Evaluation of Shielding Performance of Gamma Ray Shielding Tungsten Polymer Composite with LBL-Type Layered Structure
Abstract
:1. Introduction
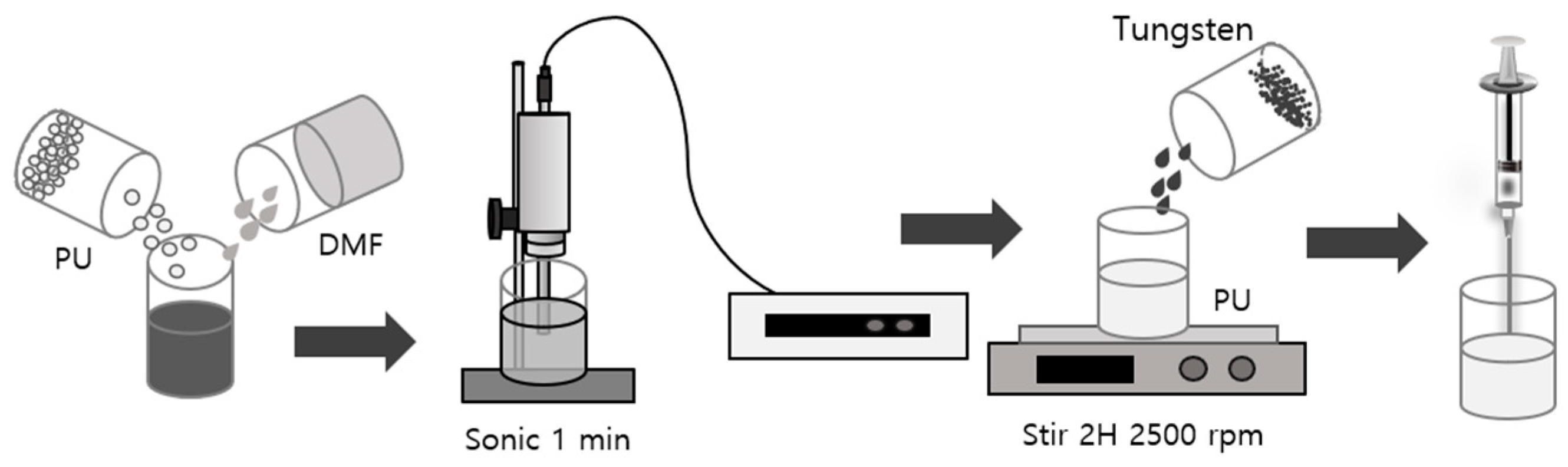
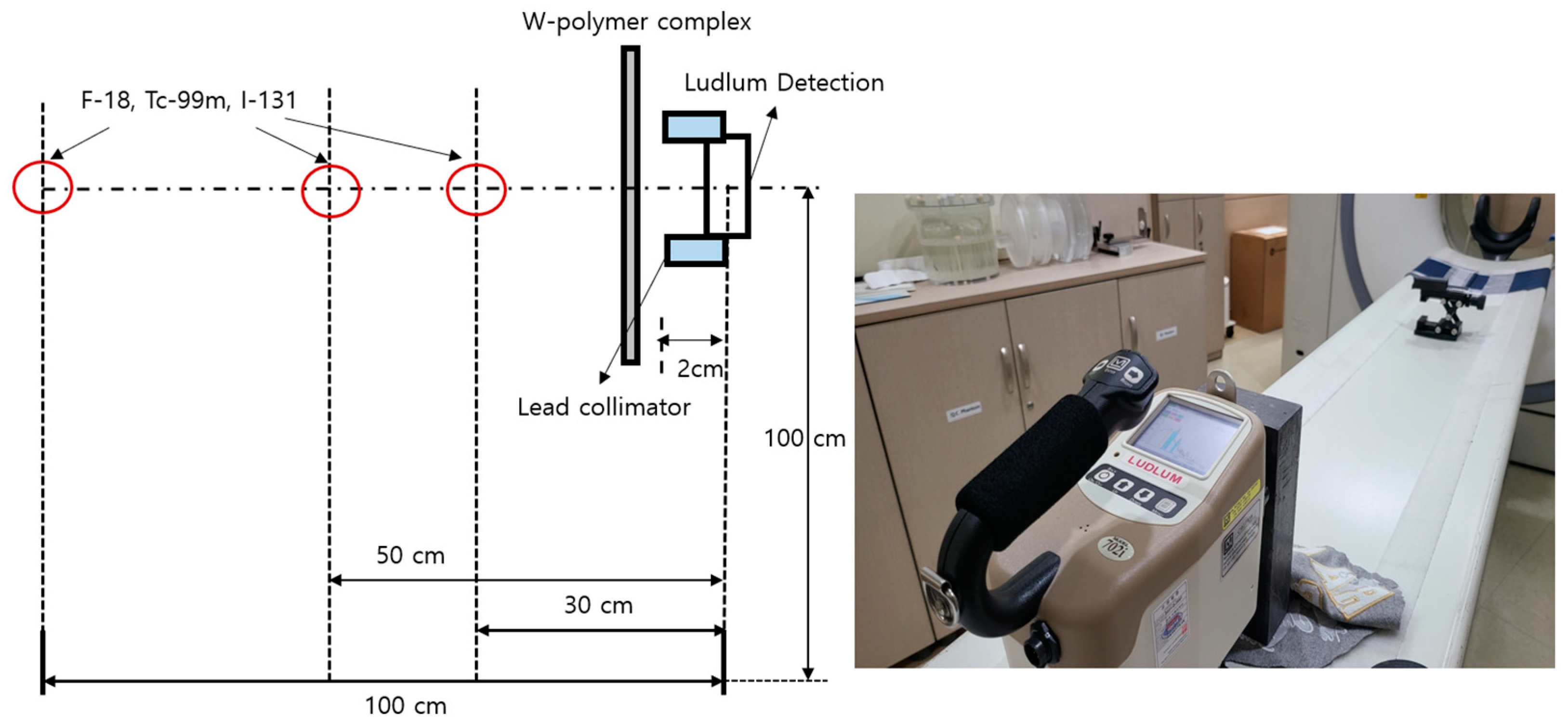
2. Materials and Methods
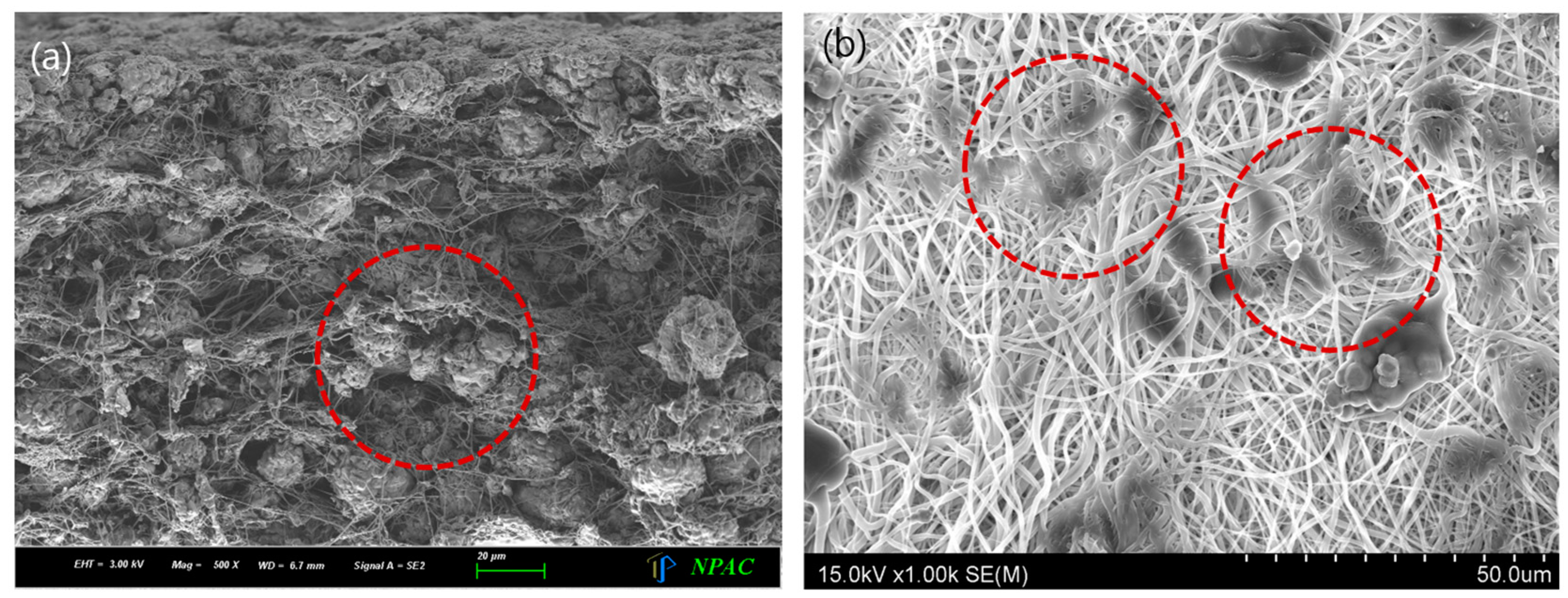
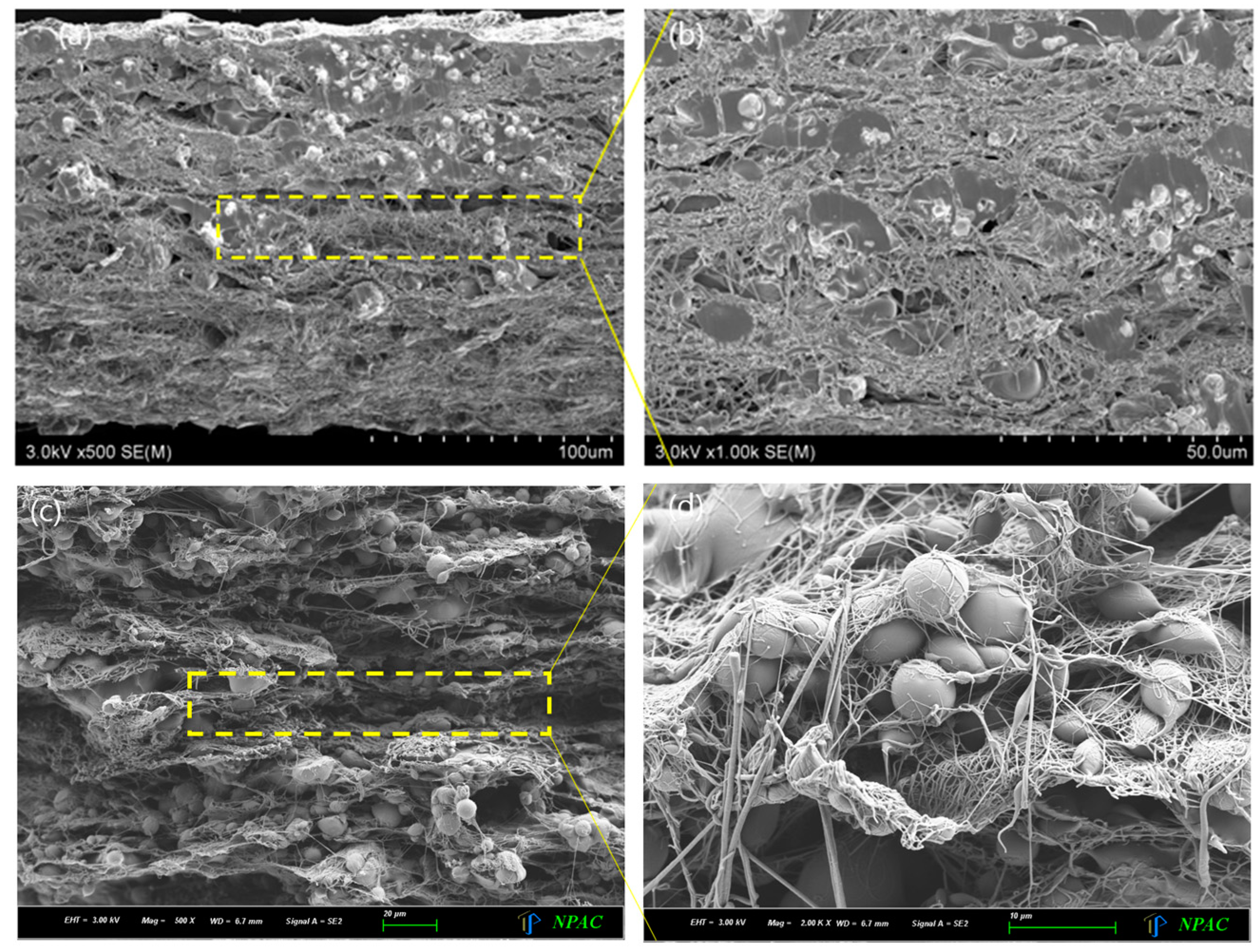
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramamoorthy, N. Impact of Nuclear Medicine and Radiopharmaceuticals on Health-Care Delivery: Advances, Lessons, and Need for an Objective Value-Matrix. Indian J. Nucl. Med. 2018, 33, 273–276. [Google Scholar] [CrossRef]
- Payolla, F.B.; Massabni, A.C.; Orvig, C. Radiopharmaceuticals for Diagnosis in Nuclear Medicine: A Short Review. Eclética Quim. 2019, 44, 11–19. [Google Scholar] [CrossRef]
- Rajkhowa, S.; Sarma, J.; Das, A.R. Chapter 15—Radiological Contaminants in Water: Pollution, Health Risk, and Treatment. In Contamination of Water; Ahamad, A., Siddiqui, S.I., Singh, P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 217–236. ISBN 9780128240588. [Google Scholar] [CrossRef]
- Chida, K. What Are Useful Methods to Reduce Occupational Radiation Exposure among Radiological Medical Workers, Especially for Interventional Radiology Personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef]
- Elsafi, M.; El-Nahal, M.A.; Sayyed, M.I.; Saleh, I.H.; Abbas, M.I. Effect of Bulk and Nanoparticle Bi2O3 on Attenuation Capability of Radiation Shielding Glass. Ceram. Int. 2021, 47, 19651–19658. [Google Scholar] [CrossRef]
- Al Hamrashdi, H.; Cheneler, D.; Monk, S.D. A Fast and Portable Imager for Neutron and Gamma Emitting Radionuclides. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 953, 163253. [Google Scholar] [CrossRef]
