The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
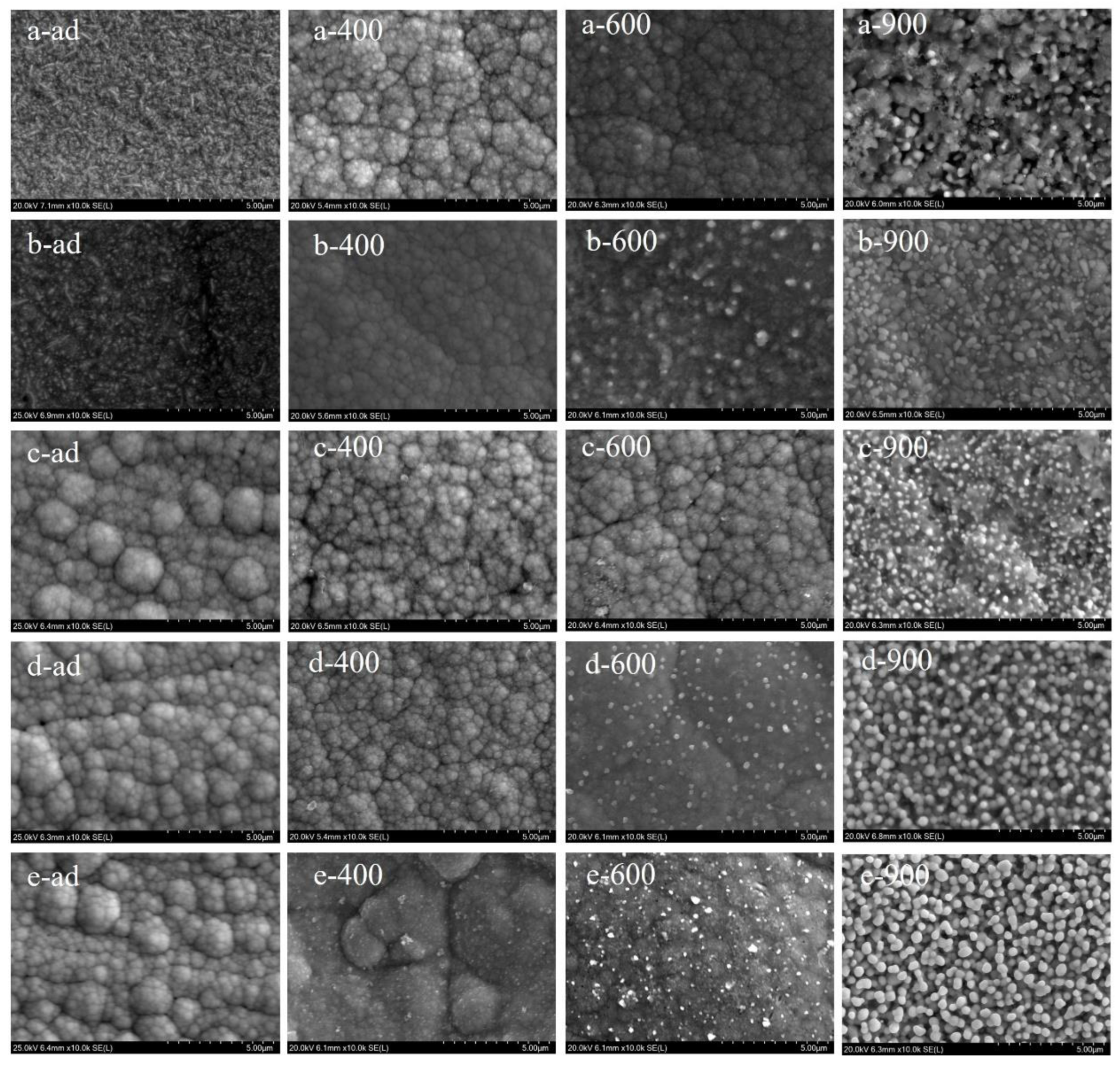
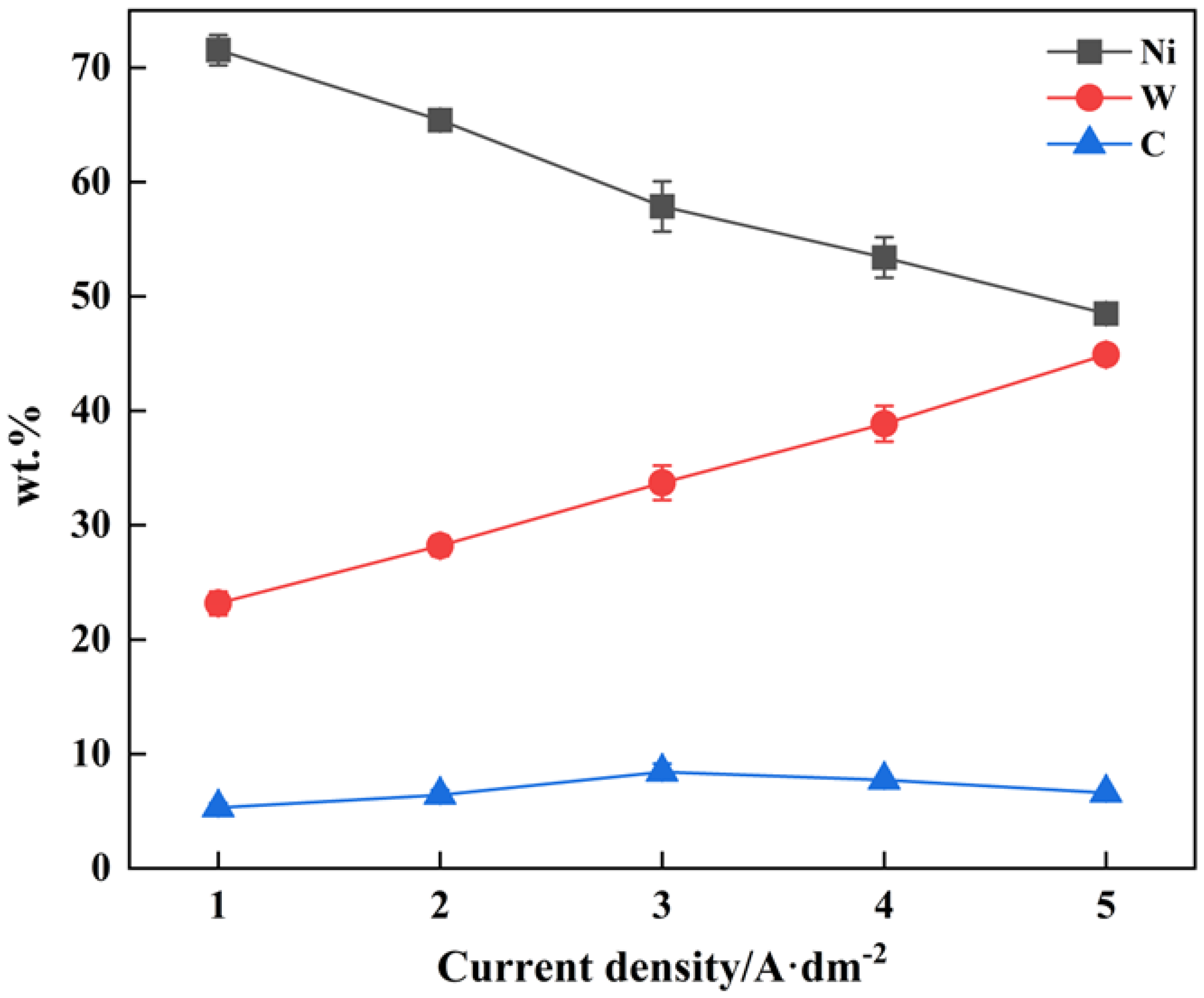
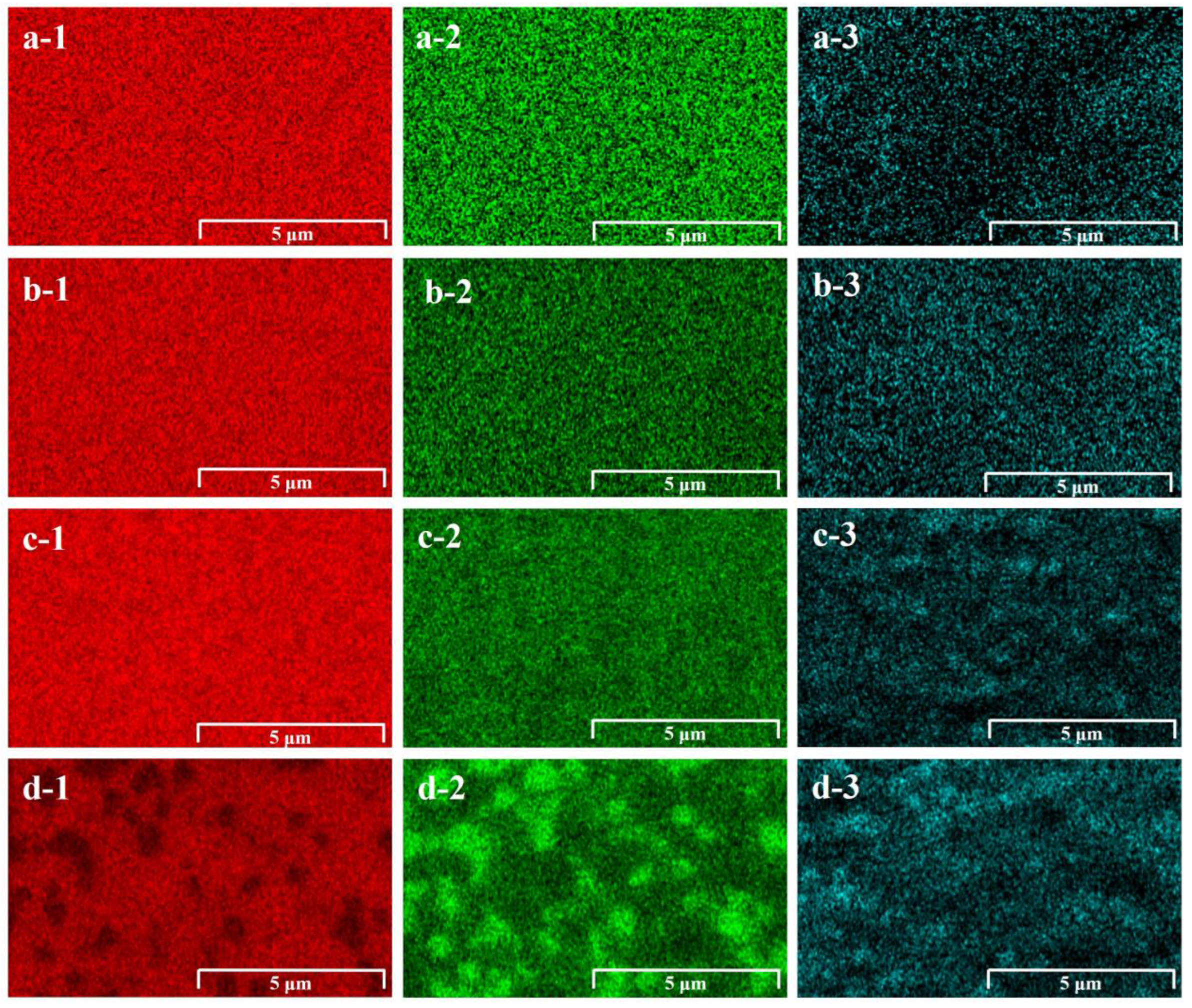
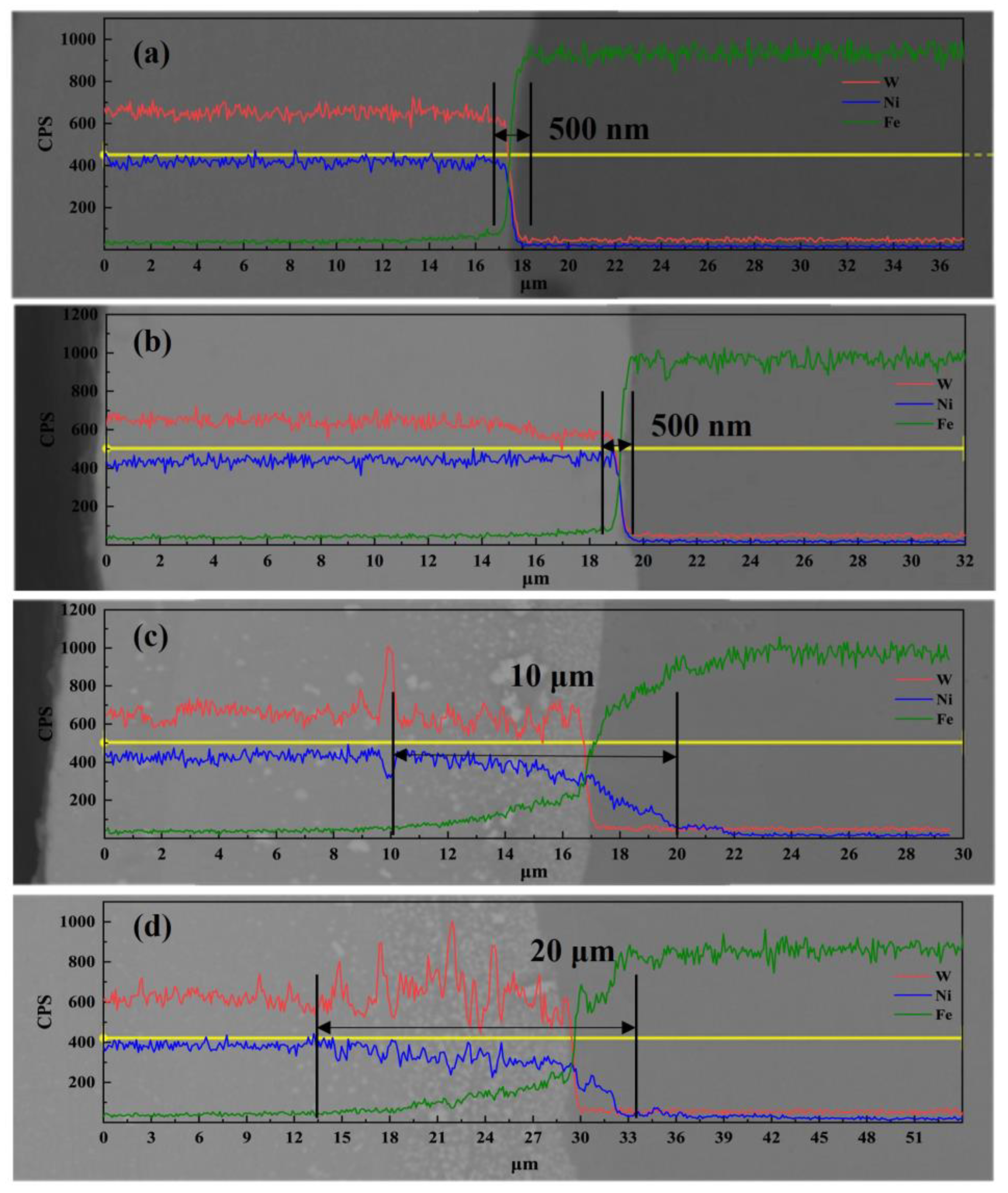
3.1. Surface Morphology and Composition
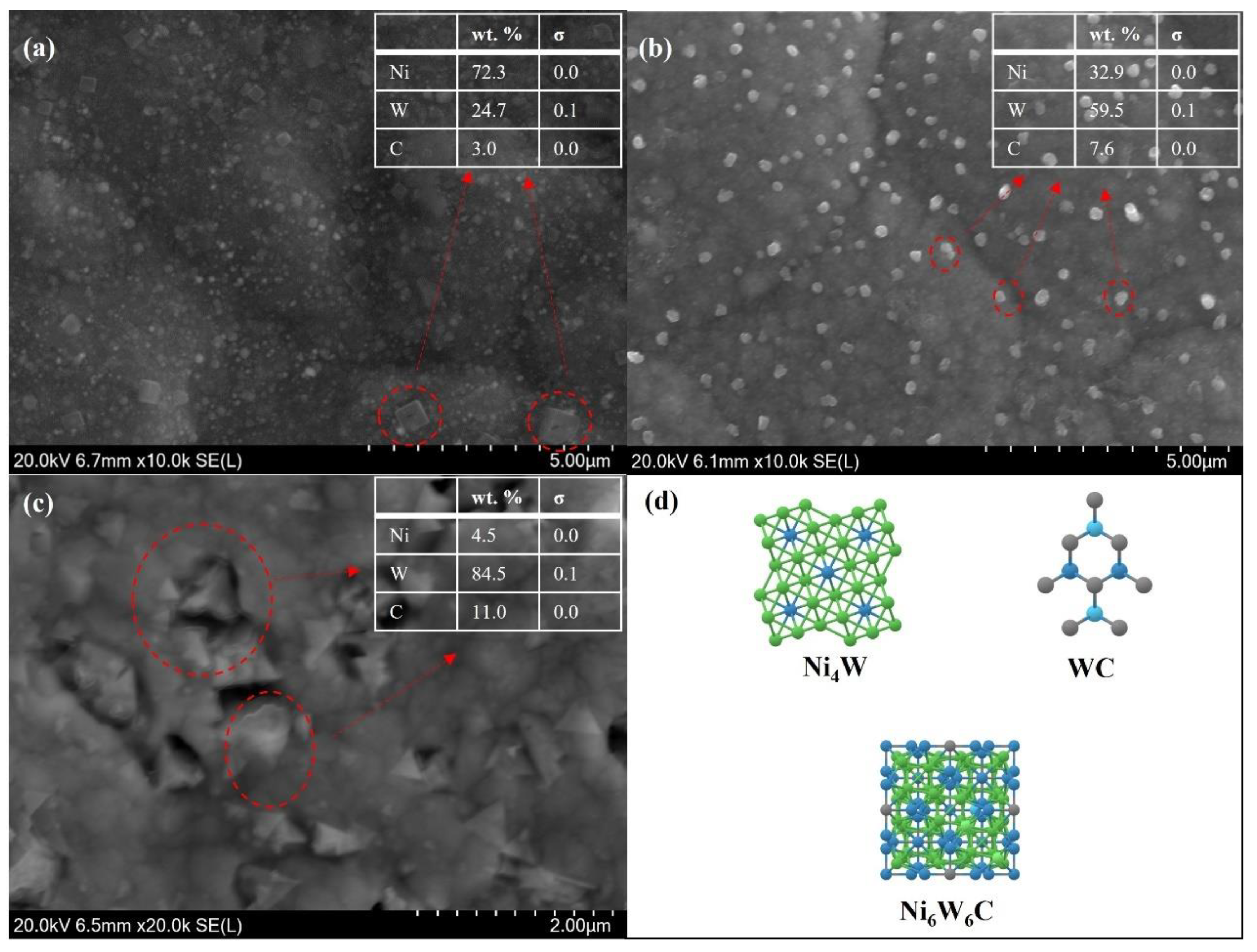
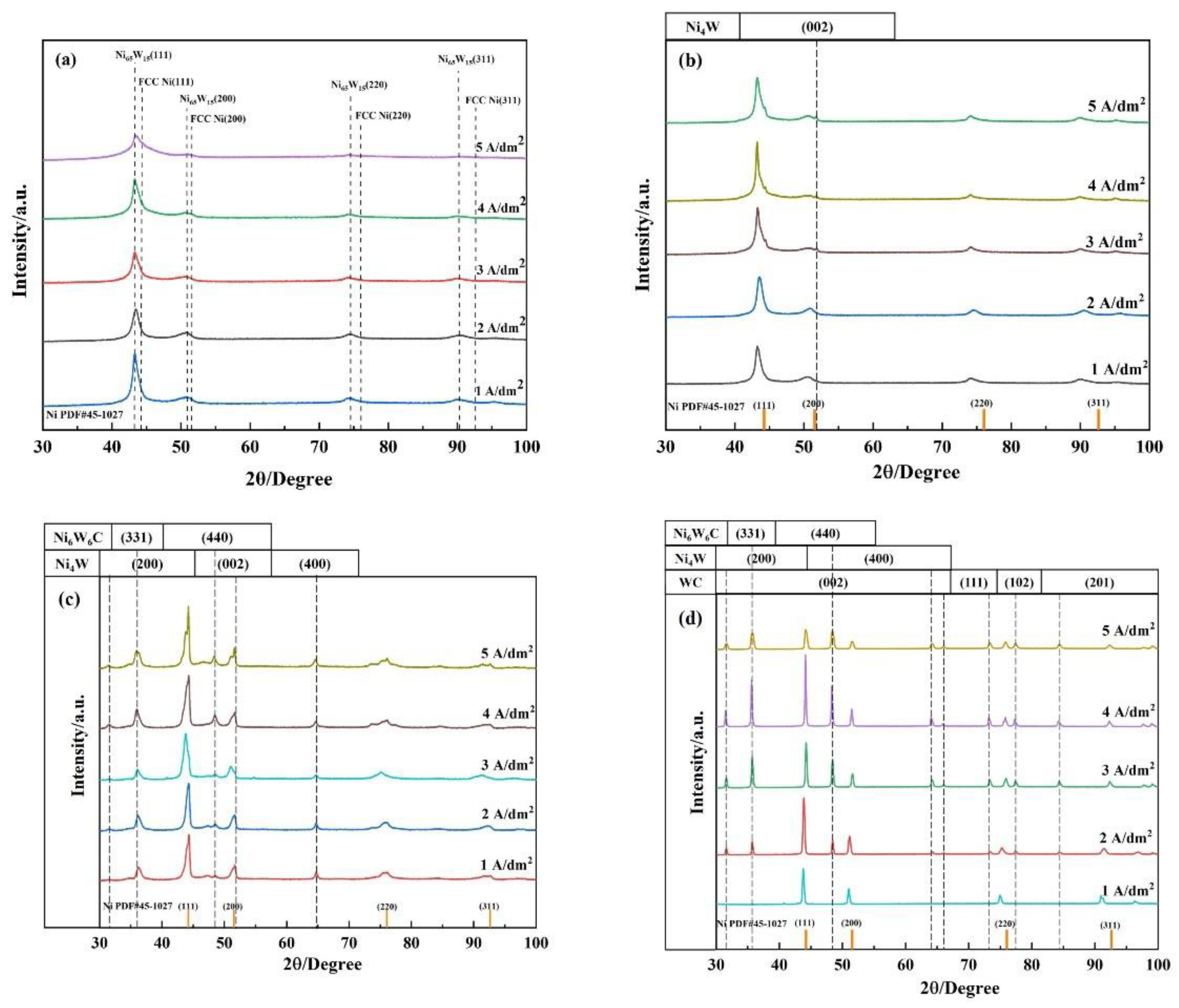
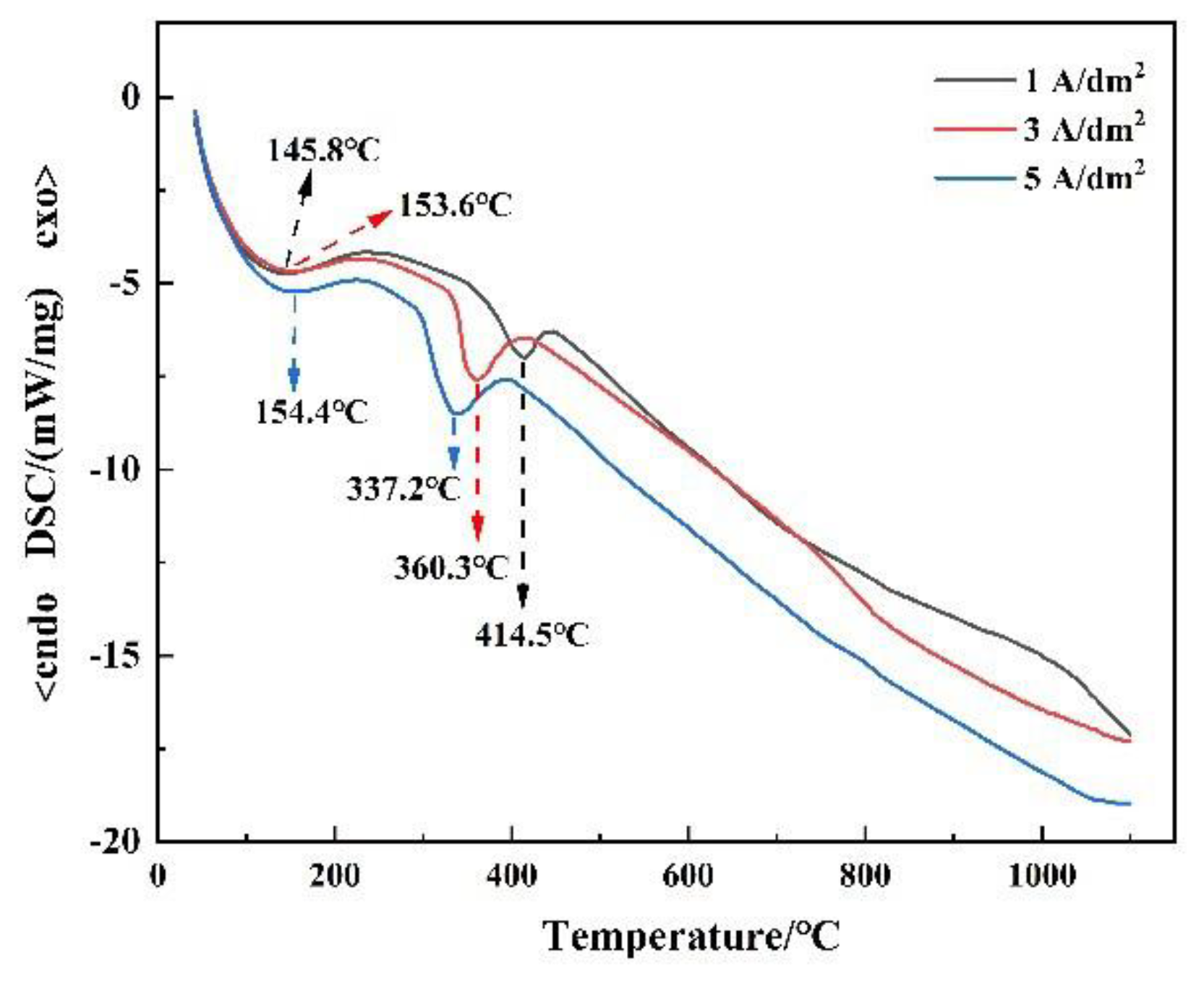
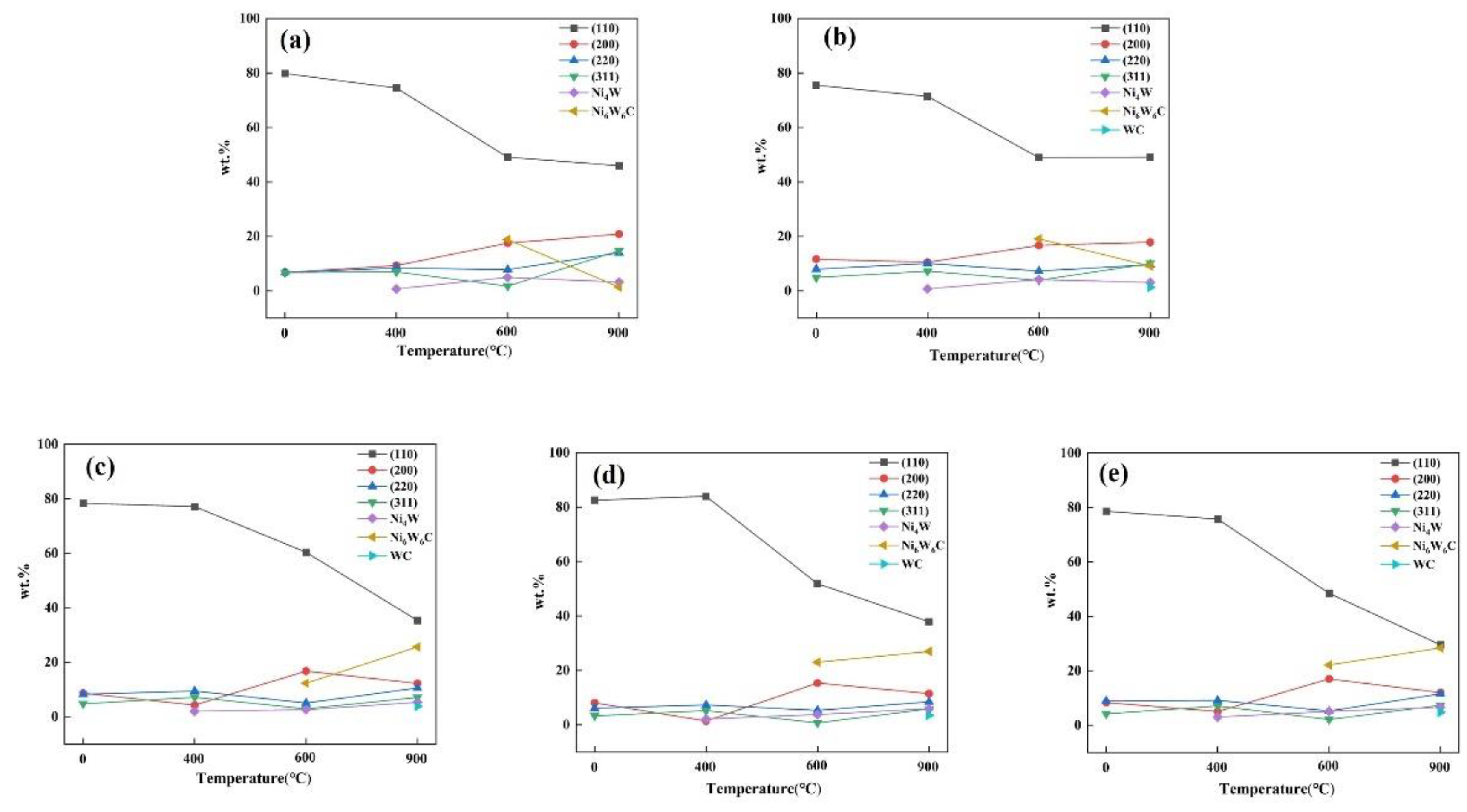
3.2. Phase Analysis
3.3. Mechanical Properties
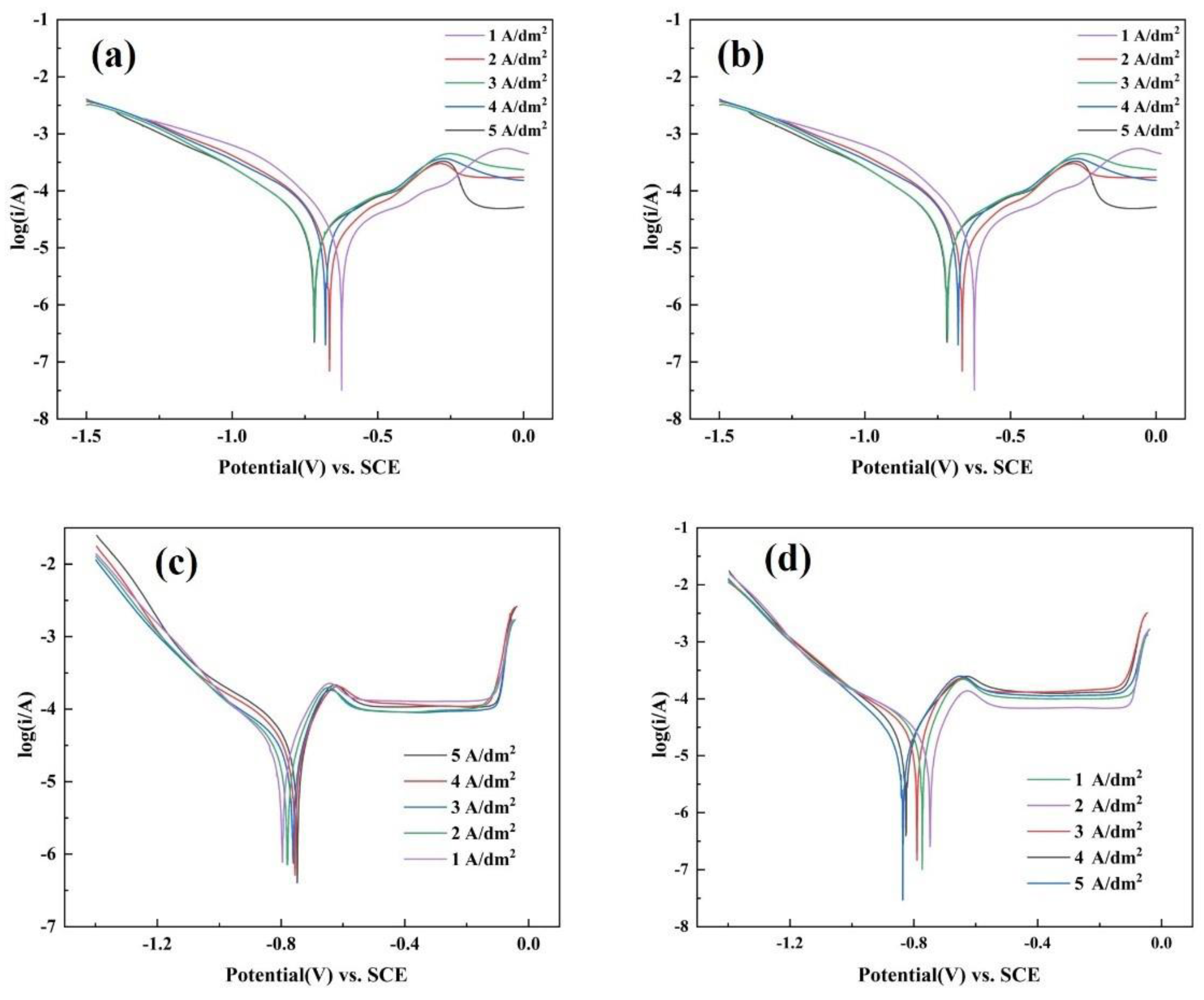
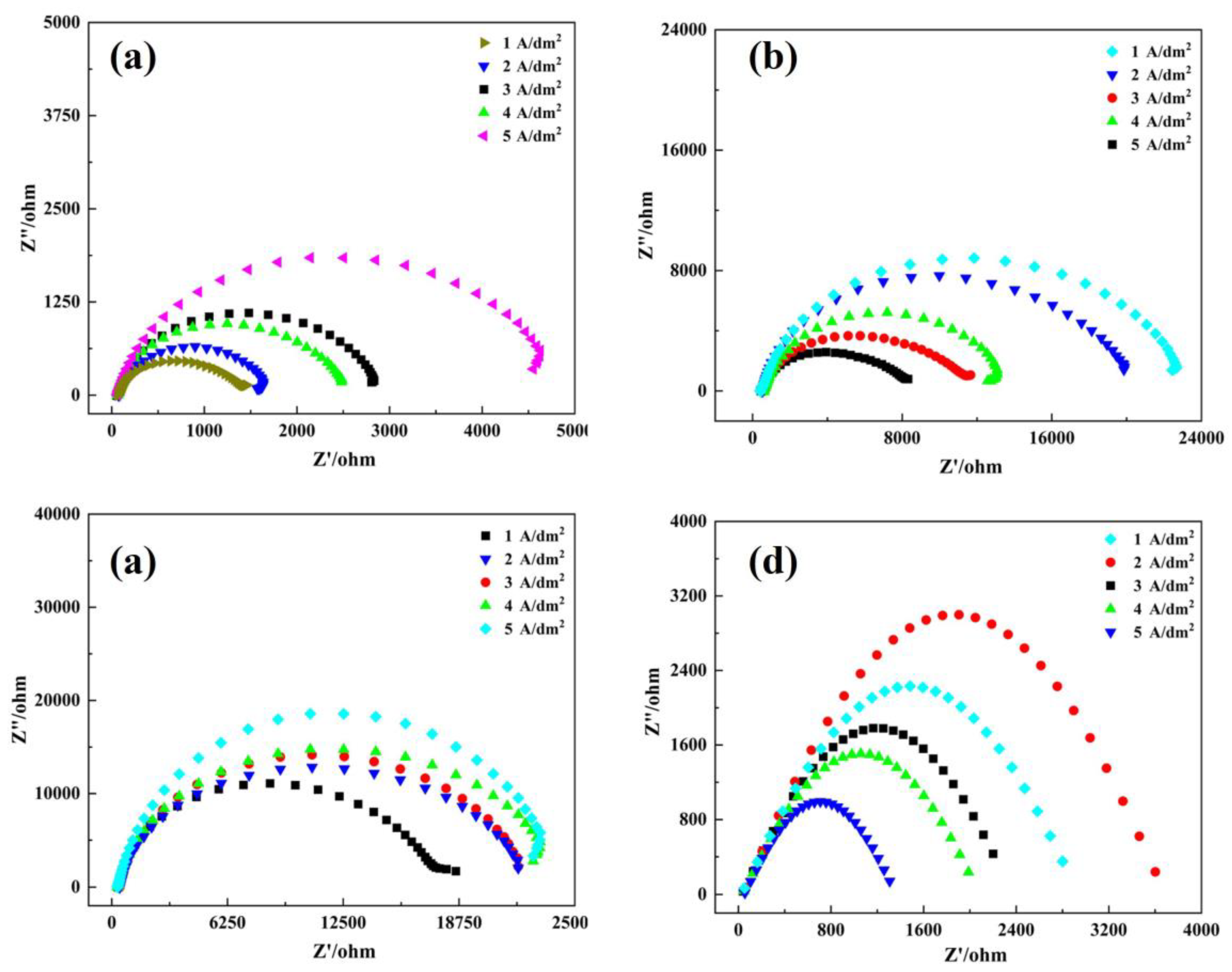
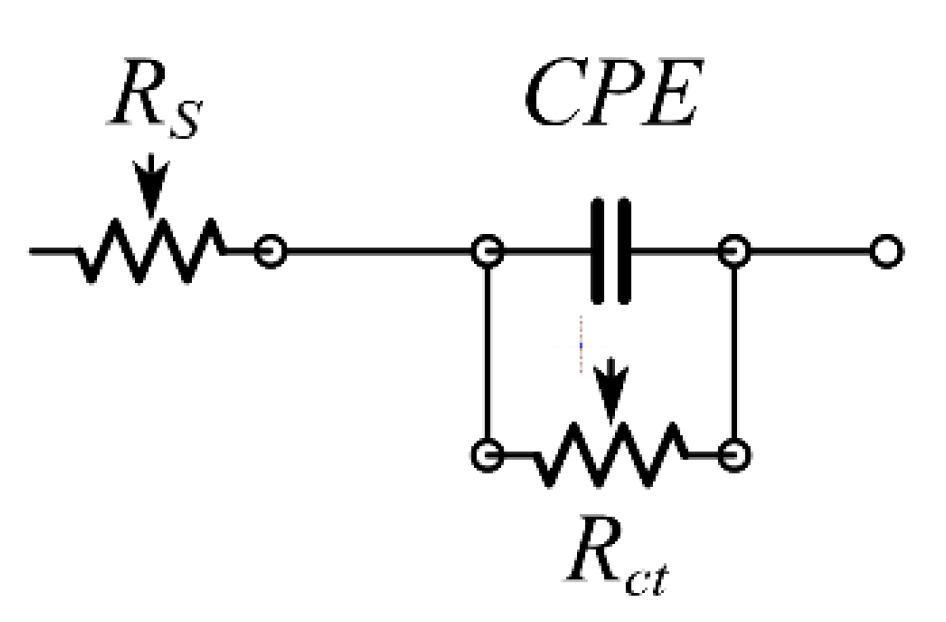
3.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García, J.; Ciprés, V.C.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mater. 2019, 80, 40–68. [Google Scholar] [CrossRef]
- Cinca, N.; Beake, B.; Harris, A.; Tarrés, E. Micro-scale impact testing on cemented carbides. Int. J. Refract. Met. Hard Mater. 2019, 84, 105045. [Google Scholar] [CrossRef]
- Huang, L.; Dong, J.; Yang, F.; Xu, S.; Zhou, S. Studies on the Mechanism, Structure and Microhardness of Ni-W Alloy Electrodeposits. Trans. IMF 1999, 77, 185–187. [Google Scholar] [CrossRef]
- de Lima-Neto, P.; Correia, A.N.; Santana, R.A.; Colares, R.P.; Barros, E.B.; Casciano, P.N.; Vaz, G.L. Morphological, structural, microhardness and electrochemical characterisations of electrodeposited Cr and Ni–W coatings. Electrochim. Acta 2010, 55, 2078–2086. [Google Scholar] [CrossRef]
- Imaz, N.; Díez, J.A.; Pellicer, E.; Sort, J.; Grande, H.; García-Lecina, E. Thermal treatment effect on the mechanical, tribological and corrosion properties of Ni–W alloy obtained by direct and pulse plating electrodeposition. Trans. IMF 2017, 95, 31–38. [Google Scholar] [CrossRef]
