Influence of Tooling on the Properties of the Surface Layer in HEA Alloy Sinters Produced by the SPS Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Hardness
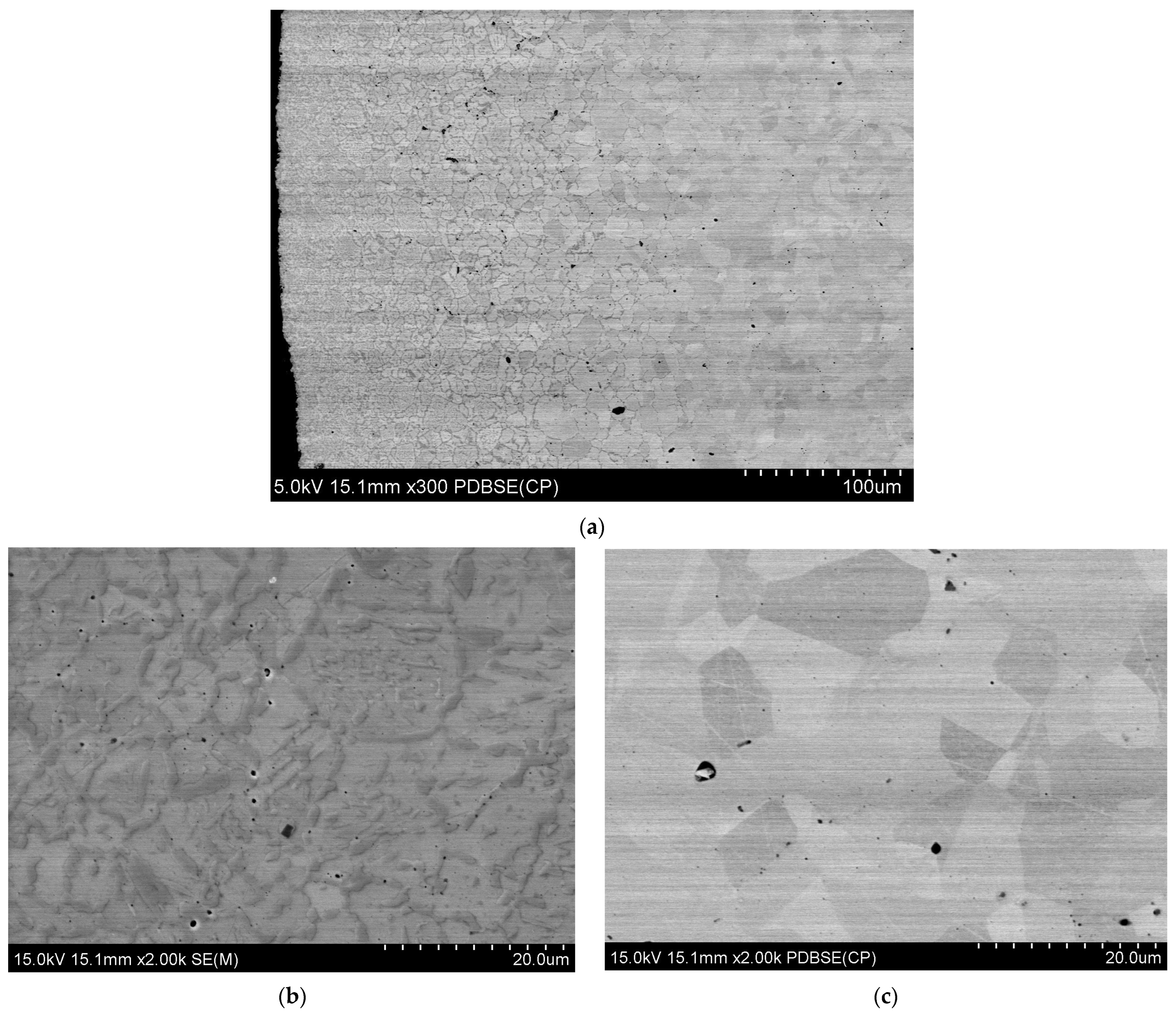
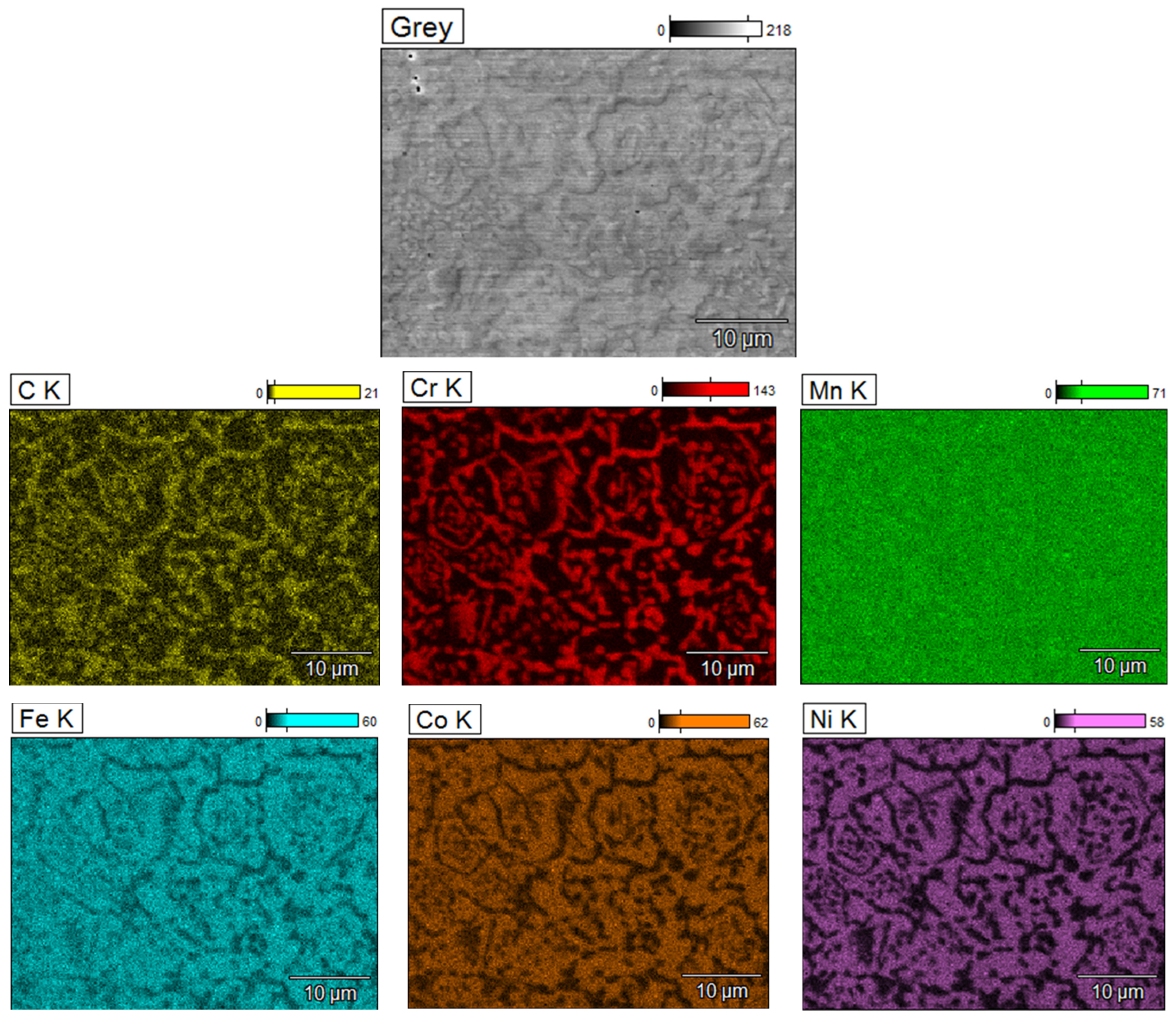
3.2. Microstructure of HEA Materials
4. Conclusions
- The sintering of the HEA powder at a temperature of 1050 °C and a compaction pressure of 50 MPa using the SPS method makes it possible to obtain specimens with a density similar to that of solid material, with a few fine pores uniformly distributed in the microstructure.