- Jang, D.-G.; Park, C.-W.; Park, E.-T. Scattering Measurement of Syringe Shield Used in PET/CT. J. Radiolog. Sci. Technol. Kor. Soc. Radiolog. Sci. 2020, 43, 375–382. [Google Scholar] [CrossRef]
- Park, M.; Kwon, T.E.; Ha, W.H.; Kim, C.H.; Park, S.; Jin, Y.W. Counting Efficiencies Determined by Monte Carlo Methods for In Vivo Measurement of 131I Activity in Thyroid. Health Phys. 2019, 117, 388–395. [Google Scholar] [CrossRef]
- Rani, N.; Vermani, Y.K.; Singh, T. Gamma Radiation Shielding Properties of Some Bi-Sn-Zn Alloys. J. Radiol. Prot. 2020, 40, 296–310. [Google Scholar] [CrossRef]
- Arif Sazali, M.; Alang, M.; Hamzah, K. A Review on Multilayer Radiation Shielding. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 555, p. 012008. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Moore, P.R.; Papiz, M.Z.; Smith, J.M.A. Measurement of Absorption Curves for Protein Single Crystals on the Oscillation Camera with Time Decaying Incident-Beam Intensity and Variable-Wavelength Synchrotron X-Radiation. J. Appl. Crystallogr. 1984, 17, 417–419. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric Composite Materials for Radiation Shielding: A Review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Khan, N.S. Study of two dimensional boundary layer flow of a thin film second grade fluid with variable thermo-physical properties in three dimensions space. Filomat 2019, 33, 5387–5405. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V. Research of the Shielding Effect and Radiation Resistance of Composite CuBi2O4 Films as well as Their Practical Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11729–11740. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the Layer-by-Layer Assembly of Multilayered Polymer Composites: Strategy, Structural Control and Applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Švára, D.; Kopřivová, B.; Picek, T.; Mikeš, P.; Kluk, A.; Šoóš, M. The Impact of the Lamination Pressure on the Properties of Electrospinned Nanofibrous Films, European. J. Pharm. Sci. 2022, 173, 106170. [Google Scholar] [CrossRef]
- Kijima, K.; Krisanachinda, A.; Pasawang, P.; Hanaoka, K.; Monzen, H.; Nishimura, Y. Shielding efficiency of novel tungsten rubber against radionuclides of 99mTc, 131I, 18F and 68Ga. Radiat. Phys. Chem. 2020, 172, 108755. [Google Scholar] [CrossRef]
- Osman, A. Study on gamma ray shielding performance of concretes doped with natural sepiolite mineral. Radiochimica Acta. 2018, 106, 1009–1016. [Google Scholar] [CrossRef]
- Liang, C.; Gu, Z.; Zhang, Y.; Ma, Z.; Qiu, H.; Gu, J. Structural Design Strategies of Polymer Matrix Composites for Electromagnetic Interference Shielding: A Review. Nano-Micro Lett. 2021, 13, 181. [Google Scholar] [CrossRef]
- Kim, S.-C. Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder. Appl. Sci. 2022, 12, 968. [Google Scholar] [CrossRef]
- Oshina, I.; Spigulis, J. Beer–Lambert law for optical tissue diagnostics: Current state of the art and the main limitations. J. Biomed. Opt. 2021, 26, 100901. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 179. [Google Scholar] [CrossRef]
- Aldhuhaibat, M.J.R.; Amana, M.S.; Jubier, N.J.; Salim, A.A. Improved gamma radiation shielding traits of epoxy composites: Evaluation of mass attenuation coefficient, effective atomic and electron number. Radiat. Phys. Chem. 2021, 179, 109183. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, Y.; Zhou, W.; Xu, W.; Pang, L.; Fan, X.; Jiang, S. Electrospun fibrous materials and their applications for electromagnetic interference shielding: A review. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106309. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.-U.-Z.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Hou, J.; Park, C.; Jang, W.; Byun, H. Facile fabrication and characterization of aliphatic polyketone (PK) micro/nano fiber membranes via electrospinning and a post treatment process. RSC Adv. 2021, 11, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Lamichhane, S.; Lee, S. Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin. Pharmaceutics 2021, 13, 565. [Google Scholar] [CrossRef]
- Liang, X.; Gao, G.; Wu, G. Statistical analysis on hollow and core-shell structured vanadium oxide microspheres as cathode materials for Lithium ion batteries. Data Brief. 2018, 18, 719–722. [Google Scholar] [CrossRef]
- Kim, S.C. Construction of a medical radiation-shielding environment by analyzing the weaving characteristics and shielding performance of shielding fibers using X-ray-impermeable materials. Appl. Sci. 2021, 11, 1705. [Google Scholar] [CrossRef]
- Boice, J., Jr.; Dauer, L.T.; Kase, K.R.; Mettler, F.A., Jr.; Vetter, R.J. Evolution of radiation protection for medical workers. Brit. J. Radiol. 2020, 93, 20200282. [Google Scholar] [CrossRef]
- AbuAlRoos, N.J.; Baharul Amin, N.A.; Zainon, R. Conventional and New Lead-Free Radiation Shielding Materials for Radiation Protection in Nuclear Medicine: A Review. Radiat. Phys. Chem. 2019, 165, 108439. [Google Scholar] [CrossRef]
- Klaassen, N.J.M.; Arntz, M.J.; Arranja, G.A.; Roosen, J.; Nijsen, J.F.W. The various therapeutic applications of the medical isotope holmium-166: A narrative review. EJNMMI Radiopharm. Chem. 2019, 4, 19. [Google Scholar] [CrossRef]