- Vamsi, M.; Wasekar, N.P.; Sundararajan, G. Influence of heat treatment on microstructure and mechanical properties of pulse electrodeposited Ni-W alloy coatings. Surf. Coat. Technol. 2017, 319, 403–414. [Google Scholar] [CrossRef]
- Marvel, C.J.; Cantwell, P.R.; Harmer, M.P. The critical influence of carbon on the thermal stability of nanocrystalline Ni–W alloys. Scr. Mater. 2015, 96, 45–48. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Rouhaghdam, A.S. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Marvel, C.; Smeltzer, J.; Hornbuckle, B.; Darling, K.; Harmer, M. On the reduction and effect of non-metallic impurities in mechanically alloyed nanocrystalline Ni-W alloys. Acta Mater. 2020, 200, 12–23. [Google Scholar] [CrossRef]
- Tao, X.P.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Chen, J.; Abdullah, A.O. In situ synthesised WC-reinforced Co-based alloy layer by vacuum cladding. Surf. Eng. 2017, 34, 316–323. [Google Scholar] [CrossRef]
- Shu, D.; Li, Z.; Yao, C.; Li, D.; Dai, Z. In situ synthesised WC reinforced nickel coating by laser cladding. Surf. Eng. 2017, 34, 276–282. [Google Scholar] [CrossRef]
- Su, C.-W.; Zhao, L.; Bai, Y.; Tian, L.; Wen, B.; Guo, J. Microhardness Improvement of Ni-W/SiC Composite Coatings by High Frequency Induction Heat Treatment. J. Electrochem. Soc. 2019, 166, D301–D307. [Google Scholar] [CrossRef]
- Lee, J.; Lear, C.R.; Zhang, X.; Bellon, P.; Averback, R.S. Irradiation-Induced Nanoprecipitation in Ni-W Alloys. Met. Mater. Trans. A 2014, 46, 1046–1061. [Google Scholar] [CrossRef]
