Investigation of High-Temperature Oxidation of Homogeneous and Gradient Ni-Cr-Al Coatings Obtained by Detonation Spraying
Abstract
:1. Introduction
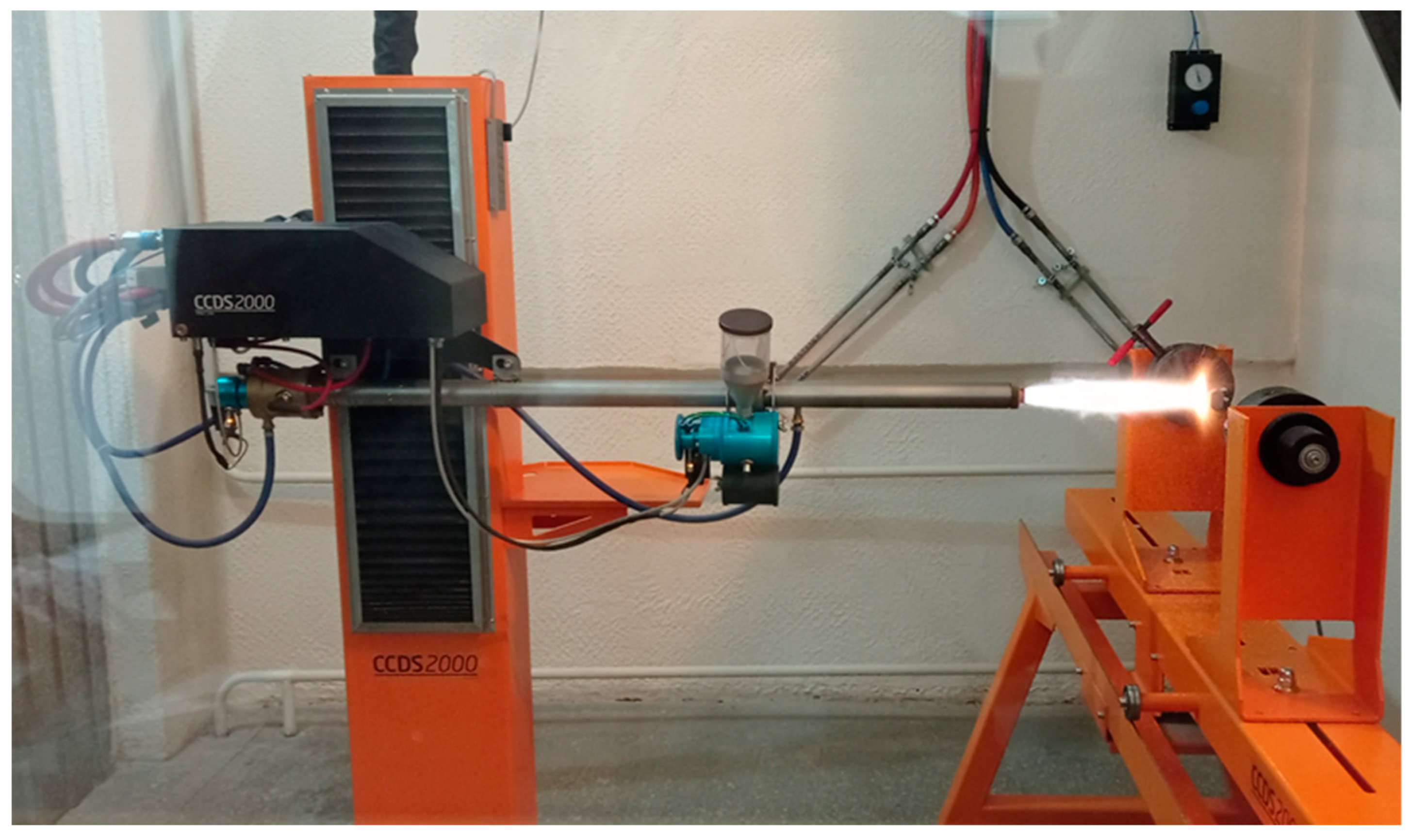
2. Materials and Methods
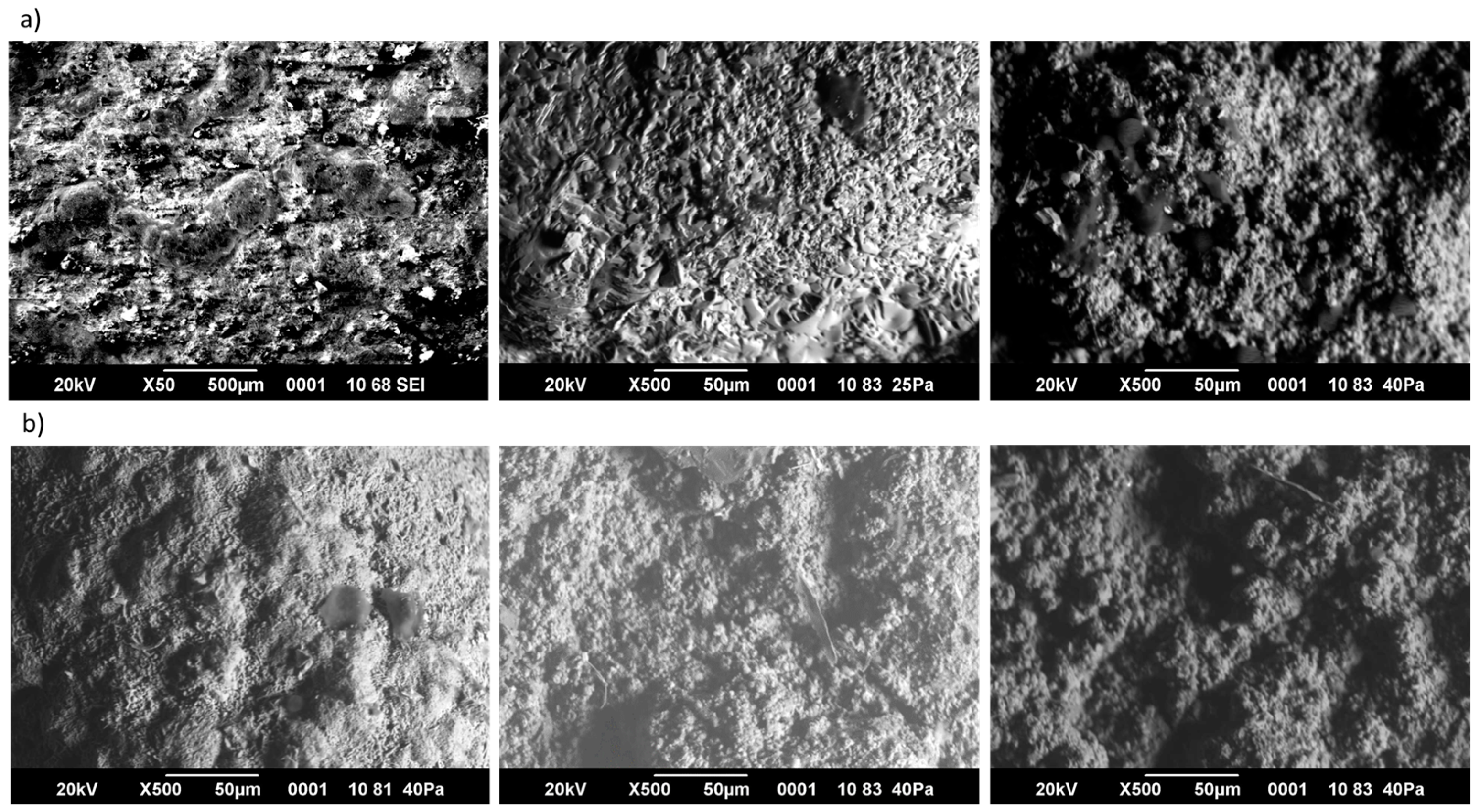
3. Results and Discussion
4. Conclusions
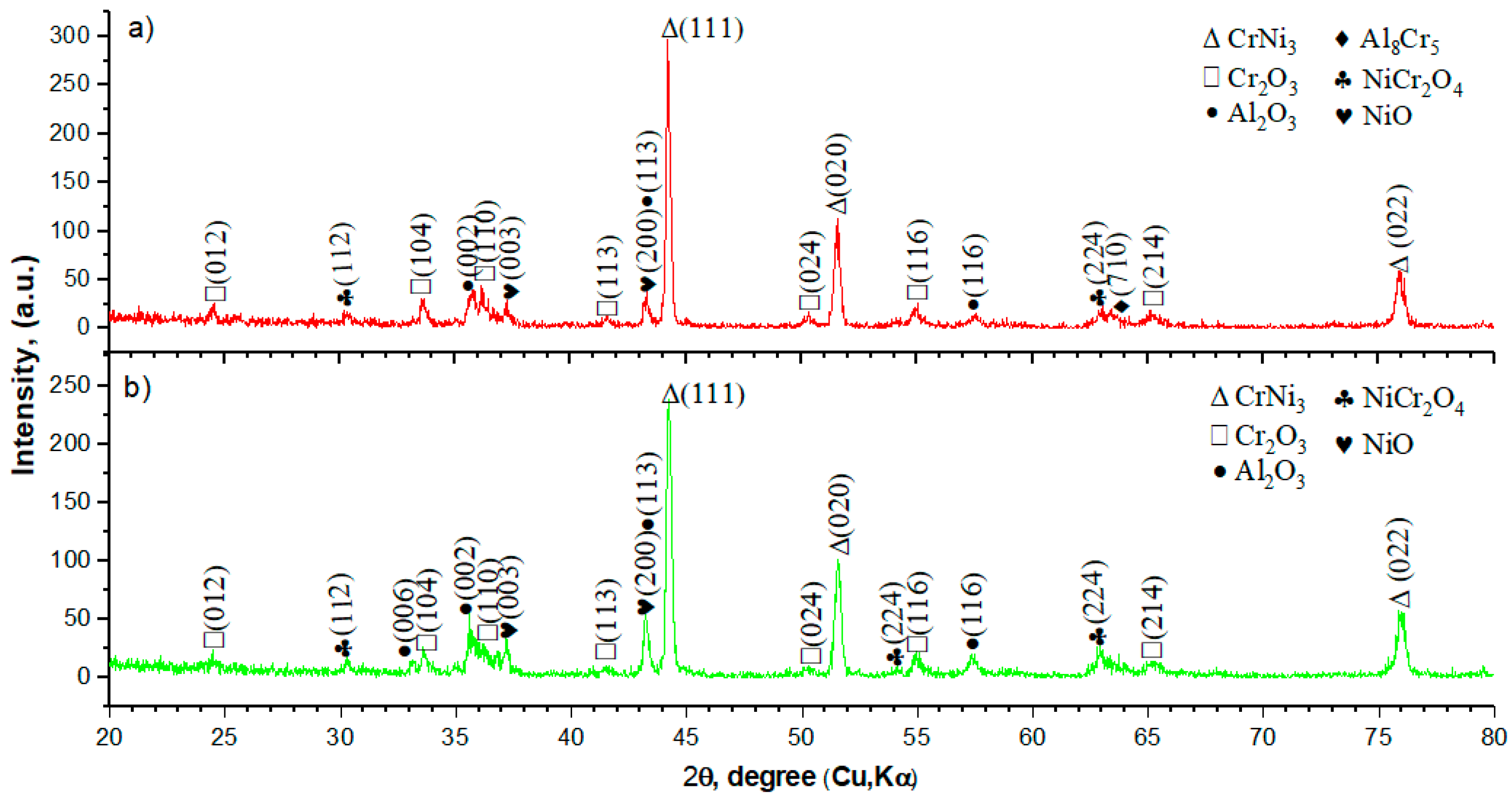
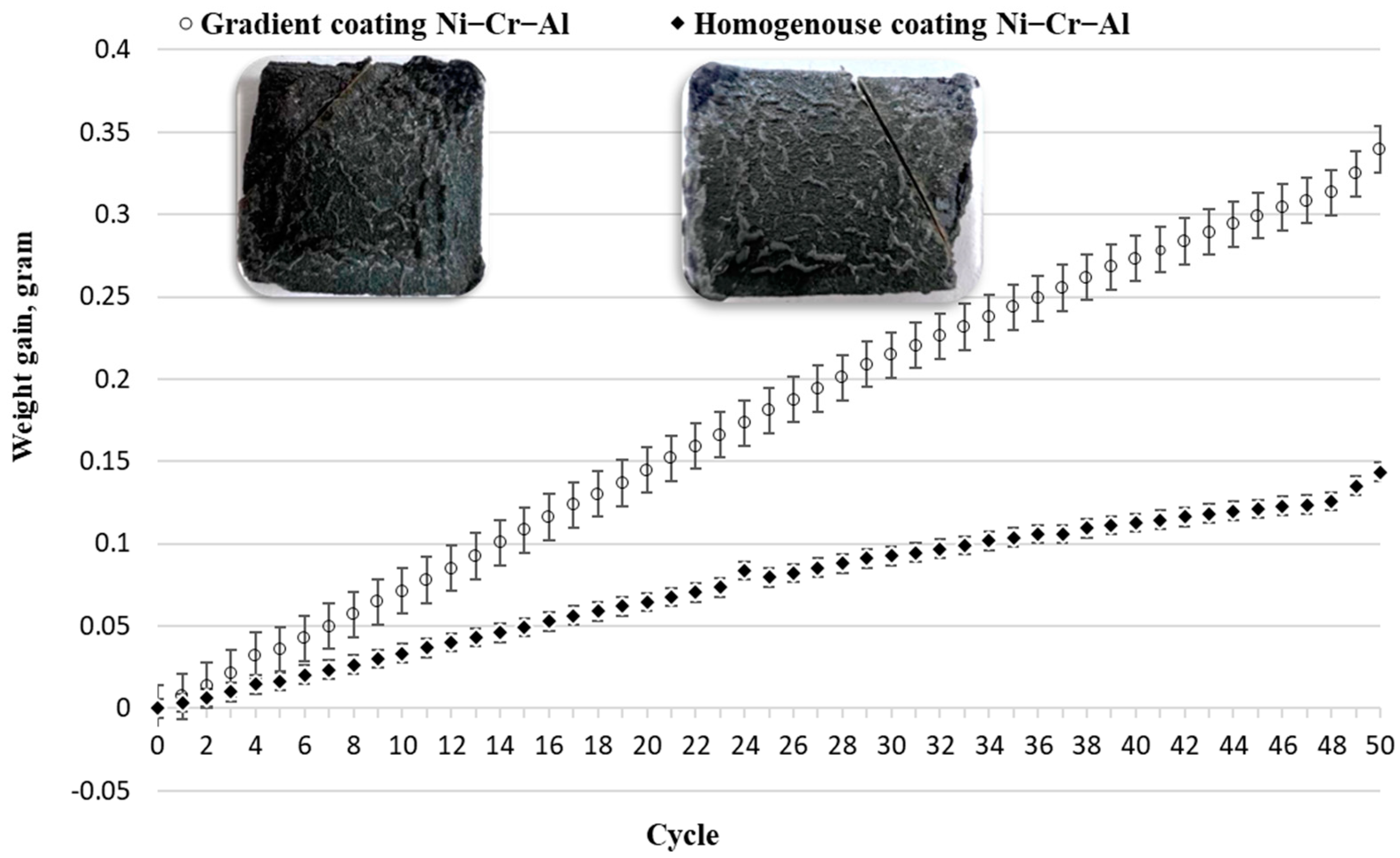
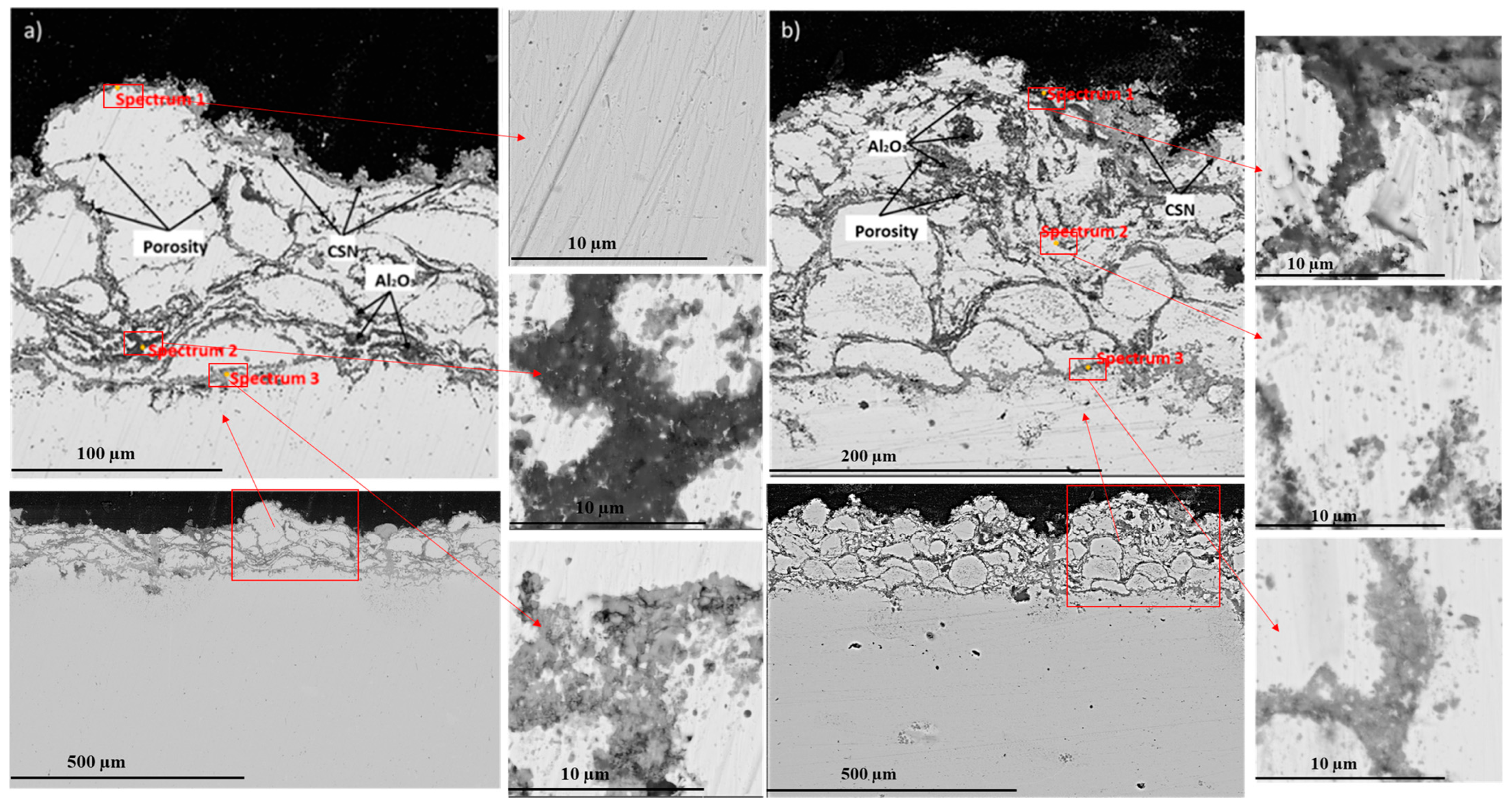
- The results of X-ray phase analysis showed that after high-temperature oxidation, the protective oxides Cr2O3 and Al2O3 are formed, as well as the secondary oxides NiCr2O4 and NiO. The results showed that the surface of the samples with coatings of homogeneous Ni-Cr-Al or gradient Ni-Cr-Al remains undamaged. A sample with a homogeneous Ni-Cr-Al coating showed a smaller weight gain than a sample with a gradient Ni-Cr-Al coating;
- The results of the elemental analysis showed that after the cyclic high-temperature oxidation of the near-surface layer, the CSN content of the homogeneous coatings is higher compared to that of the gradient coating. Ni-Cr-Al gradient coatings retain a high chromium content compared to homogeneous coatings, which can slow down mixing or diffusion between different phases of the material at the interface. This, in turn, contributes to an increase in the resistance of gradient coatings to oxidation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghadami, F.; Davoudabadi, M.A.; Ghadami, S. Cyclic oxidation properties of the nanocrystalline AlCrFeCoNi high-entropy alloy coatings applied by the atmospheric plasma spraying technique. Coatings 2022, 12, 372. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; Dahm, K.L.; Wang, F. Improved oxide spallation resistance of microcrystalline Ni-Cr-Al coatings. Oxid. Met. 1998, 50, 51–69. [Google Scholar] [CrossRef]
- Ghadami, F.; Sabour Rouh Aghdam, A.; Ghadami, S. Microstructural characteristics and oxidationvehavior of the modified MCrAlX coatings: A critical review. Vacuum 2021, 185, 109980. [Google Scholar] [CrossRef]
- Tang, F.; Ajdelsztajn, L.; Schoenung, J.M. Influence of cryomilling on the morphology and composition of the oxide scales formed on HVOF CoNiCrAlY coatings. Oxid. Met. 2004, 61, 219–238. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.K.; Choi, H.S.; Ahn, H.S. Phase transformation and bond coat oxidation behavior of plasma-sprayed zirconia thermal barrier coating. Surf. Coat. Technol. 2000, 124, 1–12. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Zhen, H.J.; Dong, Z.H.; Yang, X.Y.; Peng, X. Preparation and performance of a novel wear-resistant and high-temperature oxidation-resistant NiCrAlSiC composite coating. Acta Metall. Sin. 2019, 55, 902–910. [Google Scholar]
- Dong, Z.H.; Xie, Y.; Peng, X. Effect of Cr particle size on the development of a chromia scale on Ni-Cr composites. Corros. Sci. 2022, 194, 109915. [Google Scholar] [CrossRef]