- Kumar, A.; Gaikwad, D.K.; Obaid, S.S.; Tekin, H.O.; Agar, O.; Sayyed, M.I. Experimental studies and Monte Carlo simulations on gamma ray shielding competence of (30 + x)PbO10WO3 10Na2O − 10MgO − (40 − x)B2O3 glasses. Prog. Nucl. Energy 2020, 119, 103047. [Google Scholar] [CrossRef]
- Kharrati, H.; Agrebi, A.; Karaoui, M.-K. Monte Carlo Simulation of X-ray Buildup Factors of Lead and Its Applications in Shielding of Diagnostic X-ray Facilities. Med. Phys. 2007, 34, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- AbuAlRoos, N.A.; Azman, M.N.; Amin, N.A.B.; Zainon, R. Tungsten-based material as promising new lead-free gamma radiation shielding material in nuclear medicine. Phys. Medica 2020, 78, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Mousavi, S.H.; Mohsenizadeh, S.A.; Khoshfetrat, M.; Arefizadeh, R. Investigation of the Efficiency and Quality of Lightweight Gowns with Multi-Layered Nanoparticles Compositions of Bismuth, Tungsten, Barium, and Copper. J. Biomed. Phys. Eng. 2023, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C. Performance Evaluation According to Polymer Encapsulation Characteristics of Eco-Friendly Plastic Gamma-Ray Shield. Coatings 2022, 12, 1621. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.; Xie, J.; Ji, C.; Gu, Z. Eco-friendly and Scalable Synthesis of Fullerenols with High Free Radical Scavenging Ability for Skin Radioprotection. Small 2021, 17, 202102035. [Google Scholar] [CrossRef]
- Laurenzi, S.; de Zanet, G.; Santonicola, M.G. Numerical investigation of radiation shielding properties of polyethylene-based nanocomposite materials in different space environments. Acta Astronaut. 2020, 170, 530–538. [Google Scholar] [CrossRef]
