Surface Modification of Nano-Al2O3 with Silane Coupling Agent and Its Effect on the Compressive Strength of PI/Al2O3 Composites
Abstract
:1. Introduction
2. Materials and Experimental Procedures
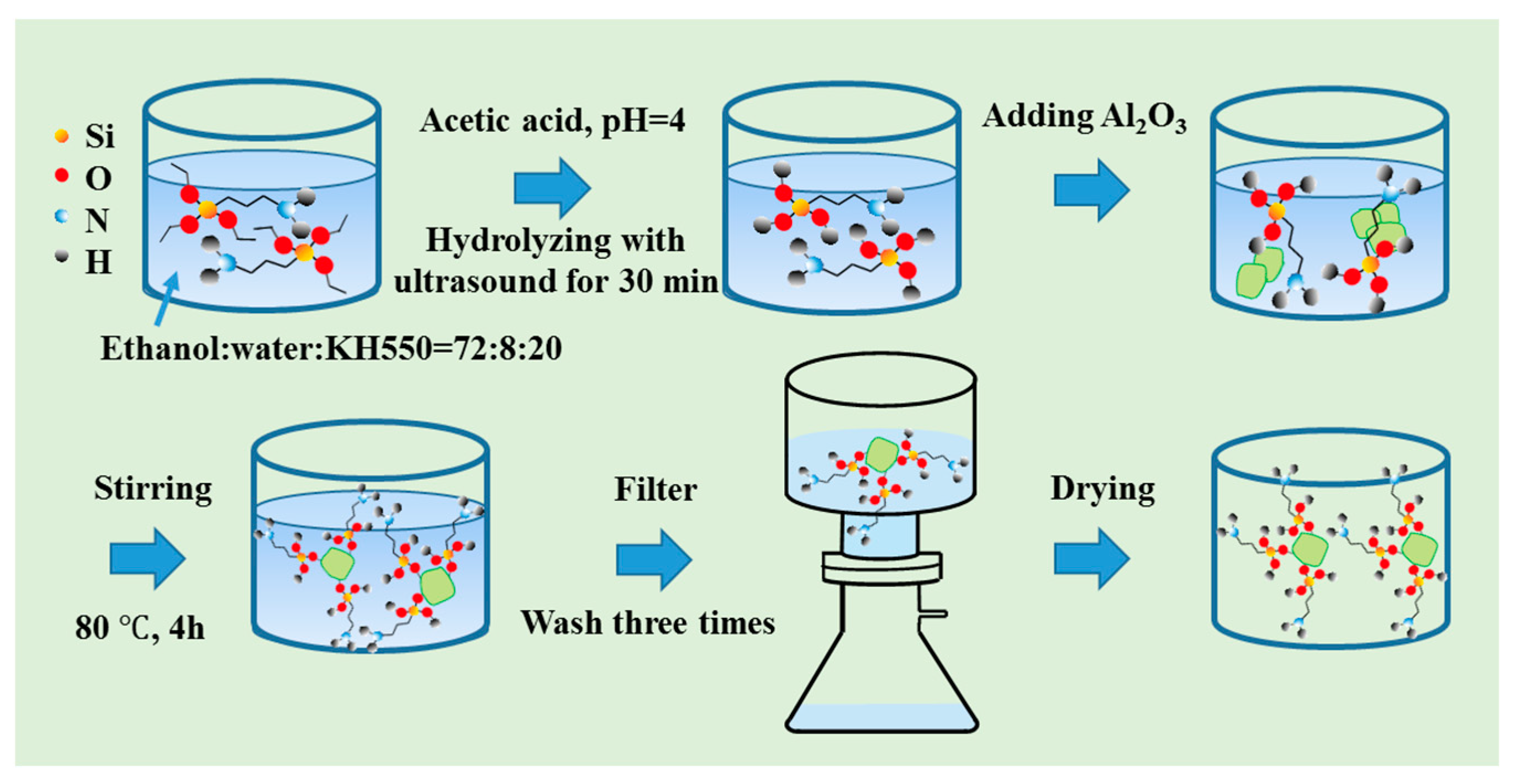
