Effect of Re Addition on Microstructure and Oxidation Resistance of Co-Cr-Ta-Ti-C Alloys
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussions
3.1. Microstructures of the Alloys
3.2. Evolution of Melting Temperatures
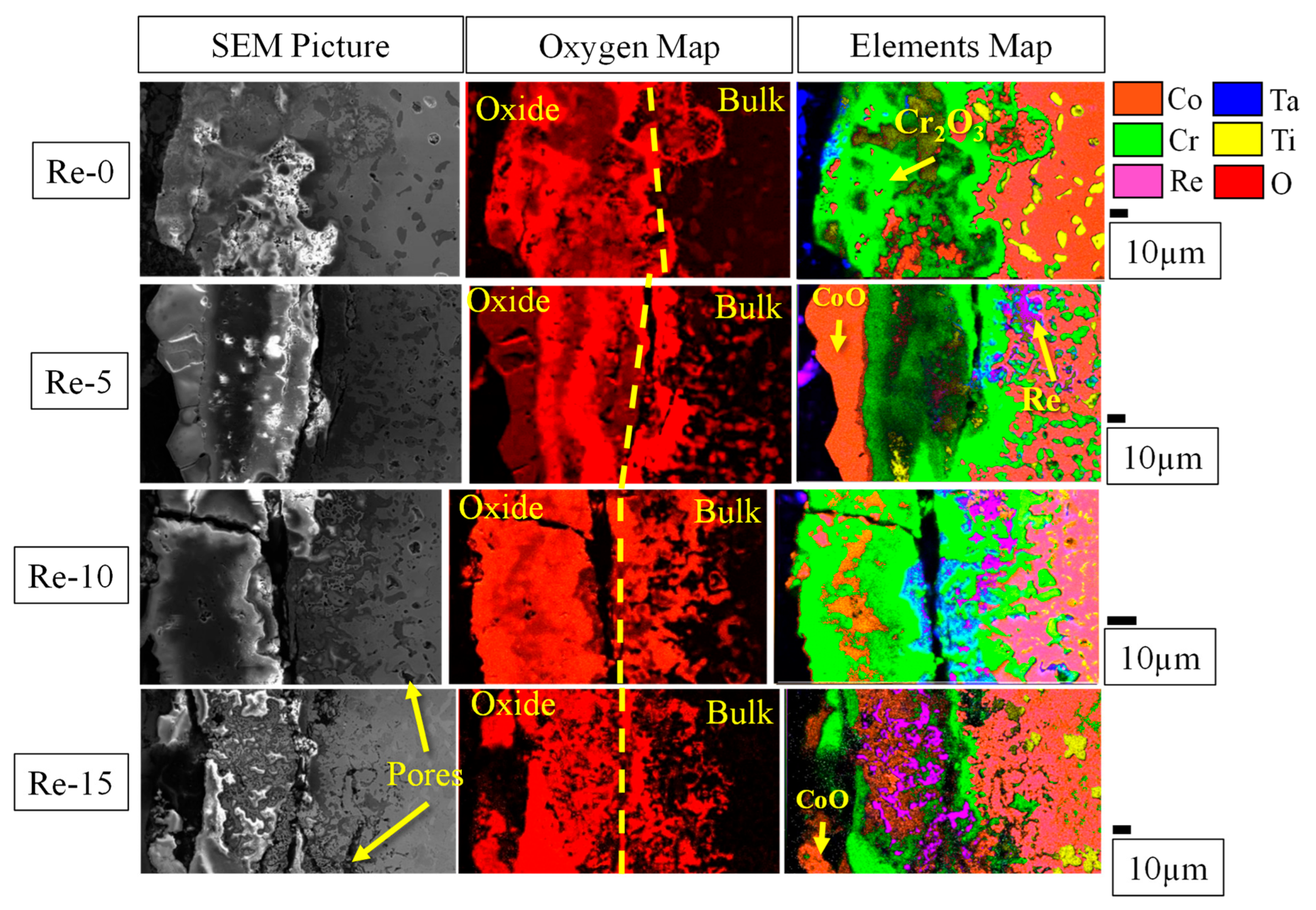
3.3. Oxidation Resistance at 1200 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, L.G.; Gibbons, T.B. Recent developments in gas turbine materials and technology and their implications for syngas firing. Int. J. Hydrogen Energy 2007, 32, 3610–3621. [Google Scholar] [CrossRef]
- Mouritz, A.P. Superalloys for Gas Turbine Engines, Introduction to Aerospace Materials; Woodhead Publishing: Sawston, UK, 2012; pp. 251–267. ISBN 9781855739468. [Google Scholar] [CrossRef]
- Kirka, M.M.; Fernandez-Zelaia, P. Additive Materials for High Temperature Applications. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 529–536. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys, Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006; ISBN 0-521-85904-2. [Google Scholar]