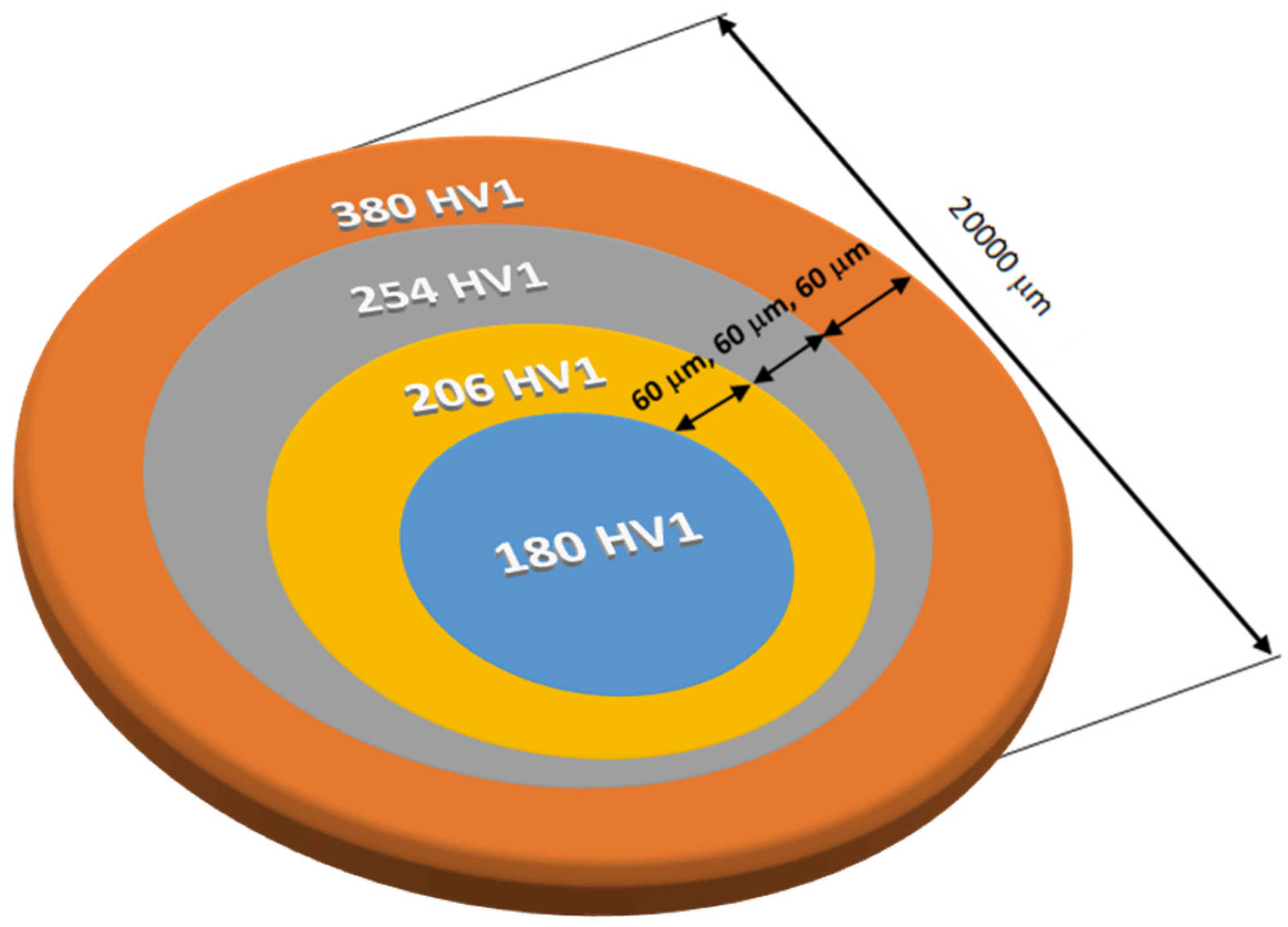
- The hardness test on the surface and on the cross-section revealed significant differences depending on the location of the test. The hardness on the surface was, respectively, 402 HV1, while at a distance of over 180 μm, it decreased to 180 HV1.