2.1. Materials and Surface Modification of Nano Al2O3
2.2. Preparation of PI/Al2O3 Composites
2.3. Microstructural Characterization, Thermal Stability and Mechanical Properties
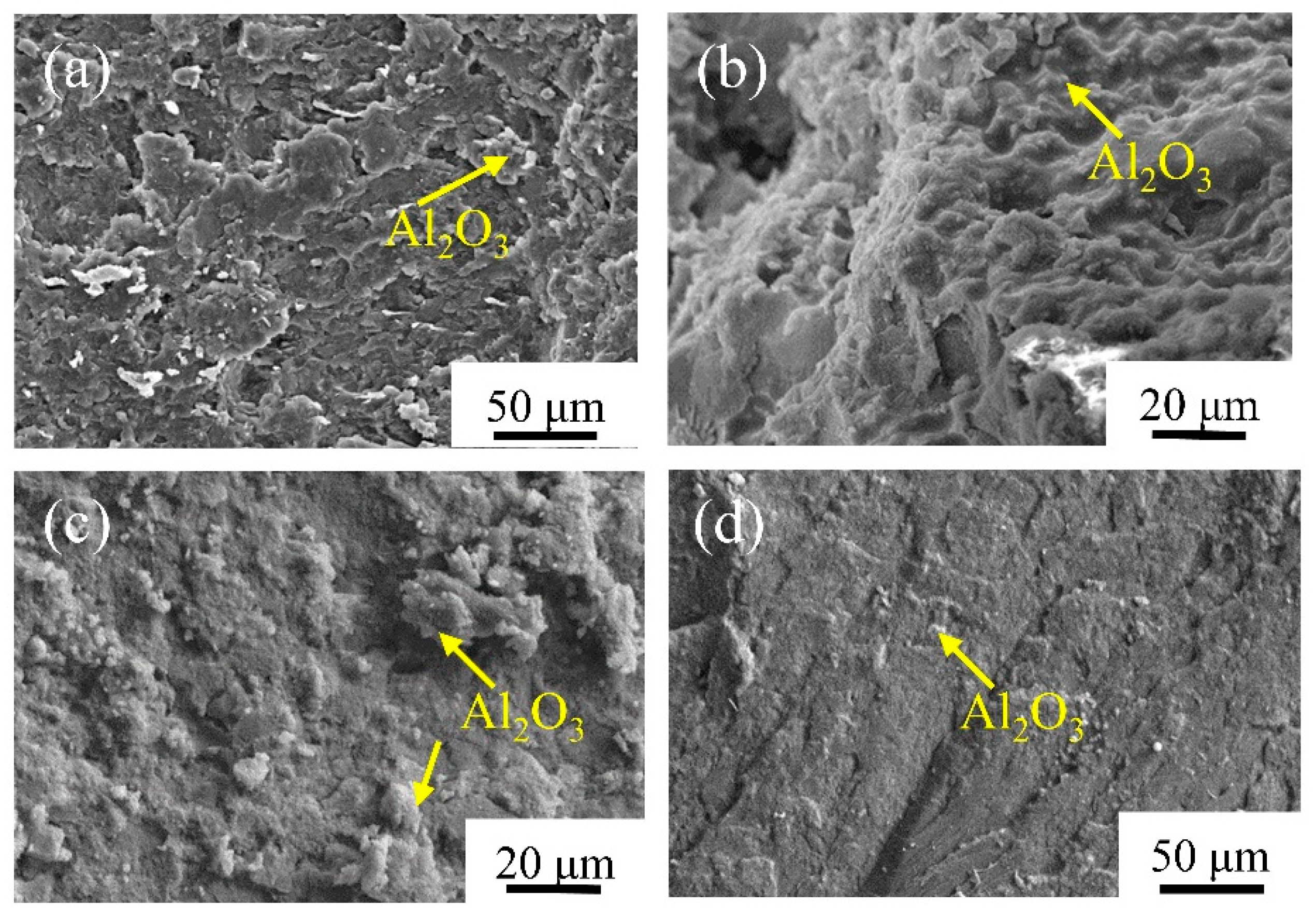
3. Results