- Yang, X.; Peng, X.; Xu, C.; Wang, F. Electrochemical assembly of Ni-xCr-yAl nanocomposites with excellent high-temperature oxidation resistance. J. Electrochem. Soc. 2009, 156, 167–175. [Google Scholar] [CrossRef]
- Kilic, M.; Ozkan, D.; Gok, M.S.; Karaoglanli, A.C. Room- and high temperature wear resistance of MCrAlY coatings deposited by detonation gun (D-gun) and supersonic plasma spraying (SSPS) techniques. Coatings 2020, 10, 1107. [Google Scholar] [CrossRef]
- Ke, P.L.; Wu, Y.N.; Wang, Q.M.; Gong, J.; Sun, C.; Wen, L.S. Study on thermal barrier coatings deposited by detonation gun spraying. Surf. Coat. Technol. 2005, 7, 2271–2276. [Google Scholar] [CrossRef]
- Yuan, F.H.; Chen, Z.X.; Huang, Z.W.; Wang, Z.G.; Zhu, S.J. Oxidation behavior of thermal barrier coatings with HVOF and detonation-sprayed NiCrAlY bondcoats. Corros. Sci. 2008, 50, 1608–1617. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.C.; Park, C.G. Evaluation of functionally graded thermal barrier coatings fabricated by detonation gun spray technique. Surf. Coat. Technol. 2003, 168, 275–280. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Tyurin, Y.; Kakimzhanov, D.; Baizhan, D.; Kolisnichenko, O.; Zhurerova, L. Deposition of duplex Cr3C2-NiCr coatings on steel using a combined technique of gas detonation spraying and pulse-plasma treatment. High Temp. Mater. Process. 2021, 25, 25–37. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Patnaik, P.C. The Growth and Influence of Thermally Grown Oxide in a Thermal Barrier Coating. Surf. Coat. Technol. 2006, 201, 1074–1079. [Google Scholar] [CrossRef]