- The observation of the microstructure showed changes in the top layer in relation to the core of the sintered material. The precipitate network was observed in the FCC phase boundaries.
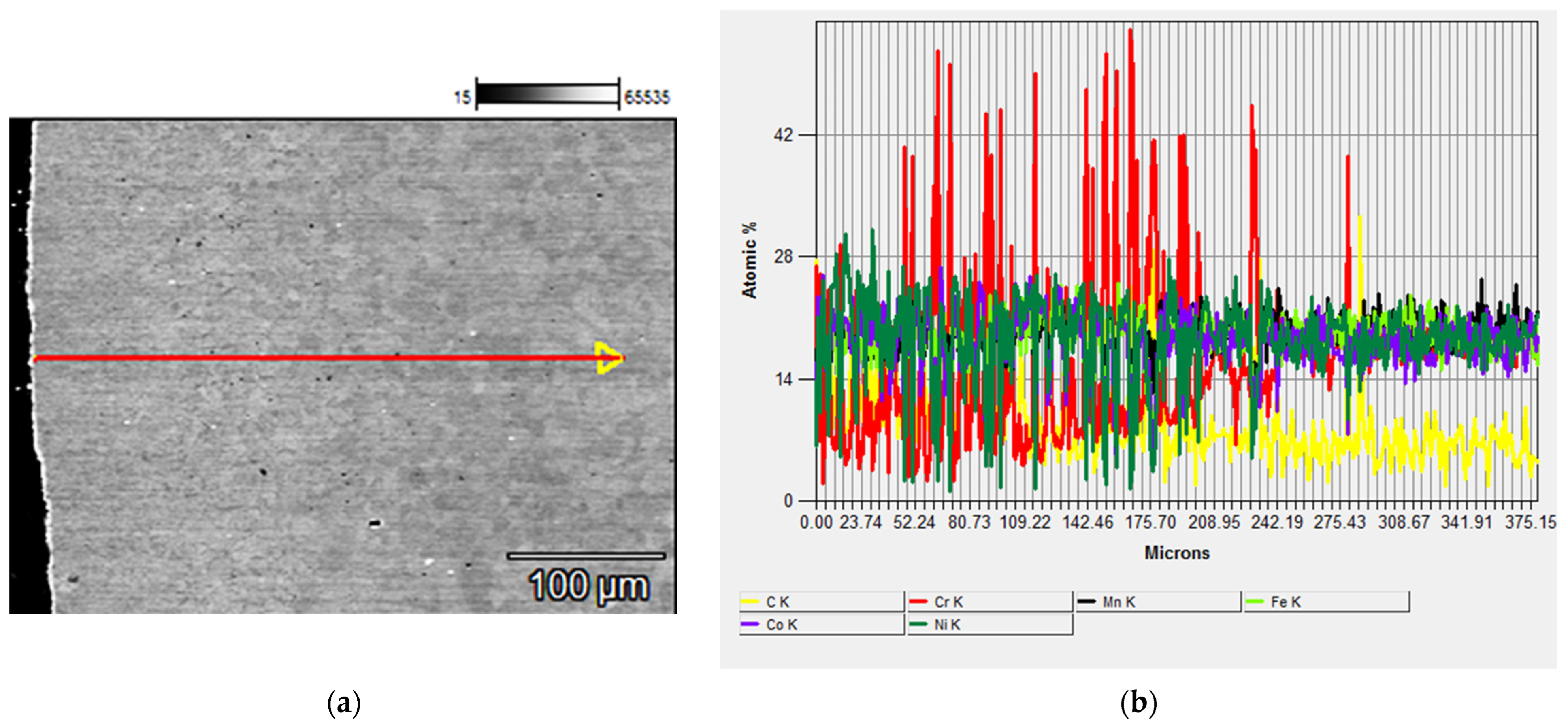
- The chemical composition analysis of the precipitates and X-ray diffraction confirmed that due to the carbon diffusion from the tool and chromium depletion of matrix grains, new Cr7C3- and Cr23C6-type chromium carbides were formed.