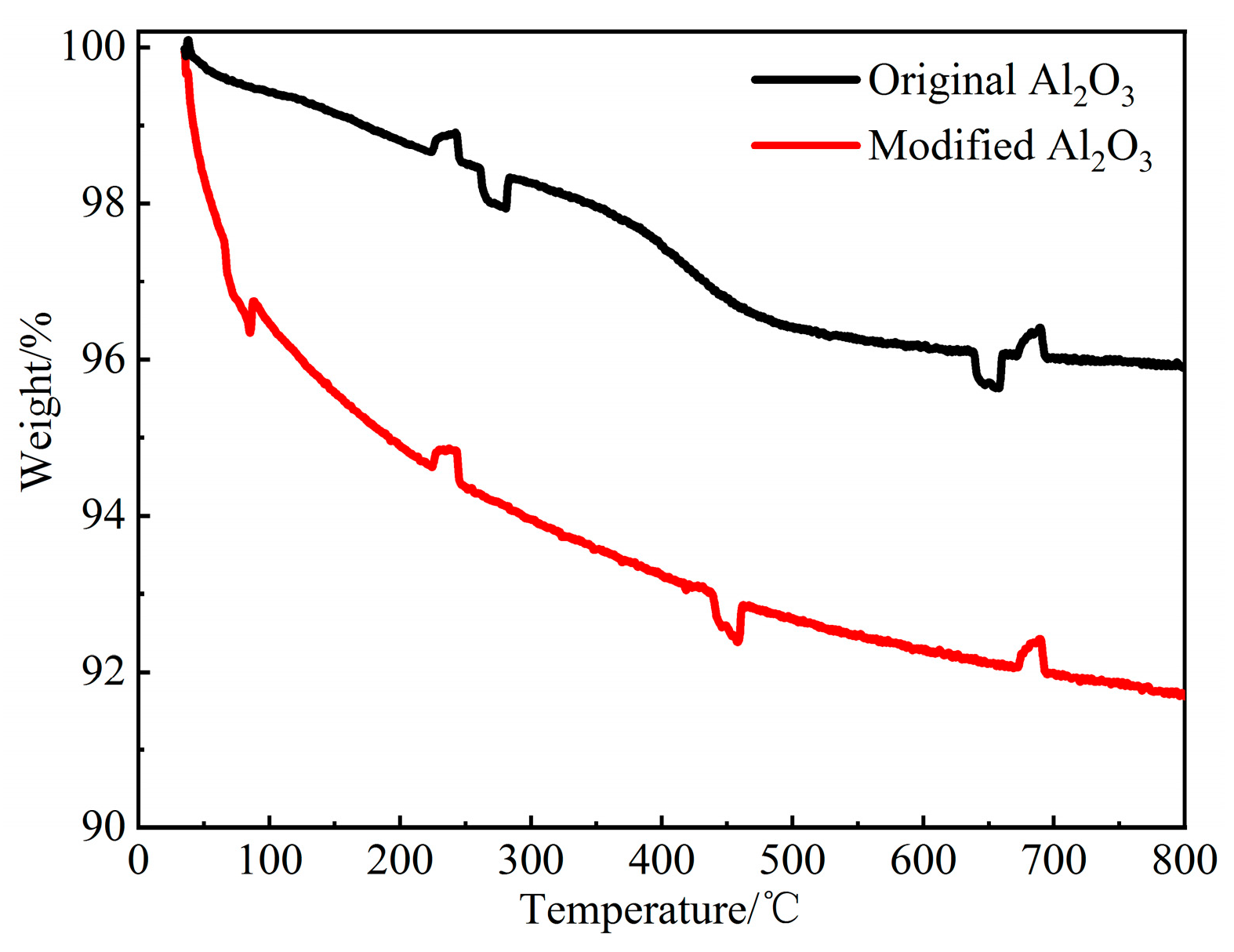
3.1. Thermal Stability of PI/Al2O3 Composites
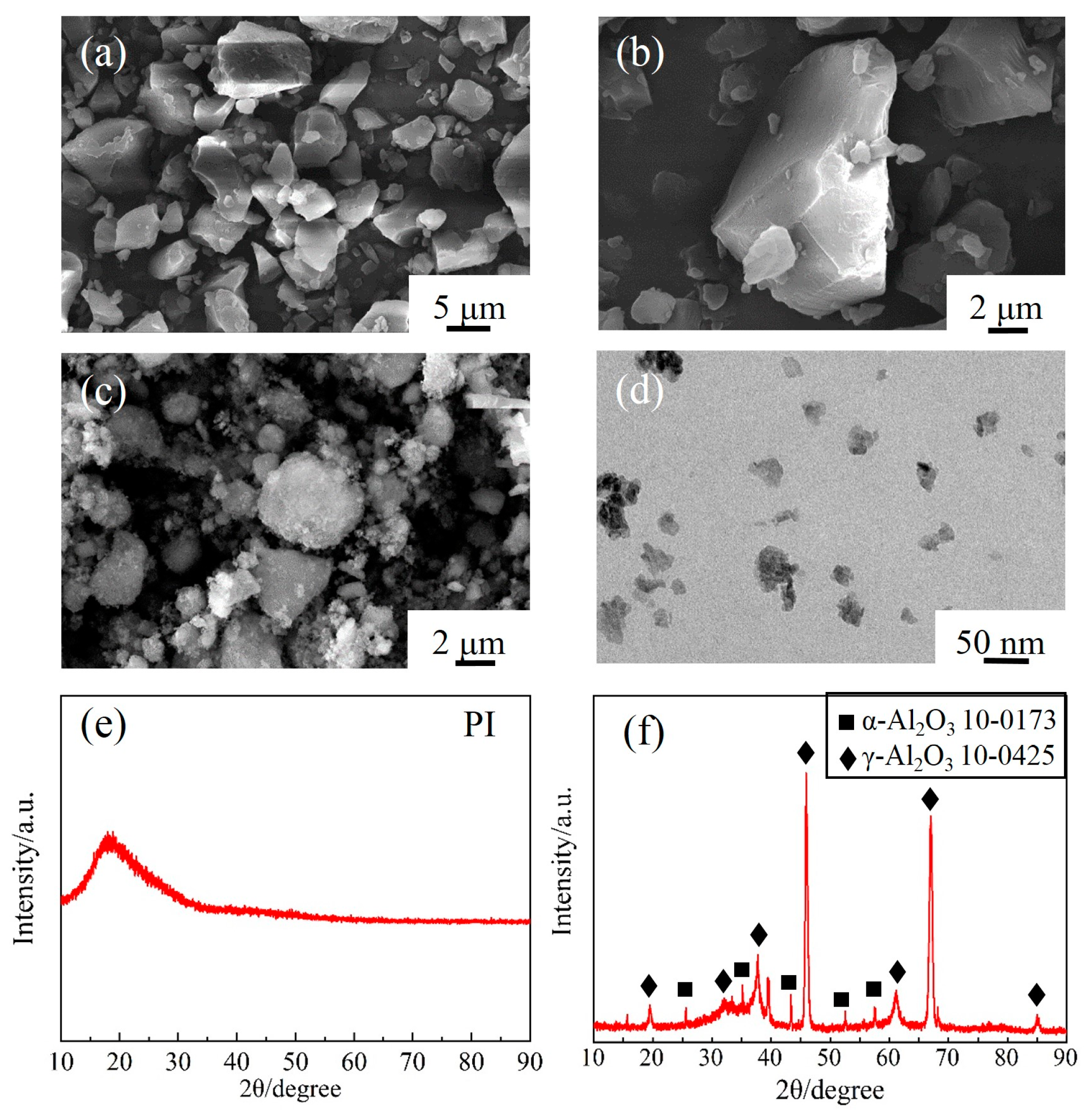
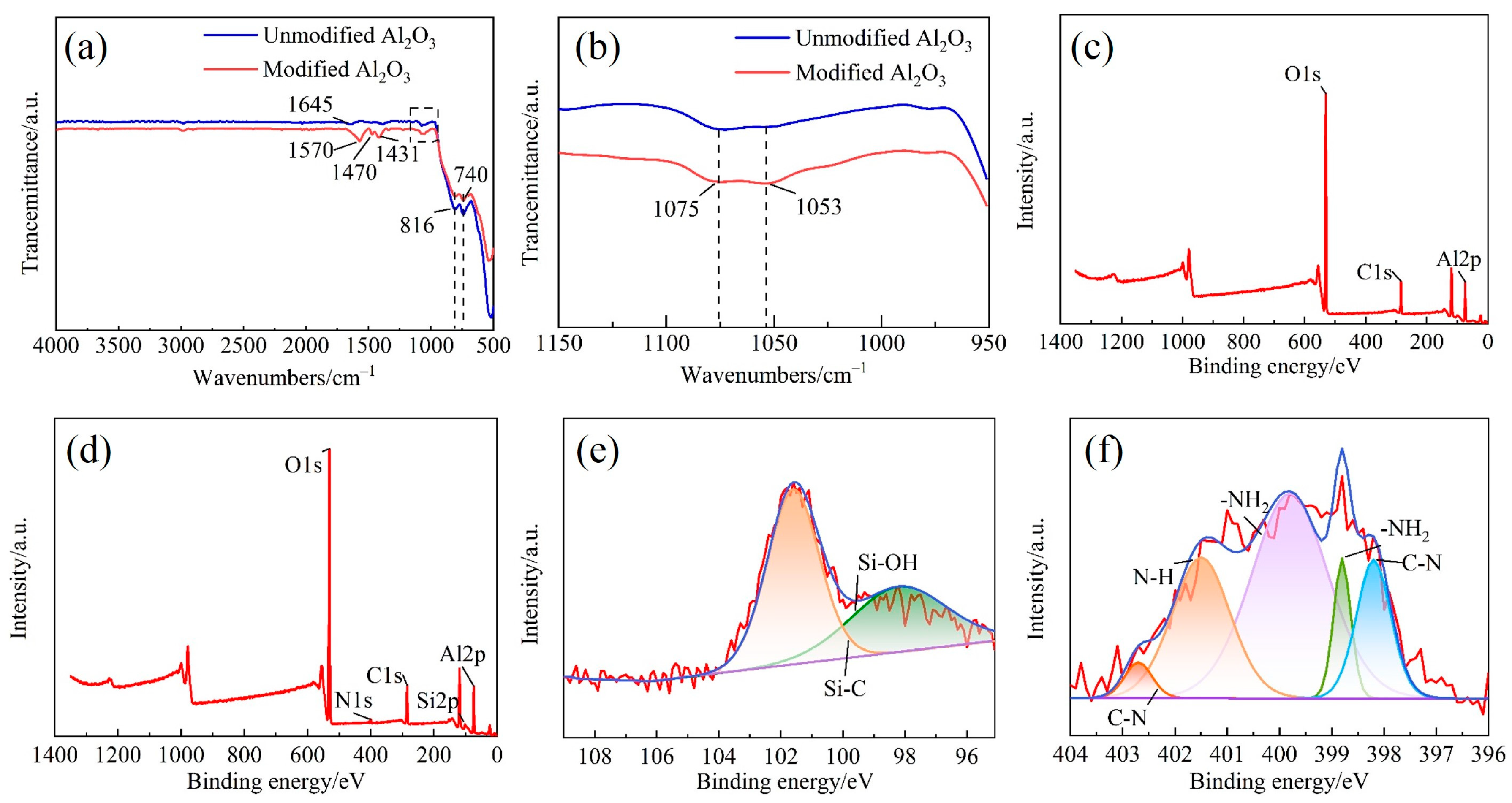
3.2. Surface Modification of Al2O3 Powder
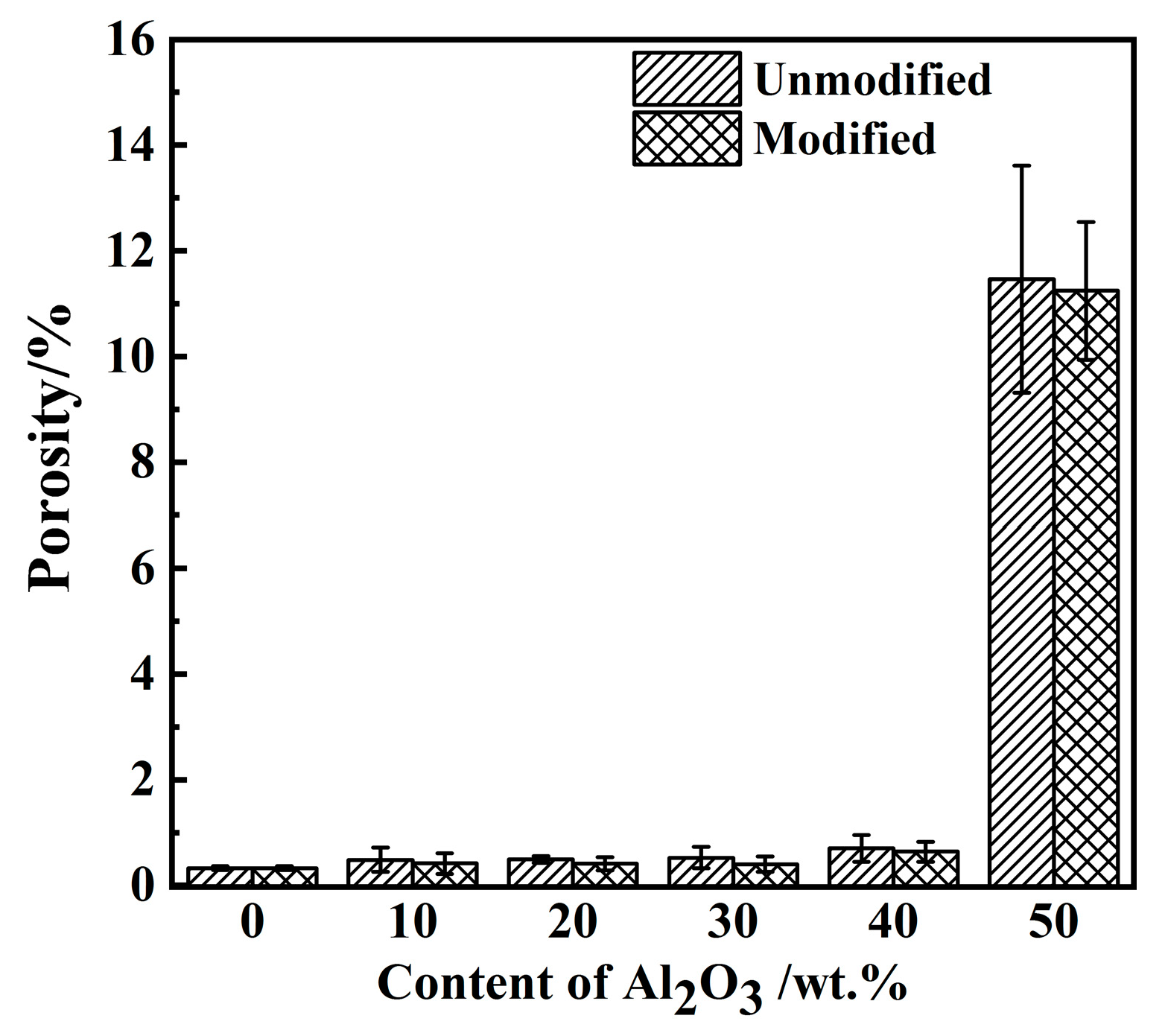