- Boonyongmaneerat, Y.; Saengkiettiyut, K.; Saenapitak, S.; Sangsuk, S. Effects of WC addition on structure and hardness of electrodeposited Ni–W. Surf. Coat. Technol. 2009, 203, 3590–3594. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Chen, W.; Liu, Y.; Zhang, J.; Wei, Y.; Zhang, W.; Jia, W. Effect of current density and deposition time on microstructure and corrosion resistance of Ni-W/TiN nanocomposite coating. Ceram. Int. 2018, 45, 4870–4879. [Google Scholar] [CrossRef]
- Bin Humam, S.; Gyawali, G.; Joshi, B.; Kim, T.H.; Lee, S.W. Influence of WC and TaC particles on the microstructure and scratch resistance of electrodeposited nickel-tungsten alloy. J. Alloys Compd. 2021, 893, 162371. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Zhang, W.; Huan, Y. Preparation of Ni-W/SiC nanocomposite coatings by electrochemical deposition. J. Alloys Compd. 2017, 702, 38–50. [Google Scholar] [CrossRef]
- Yao, Y.W. Preparation, mechanical property and wear resistance of Ni–W/Al2O3composite coatings. Surf. Eng. 2008, 24, 226–229. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Bigos, A.; Szczerba, M.; Janusz-Skuza, M.; Maj, L.; Debski, A.; Wiazania, G.; Kot, M. Heat treatment of ultrasonic electrodeposited Ni-W/ZrO2 nanocomposites. Surf. Coat. Technol. 2020, 393, 125779. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Z. Growth mechanism of in-situ WC grain in Fe-Ni-W-C alloys system. J. Alloys Compd. 2018, 738, 379–393. [Google Scholar] [CrossRef]