- Makineni, S.K.; Sharma, A.; Pandey, P.; Chattopadhyay, K. An Overview on Co-Base Alloys for High Temperature Applications. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 323–334. [Google Scholar] [CrossRef]
- Coutsouradis, D.; Davin, A.; Lamberigts, M. Cobalt-based superalloys for applications in gas turbines. Mater. Sci. Eng. 1987, 88, 11–19. [Google Scholar] [CrossRef]
- Berthod, P.; Himeur, Z.; Panteix, P.-J. Influences of the Co content and of the level of high temperature on the microstructure and oxidation of cast {Ni, Co}-based Cr-rich TaC-containing cast alloys. J. Alloy. Compd. 2018, 739, 447–456. [Google Scholar] [CrossRef]
- Bernard, J.-L.; Berthod, P.; Hericher, L.; Liebaut, C.; Michon, S. Refractory Alloy, Fibre-Forming Plate and Method for Producing Mineral Wool. U.S. Patent 826,296,4B2, 11 September 2012. [Google Scholar]
- Selvam, J.D.R.; Dinaharan, I.; Rai, R.S. Matrix and Reinforcement Materials for Metal Matrix Composites. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 615–639. ISBN 9780128197318. [Google Scholar] [CrossRef]
- Durlu, N. Titanium carbide based composites for high temperature applications. J. Eur. Ceram. Soc. 1999, 19, 2415–2419. [Google Scholar] [CrossRef]