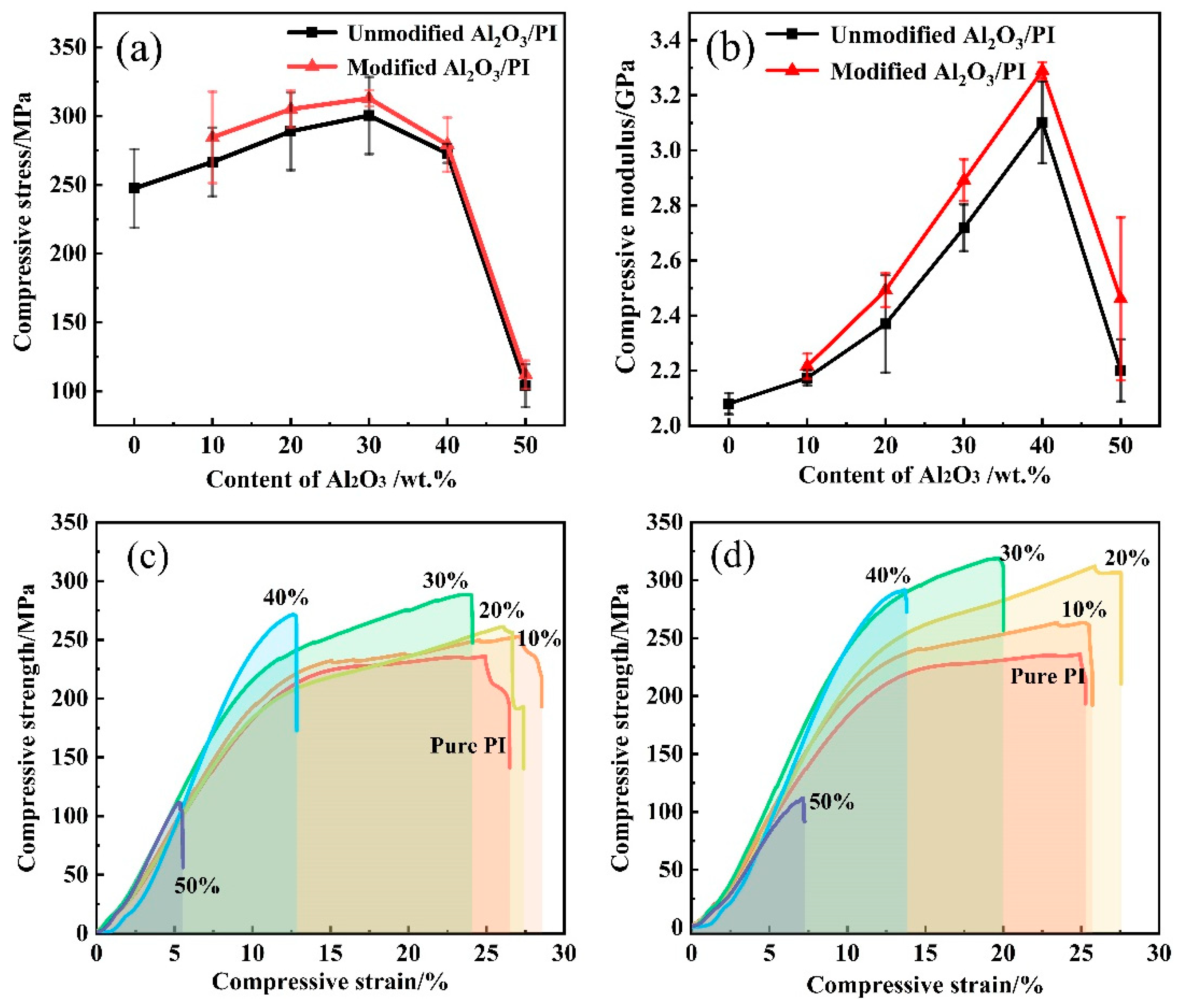
3.3. Mechanical Properties of PI/Al2O3 Composite Materials
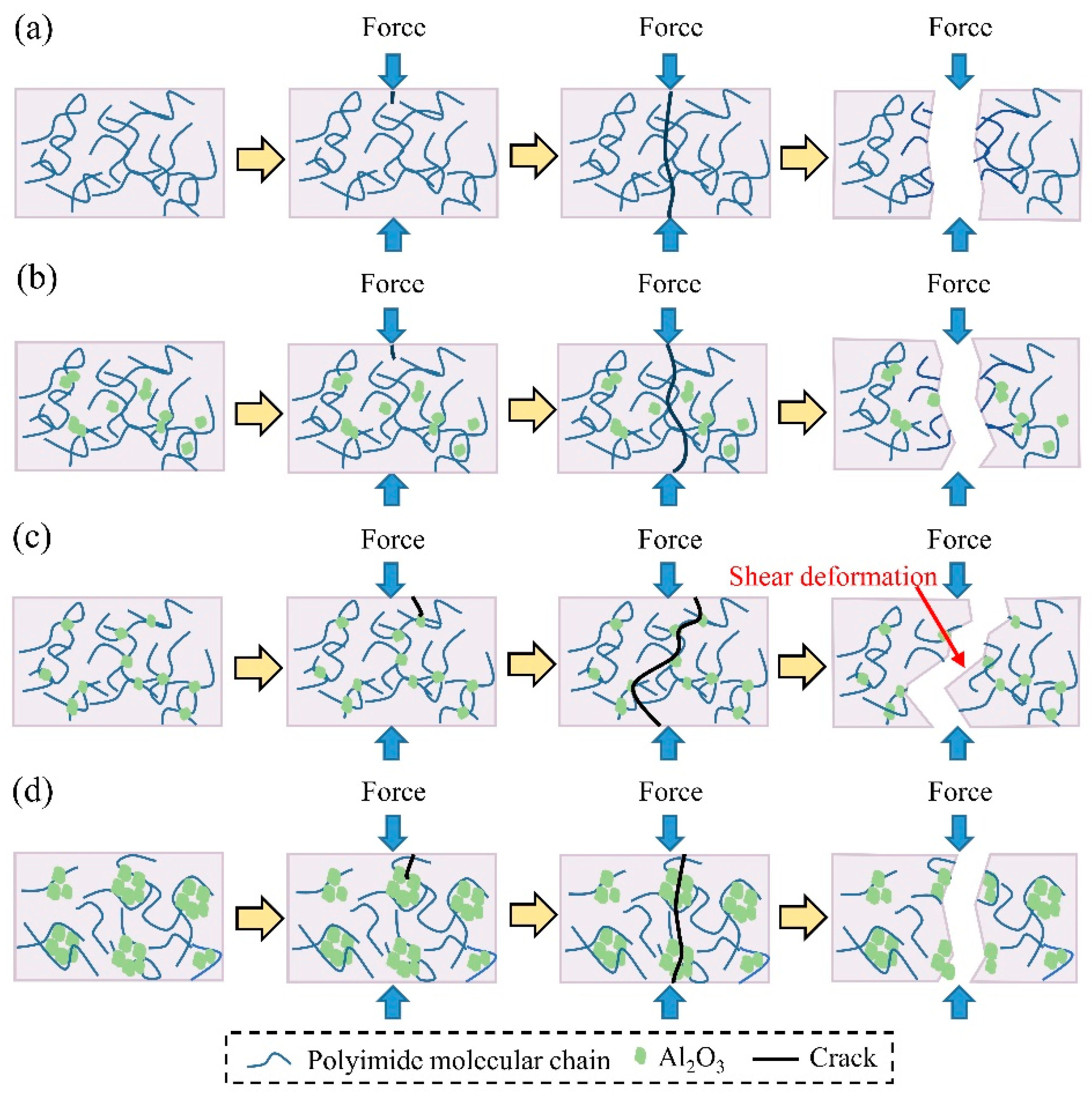
4. Discussion
5. Conclusions
- Chemical reactions occurred during the surface modification of nano-Al2O3. The appearance of -NH2, -CH2-, Si-C-H and Al-O-Si peaks in infrared spectroscopy and N and Si peaks in photoelectron spectra suggested that a modified layer was successfully prepared on the surface of Al2O3 nanoparticles.
- Surface modification enhanced the interfacial compatibility between the Al2O3 and PI matrix. The compressive strength and compressive modulus of composites with modified Al2O3 were higher than those with original Al2O3 to some extent.