- Sudarshan, R.; Kokini, K. Estimating the Fracture Resistance of Functionally Graded Thermal Barrier Coatings from Thermal Shock Tests. Surf. Coat. Technol. 2003, 173, 201–212. [Google Scholar]
- Sahin, A. An Interface Crack between a Graded Coating and a Homogeneous Substrate. Turk. J. Eng. Environ. Sci. 2004, 28, 135–148. [Google Scholar]
- Lee, W.Y.; Stinton, D.P.; Berndt, C.C.; Erdogan, F.; Lee, Y.D.; Mutasim, Z. Concept of Functionally Graded Materials for Advanced Thermal Barrier Coating Applications. J. Am. Ceram. Soc. 2005, 79, 3003–3012. [Google Scholar] [CrossRef]
- Sniezewski, J.; Vidal, V.; Lours, P.; Le Maoult, Y. Thermal Barrier Coatings Adherence and Spallation: Interfacial Indentation Resistance and Cyclic Oxidation Behaviour under Thermal Gradient. Surf. Coat. Technol. 2009, 204, 807–811. [Google Scholar] [CrossRef]
- Zhang, X.C.; Xu, B.S.; Wang, H.D.; Jiang, Y.; Wu, Y.X. Application of Functionally Graded Interlayer on Reducing the Residual Stress Discontinuities at Interfaces within a Plasma-Sprayed Thermal Barrier Coating. Surf. Coat. Technol. 2007, 201, 5716–5719. [Google Scholar] [CrossRef]
- Suresh, S. Graded materials for resistance to contact deformation and damage. Science 2001, 292, 2447–2451. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jahromi, S.A.J.; Javadpour, S.; Kobayashi, A.; Shirvani, K. Hot corrosion and microstructural change of Al-gradient CoNiCrAlYSi coatings, produced by LVPS and diffusional processes. Oxid. Met. 2012, 78, 17–30. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jahromi, S.A.J.; Javadpour, S.; Kobayashi, A. Cyclic oxidation and hot corrosion behaviors of gradient CoNiCrAlYSi coatings, produced by HVOF and diffusional processes. Oxid. Met. 2016, 86, 221–238. [Google Scholar] [CrossRef]
- Guo, M.H.; Wang, Q.M.; Gong, J.; Sun, C.; Wen, L.S. Preparation and oxidation of a gradient NiCoCrAlYSiB coating deposited by a combined system of arc ion plating and magnetron sputing. Surf. Coat. Technol. 2006, 201, 1302–1308. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Bao, Z.B.; Sun, X.F.; Guan, H.R.; Hu, Z.Q. Preparation and cyclic oxidation of gradient NiCrAlRe coatings on Ni-based superalloys. Surf. Coat. Technol. 2008, 202, 4709–4713. [Google Scholar] [CrossRef]
- Bao, Z.B.; Wang, Q.M.; Li, W.Z.; Liu, X.; Gong, J.; Xiong, T.Y.; Sun, C. Preparation and hot corrosion behaviour of an Al-gradient NiCoCrAlYSiB coating on a Ni-base superalloy. Corros. Sci. 2009, 51, 860–867. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Maulet, M.; Abilov, M.; Sagdoldina, Z.; Kozhanova, R. Structure and tribological properties of Ni-Cr-Al based gradient coating prepared by detonation spraying. Coatings 2021, 11, 218. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Maulet, M.; Kakimzhanov, D.N.; Stepanova, O.A.; Botabaeva, G.B. Comparative study of the structure and properties of homogeneous and gradient Ni-Cr-Al coatings. Eurasian J. Phys. Funct. Mater. 2022, 6, 47–55. [Google Scholar]