- Gu, Y.F.; Harada, H.; Ro, Y. Chromium and chromium-based alloys: Problems and possibilities for high-temperature service. JOM 2004, 56, 28–33. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High. Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Khalid, F.; Hussain, N.; Shahid, K. Microstructure and morphology of high temperature oxidation in superalloys. Mater. Sci. Eng. A 1999, 265, 87–94. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L: Experimental and modeling results. Mater. Sci. Eng. A 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Sidhom, H.; Philibert, J. Prediction of chromium depleted-zone evolution during aging of Ni–Cr–Fe alloys. Acta Mater. 2002, 50, 1383–1392. [Google Scholar] [CrossRef]
- Rösler, J.; Mukherji, D.; Baranski, T. Co-Re-based Alloys: A New Class of High Temperature Materials? Adv. Eng. Mater. 2007, 9, 876–881. [Google Scholar] [CrossRef]
- Mukherji, D.; Strunz, P.; Gilles, R.; Karge, L.; Rösler, J. Current status of Co-Re-based alloys being developed to supplement Ni-based superalloys for ultra-high temperature applications in gas turbines. Met. Mater. 2015, 53, 287–294. [Google Scholar] [CrossRef]
- Muktinutalapati, N.R. Materials for Gas Turbines—An Overview. In Advances in Gas Turbine Technology; Benini, E., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Bochenek, K.; Węglewski, W.; Morgiel, J.; Basista, M. Influence of rhenium addition on microstructure, mechanical properties and oxidation resistance of NiAl obtained by powder metallurgy. Mater. Sci. Eng. A 2018, 735, 121–130. [Google Scholar] [CrossRef]
- Skoczylas, P.; Kaczorowski, M. Preliminary Study of the Rhenium Addition on the Structure and Mechanical Properties of Tungsten Heavy Alloy. Materials 2021, 14, 7365. [Google Scholar] [CrossRef] [PubMed]
- Gorr, B.; Trindade, V.; Burk, S.; Christ, H.-J.; Klauke, M.; Mukherji, D.; Rösler, J. Oxidation Behaviour of Model Cobalt-Rhenium Alloys During Short-Term Exposure to Laboratory Air at Elevated Temperature. Oxid. Met. 2009, 71, 157–172. [Google Scholar] [CrossRef]
- Karge, L.; Gilles, R.; Mukherji, D.; Beran, P.; Strunz, P.; Hoelzel, M.; Rosler, J. Beyond Ni-base Superalloys: Influence of Cr Addition on Co-Re base alloys strengthened by nano-sized TaC particles. Phys. B Phys. Condens. Matter 2018, 551, 1–5. [Google Scholar] [CrossRef]