- The compressive strength and compressive modulus of the composites initially increased and then decreased with the increase of Al2O3 content. The improvement of compressive properties of composites was due to the transfer of shear stresses in the nanoparticle–matrix interface and the formation of the three-dimensional network structure. When the Al2O3 content was 30 wt.%, the compressive strength of composites reached the maximum value of 313 MPa and increased by 26.5% compared to that of the PI matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Lan, Y.; Yu, W.; Li, X.; Qian, Y.; Liu, H. Preparation of amino-functionalized graphene oxide/polyimide composite films with improved mechanical, thermal and hydrophobic properties. Appl. Surf. Sci. 2016, 362, 11–19. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Liu, B.; Chen, W.; Chen, S. Dielectric properties of polyimide/Al2O3 hybrids synthesized by in-situ polymerization. Mater. Lett. 2007, 61, 1507–1511. [Google Scholar] [CrossRef]
- Alias, A.; Ahmad, Z.; Ismail, A.B. Preparation of polyimide/Al2O3 composite films as improved solid dielectrics. Mater. Sci. Eng. B 2011, 176, 799–804. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, D.; Liu, Q.; Chen, Y.; Zhang, Q. Highly anisotropic thermally conductive polyimide composites via the alignment of boron nitride platelets. Compos. Part B Eng. 2019, 158, 311–318. [Google Scholar] [CrossRef]
- Mekuria, T.D.; Wogsato, T.A. Synthesis, characterization and properties of polyimide nanocomposite thin films reinforced with TiO2/Al2O3 hybrid nanoparticles. Mater. Today Commun. 2022, 32, 103903. [Google Scholar] [CrossRef]
- Cai, H.; Yan, F.; Xue, Q.; Liu, W. Investigation of tribological properties of Al2O3-polyimide nanocomposites. Polym. Test. 2003, 22, 875–882. [Google Scholar] [CrossRef]
- Dai, H.; Thostenson, E.T.; Schumacher, T. Comparative study of the thermoresistive behavior of carbon nanotube-based nanocomposites and multiscale hybrid composites. Compos. Part B Eng. 2021, 222, 109068. [Google Scholar] [CrossRef]
- Malik, P.; Bhasha, B.; Jain, P. Influence of Surface modified Graphene Oxide on Mechanical and Thermal Properties of Epoxy Resin. Orient. J. Chem. 2018, 34, 1597–1603. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Song, M. An investigation of the mechanism of graphene toughening epoxy. Carbon 2013, 65, 324–333. [Google Scholar] [CrossRef]
- Singh, S.; Guchhait, P.K.; Bandyopadhyay, G.G.; Chaki, T.K. Development of polyimide–nanosilica filled EPDM based light rocket motor insulator compound: Influence of polyimide–nanosilica loading on thermal, ablation, and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2013, 44, 8–15. [Google Scholar] [CrossRef]
- Omar, M.F.; Akil, H.M.; Ahmad, Z.A. Mechanical properties of nanosilica/polypropylene composites under dynamic compression loading. Polym. Composite. 2011, 32, 565–575. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mat. Sci. Eng. R. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Ning, N.; Wang, M.; Zhou, G.; Qiu, Y.; Wei, Y. Effect of polymer nanoparticle morphology on fracture toughness enhancement of carbon fiber reinforced epoxy composites. Compos. Part B Eng. 2022, 234, 109749. [Google Scholar] [CrossRef]
- Maske, S.R.; Ganesh Gupta, K.B.N.V.S.; Kumar Prusty, R.; Ray, B.C. Effect of MWCNT/Nanosilica reinforcement on the mechanical and thermal behaviour of polymer composite. Mater. Today Proc. 2022, 62, 6087–6090. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, L.; Liu, G.; Zhang, D.; Zhou, L.; Zhang, Z. The effects of alumina nanofillers on mechanical properties of high-performance epoxy resin. J. Nanosci. Nanotechnol. 2010, 10, 7526–7532. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Belgacemi, R.; Derradji, M.; Trache, D.; Mouloud, A.; Zegaoui, A.; Mehelli, O.; Khiari, K. Effects of silane surface modified alumina nanoparticles on the mechanical, thermomechanical, and ballistic impact performances of epoxy/oxidized UHMWPE composites. Polym. Composite. 2020, 41, 4526–4537. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Siew, W.H.; Given, M.; Liggat, J.; He, J. Effect of different surface treatment agents on the physical chemistry and electrical properties of polyethylene nano–alumina nanocomposites. High Volt. 2020, 5, 397–402. [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Wang, Z. Facile synthesis of soluble, self-crosslinkable and crystalline polyimides with ultrahigh thermal/chemical resistance. Polymer 2023, 268, 125717. [Google Scholar] [CrossRef]
- Nakamuraa, K.; Andoa, S.; Takeichi, T. Thermal analysis and solid-state 13C NMR study of crosslink in polyimides containing acetylene groups in the main chain. Polymer 2001, 42, 4045–4054. [Google Scholar] [CrossRef]