- Ulianitsky, V.Y.; Dudina, D.V.; Shtertser, A.; Smurov, I. Computer-controlled detonation spraying: Flexible control of the coating chemistry and microstructure. Metals 2019, 12, 1244. [Google Scholar] [CrossRef]
- Skakov, M.K.; Rakhadilov, B.K.; Batyrbekov, E.; Scheffler, M. Change of structure and mechanical properties of R6M5 steel surface layer at electrolytic-plasma nitriding. Adv. Mater. Res. 2014, 1040, 753–758. [Google Scholar] [CrossRef]
- Bala, N.; Singh, H.; Prakash, S. An overview of characterizations and high temperature behaviour of thermal spray NiCr coatings. Int. J. Mater. Sci. 2007, 2, 201–218. [Google Scholar]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Patnaik, P.C. Oxidation and crack nucleation/growth in an air-plasma-sprayed thermal barrier coating with NiCrAlY bond coat. Surf. Coat. Technol. 2005, 197, 109–115. [Google Scholar] [CrossRef]
- Singh, V.; Goyal, K.; Goyal, R. Improving the hot corrosion resistance of boiler tube steels by detonation gun sprated coatings in actual boiler of thermal power plant. Anti-Corros. Methods Mater. 2019, 66, 394–402. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Raman, S.G.; Joshi, S.V.; Sundararajan, G. Influence of detonation gun sprayed alumina coating on AA 6063 samples under cyclic loading with and withaut fretting. Tribol. Int. 2008, 41, 315–322. [Google Scholar] [CrossRef]
