; Zhou, W.; Li, X. Synthesis and evaluation of acrylate resins suspending indole derivative structure in the side chain for marine antifouling. Colloid. Surf. B 2019, 184, 110518. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Ni, C.; Yu, L. Preparation and evaluation of natural rosin-based zinc resins for marine antifouling. Prog. Org. Coat. 2021, 157, 106270. [Google Scholar] [CrossRef]
- Dong, R.; Wang, L.; Zhu, J.; Liu, L.; Qian, Y. A novel SiO2–GO/acrylic resin nanocomposite: Fabrication, characterization and properties. Appl. Phys. A-Mater. 2019, 125, 551. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, Z.; Zhang, F.; Wang, J.; Shao, Q.; Murugadoss, V.; Alhadhrami, A.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; et al. Waterborne acrylic resin co-modified by itaconic acid and γ-methacryloxypropyl triisopropoxidesilane for improved mechanical properties, thermal stability, and corrosion resistance. Prog. Org. Coat. 2022, 168, 106875. [Google Scholar] [CrossRef]
- Cevik, P.; Yildirim-Bicer, A.Z. The Effect of Silica and Prepolymer Nanoparticles on the Mechanical Properties of Denture Base Acrylic Resin. J. Prosthodont. 2018, 27, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, L.; Wang, D.; Li, M.; Yang, J.; Chen, J. Synthesis and Properties of Self-Polishing Antifouling Coatings Based on BIT-Acrylate Resins. Coatings 2022, 12, 891. [Google Scholar] [CrossRef]
- Passauer, L. A case study on the thermal degradation of an acrylate-type polyurethane wood coating using thermogravimetry coupled with evolved gas analysis. Pro Org. Coat. 2021, 157, 106331. [Google Scholar] [CrossRef]





| Samples | Weight Ratio of Monomer (%) | Mn (g mol−1) | Mw (g mol−1) | PDI |
|---|---|---|---|---|
| MMA/BA/Hx/BIT-C | ||||
| APBH0 | 40/40/0/20 | 5894 | 13,339 | 2.26 |
| APBH1 | 30/30/20/20 | 4348 | 6401 | 1.47 |
| APBH2 | 30/30/20/20 | 8318 | 19,849 | 2.39 |
| APBH3 | 30/30/20/20 | 8640 | 22,200 | 2.57 |
| APBH4 | 30/30/20/20 | 9727 | 26,465 | 2.72 |
| APBH5 | 30/30/20/20 | 6619 | 12,429 | 1.88 |
| APBH6 | 30/30/20/20 | 6741 | 14,607 | 2.16 |
| APBH7 | 30/30/20/20 | 5044 | 9005 | 1.79 |
| Samples | Oysters and Barnacle Density (per Square Meter) | Brown Algae and Green Algae Coverage Rate (%) | ||||
|---|---|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | 30 Days | 60 Days | 90 Days | |
| Blank | 719 | 864 | 1801 | 57 | 84 | 100 |
| APBH0 | 0 | 558 | 823 | 14 | 77 | 100 |
| APBH1 | 0 | 0 | 61 | 0 | 8 | 16 |
| APBH2 | 0 | 217 | 355 | 0 | 7 | 7 |
| APBH3 | 0 | 220 | 370 | 0 | 75 | 70 |
| APBH4 | 0 | 283 | 300 | 0 | 22 | 14 |
| APBH5 | 0 | 280 | 368 | 0 | 60 | 32 |
| APBH6 | 0 | 0 | 48 | 0 | 11 | 15 |
| APBH7 | 0 | 0 | 47 | 0 | 10 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, R.; Liu, X.; Hu, G.; Fu, Z.; Dong, M.; Liu, L.; Lin, X.; Zhang, P.; Chen, J.; et al. The Synthesis and Synergistic Effect of Heterocyclic Groups Grafted on Acrylic Polymers by Ester Groups for Marine Antifouling. Coatings 2023, 13, 1643. https://doi.org/10.3390/coatings13091643
Wang D, Liu R, Liu X, Hu G, Fu Z, Dong M, Liu L, Lin X, Zhang P, Chen J, et al. The Synthesis and Synergistic Effect of Heterocyclic Groups Grafted on Acrylic Polymers by Ester Groups for Marine Antifouling. Coatings. 2023; 13(9):1643. https://doi.org/10.3390/coatings13091643
Chicago/Turabian StyleWang, Dazhuang, Ruotong Liu, Xiaohui Liu, Guangwen Hu, Zhineng Fu, Miao Dong, Liju Liu, Xinrui Lin, Ping Zhang, Junhua Chen, and et al. 2023. "The Synthesis and Synergistic Effect of Heterocyclic Groups Grafted on Acrylic Polymers by Ester Groups for Marine Antifouling" Coatings 13, no. 9: 1643. https://doi.org/10.3390/coatings13091643