- Suvorov, D.V.; Gololobov, G.P.; Tarabrin, D.Y.; Slivkin, E.V.; Karabanov, S.M.; Tolstoguzov, A. Electrochemical Deposition of Ni–W Crack-Free Coatings. Coatings 2018, 8, 233. [Google Scholar] [CrossRef]
- Popczyk, M.; Kubisztal, J.; Swinarew, A.S.; Waśkiewicz, Z.; Stanula, A.; Knechtle, B. Corrosion Resistance of Heat-Treated Ni-W Alloy Coatings. Materials 2020, 13, 1172. [Google Scholar] [CrossRef]
- Schloßmacher, P.; Yamasaki, T. Structural Analysis of Electroplated Amorphous-Nanocrystalline Ni-W. Microchim. Acta 2000, 132, 309–313. [Google Scholar] [CrossRef]
- Argibay, N.; Furnish, T.; Boyce, B.; Clark, B.; Chandross, M. Stress-dependent grain size evolution of nanocrystalline Ni-W and its impact on friction behavior. Scr. Mater. 2016, 123, 26–29. [Google Scholar] [CrossRef]
- Hou, K.-H.; Chang, Y.-F.; Chang, S.-M.; Chang, C.-H. The heat treatment effect on the structure and mechanical properties of electrodeposited nano grain size Ni–W alloy coatings. Thin Solid Film. 2010, 518, 7535–7540. [Google Scholar] [CrossRef]
- Naalchian, M.; Kasiri-Asgarani, M.; Shamanian, M.; Bakhtiari, R.; Bakhsheshi-Rad, H.R.; Berto, F.; Das, O. Phase Formation during Heating of Amorphous Nickel-Based BNi-3 for Joining of Dissimilar Cobalt-Based Superalloys. Materials 2021, 14, 4600. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Grain boundary segregation, chemical ordering and stability of nanocrystalline alloys: Atomistic computer simulations in the Ni–W system. Acta Mater. 2007, 55, 4221–4232. [Google Scholar] [CrossRef]