- The quantity and size of carbides decreased with an increasing distance from the surface that was in contact with the graphite during the sintering process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish Diffusion in Co–Cr–Fe–Mn–Ni High-Entropy Alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Yeh, J.W. Physical Metallurgy of High-Entropy Alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Cao, B.X.; Wang, C.; Yang, T.; Liu, C.T. Cocktail Effects in Understanding the Stability and Properties of Face-Centered-Cubic High-Entropy Alloys at Ambient and Cryogenic Temperatures. Scr. Mater. 2020, 187, 250–255. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and Corrosion Properties of CrMnFeCoNi High Entropy Alloy Coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Han, Z.; Ren, W.; Yang, J.; Tian, A.; Du, Y.; Liu, G.; Wei, R.; Zhang, G.; Chen, Y. The Corrosion Behavior of Ultra-Fine Grained CoNiFeCrMn High-Entropy Alloys. J. Alloys Compd. 2020, 816, 152583. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Pradeep, K.G.; Kubenova, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the Single-Phase High-Entropy Alloy CrMnFeCoNi after Prolonged Anneals at Intermediate Temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef]
- Li, L.; Li, Z. Aging Induced Segregation and Nanoprecipitation in a Severely Deformed Equiatomic High-Entropy Alloy. Mater. Charact. 2020, 165, 110369. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Ozerov, M.S.; Zherebtsov, S.V.; Salishchev, G.A. Second Phase Formation in the CoCrFeNiMn High Entropy Alloy after Recrystallization Annealing. Mater. Lett. 2016, 185, 1–4. [Google Scholar] [CrossRef]
- Hadraba, H.; Chlup, Z.; Dlouhy, A.; Dobes, F.; Roupcova, P.; Vilemova, M.; Matejicek, J. Oxide Dispersion Strengthened CoCrFeNiMn High-Entropy Alloy. Mater. Sci. Eng. A 2017, 689, 252–256. [Google Scholar] [CrossRef]
- Yang, T.; Cai, B.; Shi, Y.; Wang, M.; Zhang, G. Preparation of Nanostructured CoCrFeMnNi High Entropy Alloy by Hot Pressing Sintering Gas Atomized Powders. Micron 2021, 147, 103082. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, S.; Shang, C.; Huang, S.; Wang, Y. Microstructure and Properties of CoCrFeNi(WC) High-Entropy Alloy Coatings Prepared Using Mechanical Alloying and Hot Pressing Sintering. Coatings 2019, 9, 16. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Liang, J.; Hu, M.; Yang, Y. Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process. Materials 2023, 16, 1547. [Google Scholar] [CrossRef] [PubMed]