- Ma, S.; Ding, Q.; Wei, X.; Zhang, Z.; Bei, H. The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys. Materials 2022, 15, 7352. [Google Scholar] [CrossRef] [PubMed]
- Whittle, D.P.; Stringer, J. Improvements in high temperature oxidation resistance by additions of reactive elements or oxide dispersions. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 295, 309–329. [Google Scholar] [CrossRef]
- Etienne, M.L.; Stéphane, G.; Guillaume, L.; Hideyuki, M. Microstructure and oxidation behavior of Co–Cr–Ta ternary alloys. J. Alloys Compd. 2023, 936, 167968. [Google Scholar] [CrossRef]
- Faber, J.; Fawcett, T. The Powder Diffraction File: Present and future. Acta Cryst. 2002, B58, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Principle of Differential Thermal Analysis (DTA). Available online: https://www.hitachi-hightech.com/global/products/science/tech/ana/thermal/descriptions/dta.html (accessed on 1 July 2022).
- Pandat Software: CALPHAD-Based Materials Design. Available online: https://computherm.com/software (accessed on 1 July 2020).
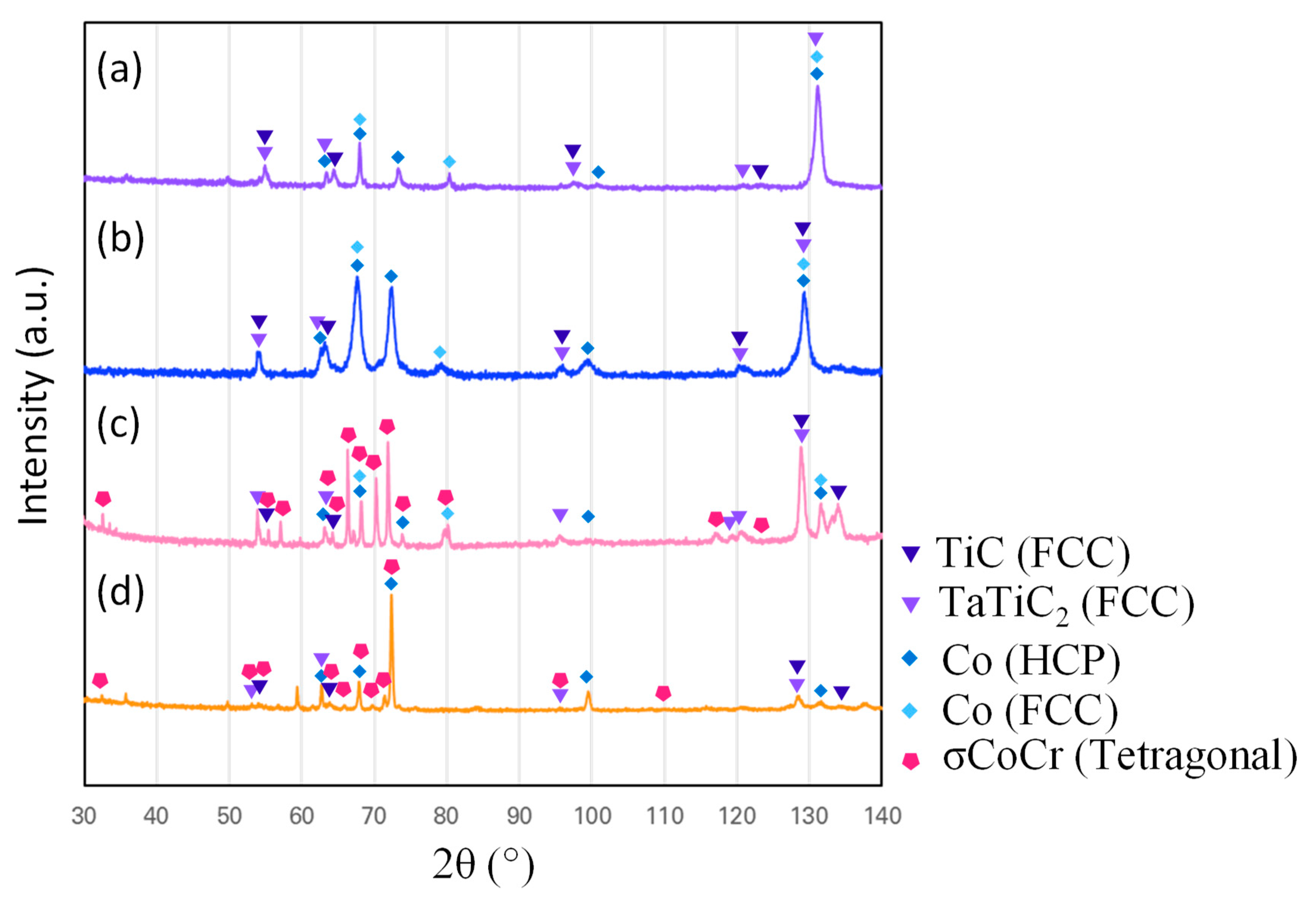