- Chookajorn, T.; Schuh, C.A. Thermodynamics of stable nanocrystalline alloys: A Monte Carlo analysis. Phys. Rev. B 2014, 89, 064102. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Shan, Q. Quantitative deviation of nanocrystals using the RIR method in X-ray diffraction (XRD). Nanomaterials 2022, 12, 2320. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Zhang, J.; Chen, W.; Zhang, W. Electrodeposition of Ni-W/TiN-Y2O3 nanocrystalline coating and investigation of its surface properties and corrosion resistance. J. Alloys Compd. 2019, 787, 952–962. [Google Scholar] [CrossRef]
- Giga, A.; Kimoto, Y.; Takigawa, Y.; Higashi, K. Demonstration of an inverse Hall–Petch relationship in electrodeposited nanocrystalline Ni–W alloys through tensile testing. Scr. Mater. 2006, 55, 143–146. [Google Scholar] [CrossRef]
- Rupert, T.J.; Cai, W.; Schuh, C.A. Abrasive wear response of nanocrystalline Ni–W alloys across the Hall–Petchbreakdown. Wear 2013, 298–299, 120–126. [Google Scholar] [CrossRef]
- Sunwang, N.; Wangyao, P.; Boonyongmaneerat, Y. The effects of heat treatments on hardness and wear resistance in Ni–W alloy coatings. Surf. Coat. Technol. 2011, 206, 1096–1101. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Yao, J.-Y.; Li, X.-P.; Ding, G.-F.; RUI LIUKey Laboratory for Thin Film and Microfabrication Technology of Ministry of Education; National Key Laboratory of Micro/Nano Fabrication Technology; Research Institute of Micro/Nano Science and Technology; Shanghai Jiao Tong University; Yang, Y.; et al. Preparing Ni–W alloy films with low internal stress and high hardness by heat treating. Surf. Rev. Lett. 2007, 14, 1107–1112. [Google Scholar] [CrossRef]