- Sagdoldina, Z.; Rakhadilov, B.; Kurbanbekov, S.; Kozhanova, R.; Kengesbekov, A. Effect of irradiation with Si+ ions on phase transformations in Ti–Al system during thermal annealing. Coatings 2021, 11, 205. [Google Scholar] [CrossRef]
- Sidhu, T.; Prakash, S.; Agrawal, R. Hot corrosion studies of HVOF sprayed Cr3C2-NiCr and Ni-20Cr coatings on nickel-based supperalloy at 900 °C. Surf. Coat. Technol. 2006, 201, 792–800. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. High temperature hot corrsion behaviour of NiCr and Cr3C2-NiCr coatings on T91 boiler steel in an aggressive environment at 750 °C. Surf. Coat. Technol. 2012, 206, 3829–3850. [Google Scholar] [CrossRef]
- Jiang, S.M.; Li, H.Q.; Ma, J.; Xu, C.Z.; Gong, J.; Sun, C. High temperature corrosion behaviour of gradient NiCoCrAlYSi coating II: Oxidation and hot corrosion. Corros. Sci. 2010, 52, 2316–2322. [Google Scholar] [CrossRef]


| C | Si | Mn | Ni | S | P | Cr | Mo | V | Cu |
|---|---|---|---|---|---|---|---|---|---|
| 0.1–0.15 | 0.17–0.37 | 0.4–0.7 | to 0.3 | to 0.025 | to 0.03 | 0.9–1.2 | 0.25–0.35 | 0.15–0.3 | to 0.2 |
| Name | Ratio O2/C2H2 | Barrel Filling Volume, % | Spraying Distance, mm | Shot Number |
|---|---|---|---|---|
| Homogeneous Ni-Cr-Al coating | 1.856 | 50% | 250 | 20 |
| Gradient Ni-Cr-Al coating | 1.856 | 50% | 250 | 5 |
| 40% | 5 | |||
| 30% | 5 | |||
| 25% | 5 |
| Sample Name | Test Time at 1000 °C | |||||
|---|---|---|---|---|---|---|
| Before Oxidation Test | After 10 Cycle | After 20 Cycle | After 30 Cycle | After 40 Cycle | After 50 Cycle | |