- Sezavar, A.; Zebarjad, S.; Sajjadi, S. A Study on the Effect of Nano Alumina Particles on Fracture Behavior of PMMA. Technologies 2015, 3, 94–102. [Google Scholar] [CrossRef]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Zhu, L.; You, L.; Shi, Z.; Song, H.; Li, S. An investigation on the graphitic carbon nitride reinforced polyimide composite and evaluation of its tribological properties. J. Appl. Polym. Sci. 2017, 134, 45403. [Google Scholar] [CrossRef]
- Derradji, M.; Ramdani, N.; Zhang, T.; Wang, J.; Gong, L.D.; Xu, X.D.; Lin, Z.W.; Henniche, A.; Rahoma, H.K.S.; Liu, W.B. Thermal and mechanical properties enhancements obtained by reinforcing a bisphenol-a based phthalonitrile resin with silane surface-modified alumina nanoparticles. Polym. Composite. 2017, 38, 1549–1558. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, J.; Jiang, Z.; Dong, B.; Chen, X. Enhancing thermo-oxidative stability of thermoset polyimide composites using nano neodymium oxide particles. J. Mater. Res. Technol. 2021, 14, 2638–2649. [Google Scholar] [CrossRef]
- Opelt, C.V.; Coelho, L.A.F. Reinforcement and toughening mechanisms in polymer nanocomposites—Reinforcement effectiveness and nanoclay nanocomposites. Mater. Chem. Phys. 2016, 169, 179–185. [Google Scholar] [CrossRef]
- Opelt, C.V.; Becker, D.; Lepienski, C.M.; Coelho, L.A. Reinforcement and toughening mechanisms in polymer nanocomposites–Carbon nanotubes and aluminum oxide. Compos. Part B Eng. 2015, 75, 119–126. [Google Scholar] [CrossRef]
- Ou, X.; Lu, X.; Chen, S.; Lu, Q. Thermal conductive hybrid polyimide with ultrahigh heat resistance, excellent mechanical properties and low coefficient of thermal expansion. Eur. Polym. J. 2019, 122, 109368. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Investigating the nanostructure and thermal properties of chiralpoly (amide-imide)/Al2O3 compatibilized with 3-aminopropyltriethoxysilane. Mater. Res. Bull. 2013, 48, 3865–3872. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N.; Huang, Y.L.; Tien, H.W.; Wang, J.Y.; Ma, C.C.M.; Li, S.M.; Wang, Y.S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Leong, Y.W.; Abu Bakar, M.B.; Ishak, Z.M.; Ariffin, A.; Pukanszky, B. Comparison of the mechanical properties and interfacial interactions between talc, kaolin, and calcium carbonate filled polypropylene composites. J. Appl. Polym. Sci. 2004, 91, 3315–3326. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Viscoelastic behavior and reinforcement mechanism in rubber nanocomposites in the vicinity of spherical nanoparticles. J. Phys. Chem. B 2013, 117, 12632–12648. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Y.; Wang, H.; Duan, C.; Wang, T.; Wang, Q. Ultra-high compression and wear resistant hybrid filled polyimide composite: Synergistic effect of Fe2O3 decorated RGO. J. Appl. Polym. Sci. 2020, 137, 49222. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, X.; Dong, Y.; Ma, J.; Zhang, Q.; Song, L.; Yang, Y.; Yang, Q. Mechanical, thermal and tribological properties of polyimide/nano-SiO2 composites synthesized using an in-situ polymerization. Tribol. Int. 2016, 103, 599–608. [Google Scholar] [CrossRef]
- Idumah, C.I.; Obele, C.M. Understanding interfacial influence on properties of polymer nanocomposites. Surf. Interfaces 2021, 22, 100879. [Google Scholar] [CrossRef]




| Samples | PI/wt.% | Al2O3/wt.% | Modified through KH550 Silane Coupling Agent |
|---|---|---|---|
| PI | 100 | 0 | No |
| 10 A | 90 | 10 | No |
| 20 A | 80 | 20 | No |
| 30 A | 70 | 30 | No |
| 40 A | 60 | 40 | No |
| 50 A | 50 | 50 | No |
| 10 A-K | 90 | 10 | Yes |
| 20 A-K | 80 | 20 | Yes |
| 30 A-K | 70 | 30 | Yes |
| 40 A-K | 60 | 40 | Yes |
| 50 A-K | 50 | 50 | Yes |
| Samples | Roughness/μm |
|---|---|
| PI | 0.995 |
| 10 A | 1.176 |
| 20 A | 1.596 |
| 30 A | 2.111 |
| 40 A | 2.424 |
| 50 A | 3.058 |
| 10 A-K | 1.680 |
| 20 A-K | 1.986 |
| 30 A-K | 2.492 |
| 40 A-K | 2.542 |
| 50 A-K | 3.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wang, Y.; Liu, G.; Shang, Q.; Wang, B.; Sun, J.; Bai, Y.; Liu, M.; Yu, F.; Ma, G.; et al. Surface Modification of Nano-Al2O3 with Silane Coupling Agent and Its Effect on the Compressive Strength of PI/Al2O3 Composites. Coatings 2024, 14, 27. https://doi.org/10.3390/coatings14010027
Cao J, Wang Y, Liu G, Shang Q, Wang B, Sun J, Bai Y, Liu M, Yu F, Ma G, et al. Surface Modification of Nano-Al2O3 with Silane Coupling Agent and Its Effect on the Compressive Strength of PI/Al2O3 Composites. Coatings. 2024; 14(1):27. https://doi.org/10.3390/coatings14010027
Chicago/Turabian StyleCao, Jing, Yu Wang, Guanghua Liu, Qingyuan Shang, Bicheng Wang, Jian Sun, Yu Bai, Ming Liu, Fangli Yu, Guozheng Ma, and et al. 2024. "Surface Modification of Nano-Al2O3 with Silane Coupling Agent and Its Effect on the Compressive Strength of PI/Al2O3 Composites" Coatings 14, no. 1: 27. https://doi.org/10.3390/coatings14010027
APA StyleCao, J., Wang, Y., Liu, G., Shang, Q., Wang, B., Sun, J., Bai, Y., Liu, M., Yu, F., Ma, G., & Wang, H. (2024). Surface Modification of Nano-Al2O3 with Silane Coupling Agent and Its Effect on the Compressive Strength of PI/Al2O3 Composites. Coatings, 14(1), 27. https://doi.org/10.3390/coatings14010027