- Liu, H.; Guo, R.-X.; Zong, Y.; He, B.-Q.; Liu, Z. Comparative study of microstructure and corrosion resistance of electroless Ni-W-P coatings treated by laser and furnace-annealing. Trans. Nonferrous Met. Soc. China 2010, 20, 1024–1031. [Google Scholar] [CrossRef]
- Sabzi, M.; Anijdan, S.H.M.; Zadeh, M.R.; Farzam, M. The effect of heat treatment on corrosion behaviour of Ni–P–3 gr/lit Cu nano-composite coating. Can. Met. Q. 2018, 57, 350–357. [Google Scholar] [CrossRef]
- Long, X.; Hang, T.; Guo, Y.; Li, Y.; Wu, Y.; Ling, H.; Hu, A.; Li, M. Influence of intercolony boundary on corrosion behavior of electrodeposited Ni–W alloy for electronic connector applications. Mater. Chem. Phys. 2020, 239, 121989. [Google Scholar] [CrossRef]


| Electrolyte | Content (g/L) | Function |
|---|---|---|
| NiSO4·6H2O | 25 | Ni source |
| Na2WO4·H2O | 50 | W source |
| Na3C6H5O7·2H2O (Trisodium citrate) | 45 | Complexing agent |
| C₆H₈O (Citric Acid) | 5.5 | Complexing agent |
| C₇H₅NO₃S (Saccharin) | 0.8 | Softener |
| Parameters | Value | |
| Temperature (°C) | 65 | |
| Current density (A/dm2) | 1,2,3,4,5 | |
| pH | 8 | |
| Cathode | Q235 | |
| Anode | Stainless steel | |
| Stirring speed (rpm) | 400 | |
| Heat treatment temperature (°C) | 400,600,900 |
| Current Density (A/dm2) | RTC | |||
|---|---|---|---|---|
| (111) | (200) | (220) | (311) | |
| As-deposited | ||||
| 1 | 0.501 | 0.152 | 0.210 | 0.135 |
| 2 | 0.414 | 0.002 | 0.002 | 0.001 |
| 3 | 0.479 | 0.001 | 0.002 | 0.001 |
| 4 | 0.597 | 0.001 | 0.002 | 0.001 |
| 5 | 0.574 | 0.001 | 0.002 | 0.001 |
| 400 | ||||
| 1 | 0.450 | 0.108 | 0.247 | 0.194 |
| 2 | 0.436 | 0.150 | 0.243 | 0.170 |
| 3 | 0.476 | 0.085 | 0.257 | 0.181 |
| 4 | 0.580 | 0.081 | 0.207 | 0.131 |