- Nagarjuna, C.; Sharma, A.; Lee, K.; Hong, S.-J.; Ahn, B. Microstructure, Mechanical and Tribological Properties of Oxide Dispersion Strengthened CoCrFeMnNi High-Entropy Alloys Fabricated by Powder Metallurgy. J. Mater. Res. Technol. 2023, 22, 1708–1722. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, A.; Han, J.; Meng, J. Microstructure, Mechanical and Tribological Properties of CoCrFeNiMn High Entropy Alloy Matrix Composites with Addition of Cr3C2. Tribol. Int. 2020, 151, 106436. [Google Scholar] [CrossRef]
- Gwalani, B.; Pohan, R.M.; Waseem, O.A.; Alam, T.; Hong, S.H.; Ryu, H.J.; Banerjee, R. Strengthening of Al0.3CoCrFeMnNi-Based ODS High Entropy Alloys with Incremental Changes in the Concentration of Y2O3. Scr. Mater. 2019, 162, 477–481. [Google Scholar] [CrossRef]
- Yim, D.; Sathiyamoorthi, P.; Hong, S.J.; Kim, H.S. Fabrication and Mechanical Properties of TiC Reinforced CoCrFeMnNi High-Entropy Alloy Composite by Water Atomization and Spark Plasma Sintering. J. Alloys Compd. 2019, 781, 389–396. [Google Scholar] [CrossRef]
- Garbiec, D.; Rybak, T.; Heyduk, F.; Janczak, M. Zastosowanie urządzenia do iskrowego spiekania plazmowego SPS HP D 25. Modern Spark Plasma Sintering Device in Metal Forming Institute. Obróbka Plast. Met. 2011, XXII, 221–225. [Google Scholar]
- Ujah, C.O.; Von Kallon, D.V.; Aigbodion, V.S. High Entropy Alloys Prepared by Spark Plasma Sintering: Mechanical and Thermal Properties. Mater. Today Sustain. 2024, 25, 100639. [Google Scholar] [CrossRef]
- Moazzen, P.; Toroghinejad, M.R.; Cavaliere, P. Effect of Iron Content on the Microstructure Evolution, Mechanical Properties and Wear Resistance of FeXCoCrNi High-Entropy Alloy system Produced via MA-SPS. J. Alloys Compd. 2021, 870, 159410. [Google Scholar] [CrossRef]
- Colombini, E.; Rosa, R.; Trombi, L.; Zadra, M.; Casagrande, A.; Veronesi, P. High Entropy Alloys Obtained by Field Assisted Powder Metallurgy Route: SPS and Microwave Heating. Mater. Chem. Phys. 2018, 210, 78–86. [Google Scholar] [CrossRef]