- Parvaresh, R.; Jalili, M.; Haghparast, A.; Khoshgard, K.; Eivazi, M.T.; Ghorbani, M. Evaluations for Determination of Optimum Shields in Nuclear Medicine. J. Biomed. Phys. Eng. 2020, 10, 651–658. [Google Scholar] [CrossRef]

| Humidity (%) | Temperature (°C) | Voltage (kV) | TCD (cm) | Rate (mL/h) | Duration (h) | Needle Size (Gauge) |
|---|---|---|---|---|---|---|
| 25~30 | 22~25 | 10 | 13~15 | 1.0 | 10 | 23 |
| Distinction | Radionuclide (Unit: keV) | |||
|---|---|---|---|---|
| 99mTc | 131I | 18F | ||
| Half-life | 6.01 h | 8.04 day | 109.9 min | |
| Emitting energy | emission | 140.5 (0.89) | 364 (0.81) | 511.0 (1.94) |
| 18.4 (0.04) | ||||
| 18.3 (0.02) | ||||
| β max emission | - | 191 (0.89) | 633.5 (0.97) | |
| Weight (kg/m2) | Tungsten Weight (kg/m2) | Thickness (mm) | Density (g/cm3) | Tensile Strength (MPa) |
|---|---|---|---|---|
| 0.781 ± 0.020 | 0.452 ± 0.012 | 0.100 ± 0.015 | 2.415 ± 0.000 | 15.1 |
| Thickness (mm) | Dose Rate (mR/h) | Shielding Rate (%) | ||||
|---|---|---|---|---|---|---|
| 0.03 m | 0.05 m | 0.10 m | 0.03 m | 0.05 m | 0.10 m | |
| 0 | 15.247 | 6.211 | 1.290 | 0 | 0 | 0 |
| 0.1 | 13.869 | 5.558 | 1.120 | 9.04 | 10.51 | 13.11 |
| 0.25 mmPb | 1.522 | 5.720 | 0.530 | 90.02 | 79.07 | 58.95 |
| 0.3 | 10.299 | 4.444 | 1.014 | 32.45 | 28.45 | 21.34 |
| 0.5 | 6.352 | 2.948 | 0.651 | 58.34 | 52.54 | 49.47 |
| 0.50 mmPb | 0.796 | 1.327 | 0.397 | 94.78 | 78.63 | 69.24 |
| 1.0 | 3.748 | 1.848 | 0.418 | 75.42 | 70.24 | 67.54 |
| Thickness (mm) | Dose Rate (mR/h) | Shielding Rate (%) | ||||
|---|---|---|---|---|---|---|
| 0.03 m | 0.05 m | 0.10 m | 0.03 m | 0.05 m | 0.10 m | |
| 0 | 55.214 | 20.741 | 4.956 | 0 | 0 | 0 |