| 5 | 0.609 | 0.094 | 0.174 | 0.122 |
| 600 | ||||
| 1 | 0.317 | 0.243 | 0.239 | 0.200 |
| 2 | 0.328 | 0.285 | 0.225 | 0.160 |
| 3 | 0.458 | 0.303 | 0.151 | 0.087 |
| 4 | 0.455 | 0.178 | 0.208 | 0.157 |
| 5 | 0.380 | 0.277 | 0.203 | 0.138 |
| 900 | ||||
| 1 | 0.199 | 0.215 | 0.301 | 0.284 |
| 2 | 0.281 | 0.231 | 0.252 | 0.235 |
| 3 | 0.367 | 0.274 | 0.187 | 0.171 |
| 4 | 0.313 | 0.221 | 0.258 | 0.208 |
| 5 | 0.249 | 0.223 | 0.292 | 0.236 |
| Ecorr (V) (V vs.SCE) | Icorr(A) (A) | |
|---|---|---|
| As-deposited | ||
| 1A/dm2 | −0.71 | 2.85 × 10−4 |
| 2 A/dm2 | −0.68 | 2.63 × 10−4 |
| 3 A/dm2 | −0.67 | 2.32 × 10−4 |
| 4 A/dm2 | −0.67 | 2.14 × 10−4 |
| 5 A/dm2 | −0.65 | 1.70 × 10−4 |
| 400 °C | ||
| 1A/dm2 | −0.62 | 4.36 × 10−6 |
| 2 A/dm2 | −0.66 | 4.58 × 10−6 |
| 3 A/dm2 | −0.72 | 9.21 × 10−6 |
| 4 A/dm2 | −0.68 | 8.43 × 10−6 |
| 5 A/dm2 | −0.72 | 9.86 × 10−6 |
| 600 °C | ||
| 1A/dm2 | −0.79 | 6.35 × 10−6 |
| 2 A/dm2 | −0.78 | 3.13 × 10−6 |
| 3 A/dm2 | −0.76 | 2.64 × 10−6 |
| 4 A/dm2 | −0.76 | 2.11 × 10−6 |
| 5 A/dm2 | −0.75 | 1.96 × 10−6 |
| 900 °C | ||
| 1A/dm2 | −0.77 | 2.10 × 10−5 |
| 2 A/dm2 | −0.75 | 1.33 × 10−5 |
| 3 A/dm2 | −0.79 | 5.10 × 10−5 |
| 4 A/dm2 | −0.82 | 1.81 × 10−5 |
| 5 A/dm2 | −0.84 | 3.72 × 10−5 |
| Rs(Ω) (Ω·cm−2) | CPE-Qdl (Ω−1sn/cm2) (cm2) × 10−6(S secn) | Rct (Ω·cm−2) | |
|---|---|---|---|
| As-deposited | |||
| 1 A/dm2 | 26.57 | 2.076 × 10-4 | 533.7 |
| 2 A/dm2 | 25.32 | 1.452 × 10-4 | 856.1 |
| 3 A/dm2 | 24.13 | 9.193 × 10-5 | 1120.5 |
| 4 A/dm2 | 28.32 | 9.953 × 10-5 | 965.5 |
| 5 A/dm2 | 24.61 | 8.593 × 10-5 | 1882.4 |
| 400 °C | |||