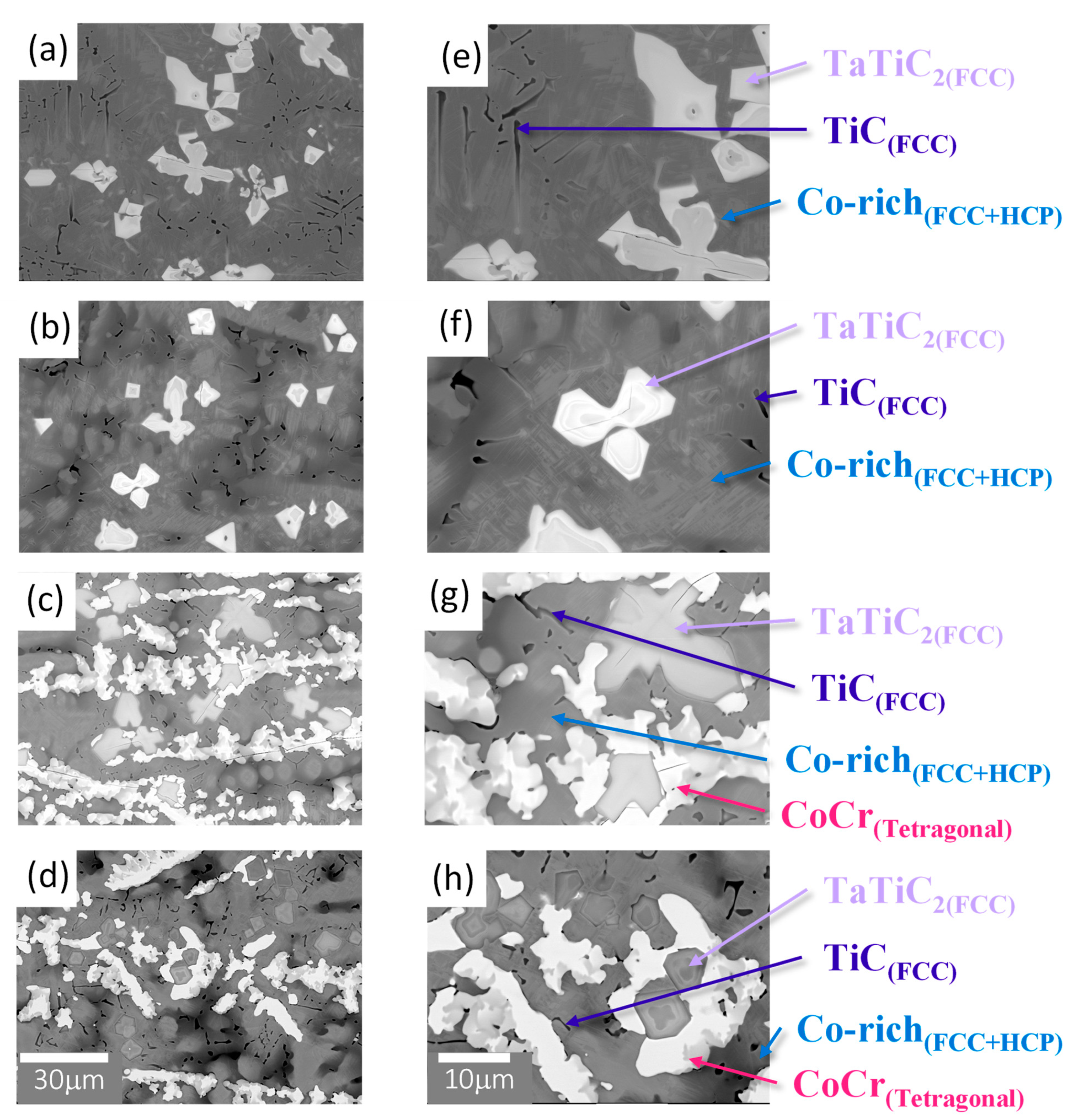


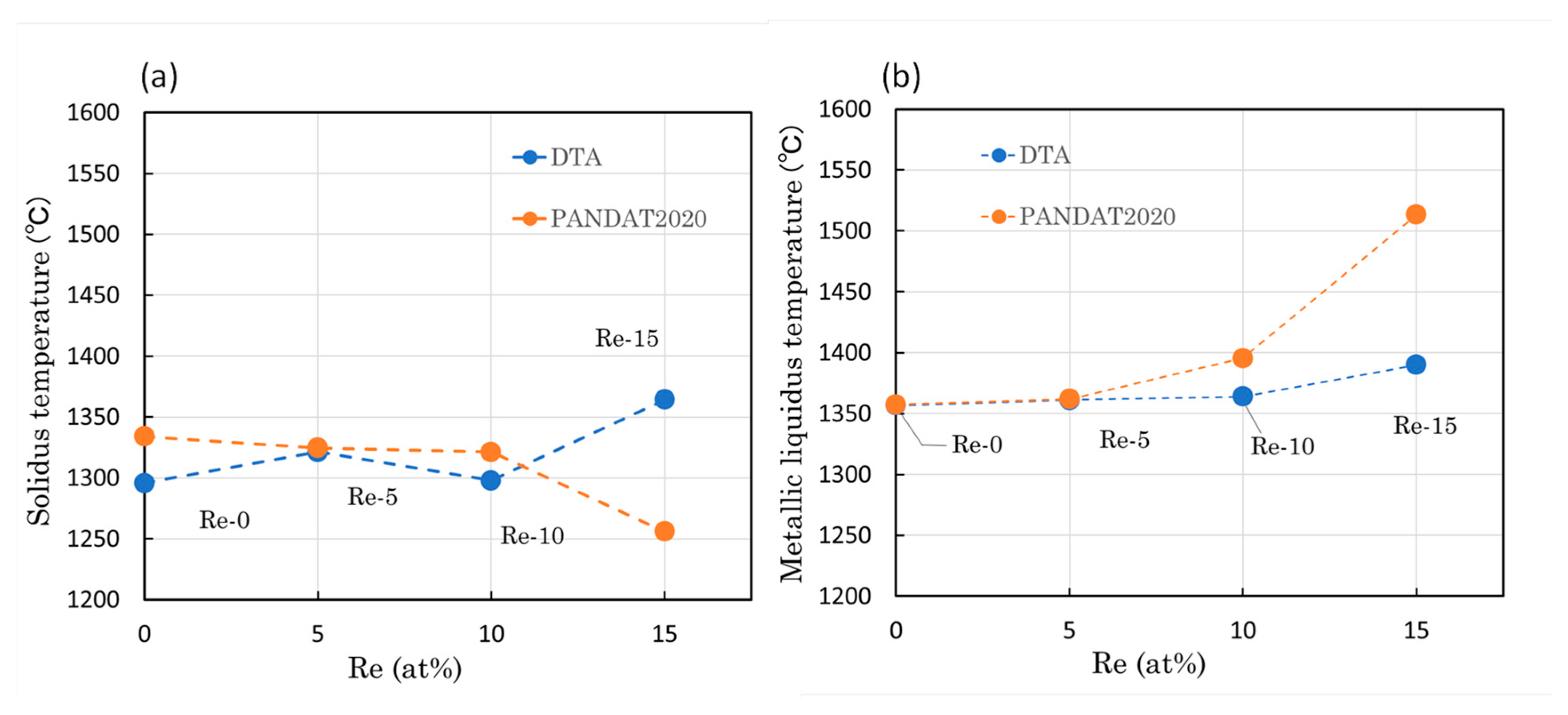

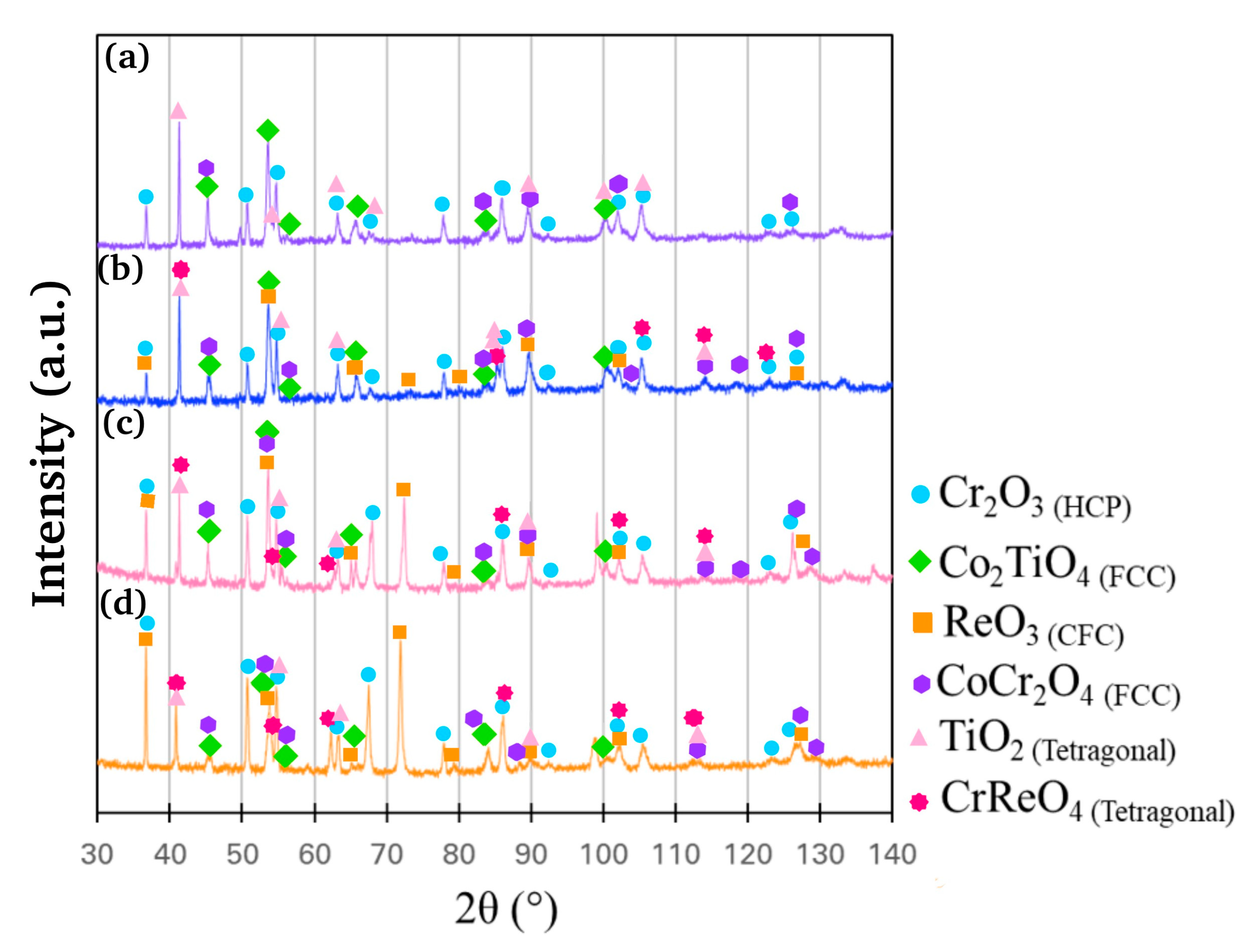
| Alloy | Co (at.%) | Cr (at.%) | Re (at.%) | Ta (at.%) | Ti (at.%) | C (at.%) |
|---|---|---|---|---|---|---|
| Re-0 | 58.8 | 27 | 1.7 | 6.4 | 6.1 | |
| Re-5 | 53.8 | 27 | 5 | 1.7 | 6.4 | 6.1 |
| Re-10 | 48.8 | 27 | 10 | 1.7 | 6.4 | 6.1 |
| Re-15 | 43.8 | 27 | 15 | 1.7 | 6.4 | 6.1 |
| Phases | Co | Cr | Re | Ta | Ti | C |
|---|---|---|---|---|---|---|
| Re-0 | 58.8 | 27 | 0 | 1.7 | 6.4 | 6.1 |
| Dark grey region (HCP+FCC) | 64.1 | 26.6 | / | 0.04 | 1.9 | 4.6 |
| Black region (TiC) | 28.7 | 15.7 | / | 4.5 | 29.3 | 21.9 |
| Light grey region (TaTiC2) | 2.1 | 4.0 | / | 19.6 | 47.1 | 27.1 |
| Re-5 | 53.8 | 27 | 5 | 1.7 | 6.4 | 6.1 |
| Dark grey region (HCP+FCC) | 60.1 | 27.8 | 2.8 | 0.3 | 3.2 | 5.7 |
| Black region (TiC) | 18.5 | 10.9 | 0.4 | 9.7 | 38.9 | 21.9 |
| Light grey region (TaTiC2) | 2.5 | 1.6 | / | 27.7 | 41.1 | 27.1 |
| Re-10 | 48.8 | 27 | 10 | 1.7 | 6.4 | 6.1 |
| Dark grey region (HCP+FCC) | 61.8 | 24.3 | 4.4 | 0.3 | 2.6 | 6.6 |
| Black region (TiC) | 23.8 | 13.9 | 2.4 | 8.4 | 29.2 | 22.4 |