- Laurent-Brocq, M.; Goujon, P.-A.; Monnier, J.; Villeroy, B.; Perrière, L.; Pirès, R.; Garcin, G. Microstructure and Mechanical Properties of a CoCrFeMnNi High Entropy Alloy Processed by Milling and Spark Plasma Sintering. J. Alloys Compd. 2019, 780, 856–865. [Google Scholar] [CrossRef]
- Tirumalasetty, G.K.; Fang, C.M.; Jansen, J.; Yokosawa, T.; Boeije, M.F.J.; Sietsma, J.; Van Huis, M.A.; Zandbergen, H.W. Structural Tale of Two Novel (Cr, Mn)C Carbides in Steel. Acta Mater. 2014, 78, 161–172. [Google Scholar] [CrossRef]
- Ye, W.; Xie, M.; Huang, Z.; Wang, H.; Zhou, Q.; Wang, L.; Chen, B.; Wang, H.; Liu, W. Microstructure and tribological properties of in-situ carbide/CoCrFeNiMn high entropy alloy composites synthesized by flake powder metallurgy. Tribol. Int. 2023, 181, 108295. [Google Scholar] [CrossRef]
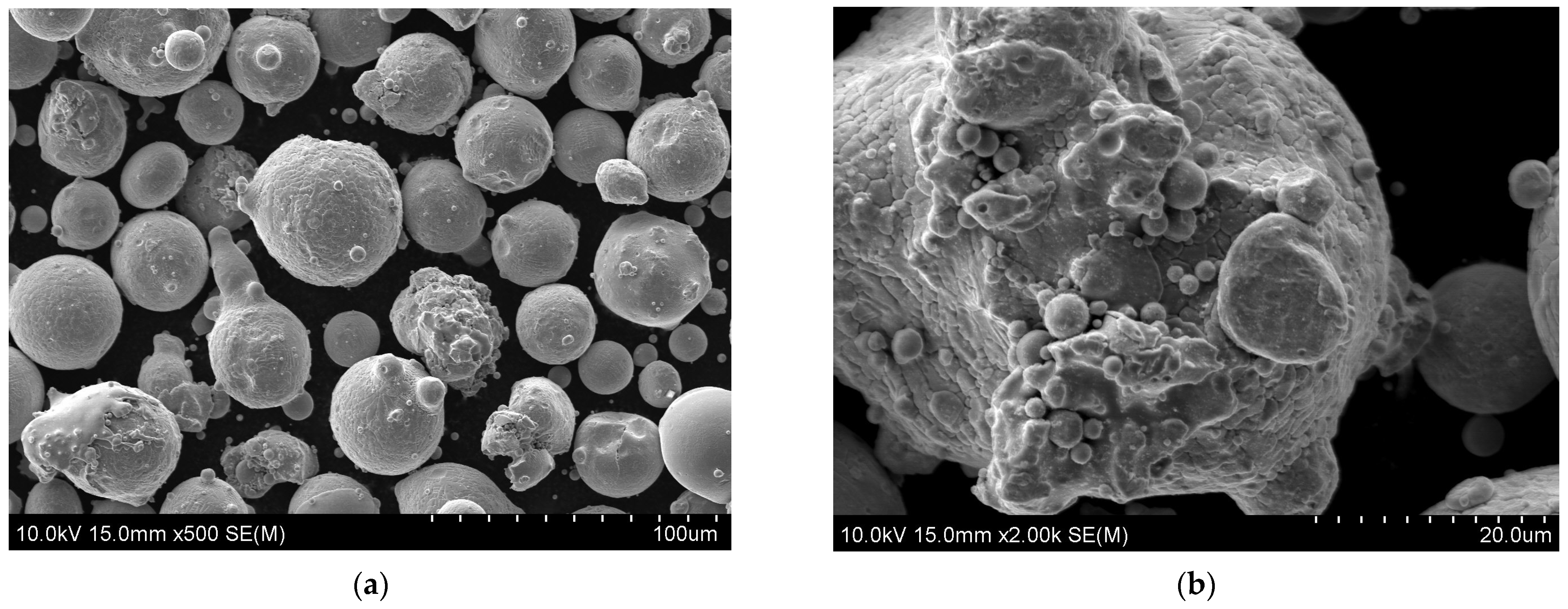



| Chemical Composition (wt. %) | ||||
| Fe | Ni | Cr | Mn | Co |
| 19.65 | 20.67 | 17.88 | 19.18 | Bal. |
| Oxygen content (ppm) | 438 | |||
| Nitrogen content (ppm) | 139 | |||
| Particle size distribution | ||||
| D10/μm | D50/μm | D90/μm | ||
| 21.6 | 34.4 | 53.7 | ||
| Flowability (s) | 16.2 | |||
| Bulk density (g/cm3) | 3.8 | |||
| Microhardness (HV) | 155.93 ± 23 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopeć-Surzyn, A.; Madej, M. Influence of Tooling on the Properties of the Surface Layer in HEA Alloy Sinters Produced by the SPS Method. Coatings 2024, 14, 186. https://doi.org/10.3390/coatings14020186
Kopeć-Surzyn A, Madej M. Influence of Tooling on the Properties of the Surface Layer in HEA Alloy Sinters Produced by the SPS Method. Coatings. 2024; 14(2):186. https://doi.org/10.3390/coatings14020186
Chicago/Turabian StyleKopeć-Surzyn, Anna, and Marcin Madej. 2024. "Influence of Tooling on the Properties of the Surface Layer in HEA Alloy Sinters Produced by the SPS Method" Coatings 14, no. 2: 186. https://doi.org/10.3390/coatings14020186
APA StyleKopeć-Surzyn, A., & Madej, M. (2024). Influence of Tooling on the Properties of the Surface Layer in HEA Alloy Sinters Produced by the SPS Method. Coatings, 14(2), 186. https://doi.org/10.3390/coatings14020186