| 1 A/dm2 | 21.37 | 1.09 × 10-4 | 2104.1 |
| 2 A/dm2 | 21.96 | 8.59 × 10-4 | 1928.1 |
| 3 A/dm2 | 21.36 | 9.693 × 10-5 | 1526.4 |
| 4 A/dm2 | 21.63 | 3.083 × 10-5 | 1598.4 |
| 5 A/dm2 | 22.22 | 2.683 × 10-6 | 1342.4 |
| 600 °C | |||
| 1A/dm2 | 27.84 | 9.875 × 10-5 | 2375.1 |
| 2 A/dm2 | 24.36 | 9.365 × 10-5 | 2472.6 |
| 3 A/dm2 | 25.63 | 8.365 × 10-5 | 2672.2 |
| 4 A/dm2 | 26.96 | 7.635 × 10-5 | 2885.7 |
| 5 A/dm2 | 24.58 | 7.132 × 10-5 | 3031.4 |
| 900 °C | |||
| 1A/dm2 | 27.32 | 2.324 × 10-5 | 863.5 |
| 2 A/dm2 | 26.32 | 1.124 × 10-5 | 1130.2 |
| 3 A/dm2 | 24.21 | 4.325 × 10-4 | 436.2 |
| 4 A/dm2 | 24.32 | 3.241 × 10-4 | 432.1 |
| 5 A/dm2 | 23.36 | 1.103 × 10-4 | 536.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, D.; Sheng, M.; Wang, H.; Guo, R.; Qu, T.; Hu, S. The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating. Coatings 2023, 13, 1651. https://doi.org/10.3390/coatings13091651
Xu Y, Wang D, Sheng M, Wang H, Guo R, Qu T, Hu S. The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating. Coatings. 2023; 13(9):1651. https://doi.org/10.3390/coatings13091651
Chicago/Turabian StyleXu, Yingjun, Deyong Wang, Minqi Sheng, Huihua Wang, Ruiqi Guo, Tianpeng Qu, and Shaoyan Hu. 2023. "The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating" Coatings 13, no. 9: 1651. https://doi.org/10.3390/coatings13091651
APA StyleXu, Y., Wang, D., Sheng, M., Wang, H., Guo, R., Qu, T., & Hu, S. (2023). The Effect of Heat Treatment on Phase Structure and Mechanical and Corrosion Resistance Properties of High Tungsten Ni-W Alloy Coating. Coatings, 13(9), 1651. https://doi.org/10.3390/coatings13091651