| Homogeneous Ni-Cr-Al |  |  |  |  |  |  |
| Gradient Ni-Cr-Al |  |  |  |  |  |  |
| Name | Element | Al, Mass % | Cr, Mass % | Ni, Mass % | O, Mass % | Total, Mass % |
|---|---|---|---|---|---|---|
| (a) Homogeneous Ni-Cr-Al coating | Spectrum 1 | - | 12.5 | 87.0 | 0.5 | 100.00 |
| Spectrum 2 | 35.1 | 20.5 | 7.1 | 37.3 | 100.00 | |
| Spectrum 3 | 2.2 | 41.8 | 28.2 | 27.9 | 100.00 | |
| (b) Gradient Ni-Cr-Al coating | Spectrum 1 | 38.4 | 13.5 | 9.0 | 39.1 | 100.00 |
| Spectrum 2 | 4.5 | 32.7 | 42.4 | 20.5 | 100.00 | |
| Spectrum 3 | 2.5 | 68.2 | 3.8 | 25.5 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhadilov, B.; Sulyubayeva, L.; Maulet, M.; Sagdoldina, Z.; Buitkenov, D.; Issova, A. Investigation of High-Temperature Oxidation of Homogeneous and Gradient Ni-Cr-Al Coatings Obtained by Detonation Spraying. Coatings 2024, 14, 11. https://doi.org/10.3390/coatings14010011
Rakhadilov B, Sulyubayeva L, Maulet M, Sagdoldina Z, Buitkenov D, Issova A. Investigation of High-Temperature Oxidation of Homogeneous and Gradient Ni-Cr-Al Coatings Obtained by Detonation Spraying. Coatings. 2024; 14(1):11. https://doi.org/10.3390/coatings14010011
Chicago/Turabian StyleRakhadilov, Bauyrzhan, Laila Sulyubayeva, Meruyert Maulet, Zhuldyz Sagdoldina, Dastan Buitkenov, and Ainur Issova. 2024. "Investigation of High-Temperature Oxidation of Homogeneous and Gradient Ni-Cr-Al Coatings Obtained by Detonation Spraying" Coatings 14, no. 1: 11. https://doi.org/10.3390/coatings14010011