| 0.1 | 51.217 | 19.492 | 4.709 | 7.24 | 6.02 | 4.98 |
| 0.25 mmPb | 21.787 | 9.983 | 3.191 | 60.54 | 51.87 | 35.62 |
| 0.3 | 40.903 | 16.296 | 4.108 | 25.92 | 21.43 | 17.12 |
| 0.5 | 38.324 | 15.512 | 3.879 | 30.59 | 25.21 | 21.74 |
| 0.50 mmPb | 15.327 | 6.629 | 1.970 | 72.24 | 68.04 | 60.25 |
| 1.0 | 35.558 | 13.847 | 3.512 | 35.60 | 33.24 | 29.12 |
| Thickness (mm) | Dose Rate (mR/h) | Shielding Rate (%) | ||||
|---|---|---|---|---|---|---|
| 0.03 m | 0.05 m | 0.10 m | 0.03 m | 0.05 m | 0.10 m | |
| 0 | 10.421 | 4.987 | 1.248 | 0 | 0 | 0 |
| 0.1 | 10.322 | 4.926 | 1.209 | 0.95 | 1.22 | 3.12 |
| 0.25 mmPb | 9.354 | 4.589 | 1.206 | 10.24 | 7.98 | 3.36 |
| 0.3 | 9.902 | 4.762 | 1.196 | 4.98 | 4.52 | 4.14 |
| 0.5 | 9.887 | 4.693 | 1.158 | 5.12 | 5.89 | 7.24 |
| 0.50 mmPb | 6.231 | 3.231 | 0.953 | 40.21 | 35.21 | 23.62 |
| 1.0 | 9.575 | 4.565 | 1.133 | 8.12 | 8.47 | 9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-C. Evaluation of Shielding Performance of Gamma Ray Shielding Tungsten Polymer Composite with LBL-Type Layered Structure. Coatings 2024, 14, 36. https://doi.org/10.3390/coatings14010036
Kim S-C. Evaluation of Shielding Performance of Gamma Ray Shielding Tungsten Polymer Composite with LBL-Type Layered Structure. Coatings. 2024; 14(1):36. https://doi.org/10.3390/coatings14010036
Chicago/Turabian StyleKim, Seon-Chil. 2024. "Evaluation of Shielding Performance of Gamma Ray Shielding Tungsten Polymer Composite with LBL-Type Layered Structure" Coatings 14, no. 1: 36. https://doi.org/10.3390/coatings14010036
APA StyleKim, S.-C. (2024). Evaluation of Shielding Performance of Gamma Ray Shielding Tungsten Polymer Composite with LBL-Type Layered Structure. Coatings, 14(1), 36. https://doi.org/10.3390/coatings14010036