| Light grey region (TaTiC2) | 3.9 | 2.5 | / | 22.3 | 44.2 | 27.0 |
| White region (σ-phase) | 42.4 | 31.7 | 10.6 | 1.4 | 6.2 | 7.6 |
| Re-15 | 43.8 | 27 | 15 | 1.7 | 6.4 | 6.1 |
| Dark grey region (HCP+FCC) | 62.4 | 21.1 | 8.7 | 0.3 | 1.4 | 6.0 |
| Black region (TiC) | 19.1 | 9.5 | 2.8 | 8.3 | 35.8 | 24.5 |
| Light grey region (TaTiC2) | 1.8 | 1.5 | / | 24.8 | 44.7 | 27.1 |
| White region (σ-phase) | 39.2 | 29.5 | 20.8 | 0.8 | 2.4 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, L.E.; Banoth, S.; Ishida, A.; Murakami, H. Effect of Re Addition on Microstructure and Oxidation Resistance of Co-Cr-Ta-Ti-C Alloys. Coatings 2024, 14, 26. https://doi.org/10.3390/coatings14010026
Moreau LE, Banoth S, Ishida A, Murakami H. Effect of Re Addition on Microstructure and Oxidation Resistance of Co-Cr-Ta-Ti-C Alloys. Coatings. 2024; 14(1):26. https://doi.org/10.3390/coatings14010026
Chicago/Turabian StyleMoreau, Louis Etienne, Santhosh Banoth, Akira Ishida, and Hideyuki Murakami. 2024. "Effect of Re Addition on Microstructure and Oxidation Resistance of Co-Cr-Ta-Ti-C Alloys" Coatings 14, no. 1: 26. https://doi.org/10.3390/coatings14010026






