Environmentally Benign Grape Seed Oil for Corrosion Inhibition: Cutting-Edge Computational Modeling Techniques Revealing the Intermolecular and Intramolecular Synergistic Inhibition Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Computational Details
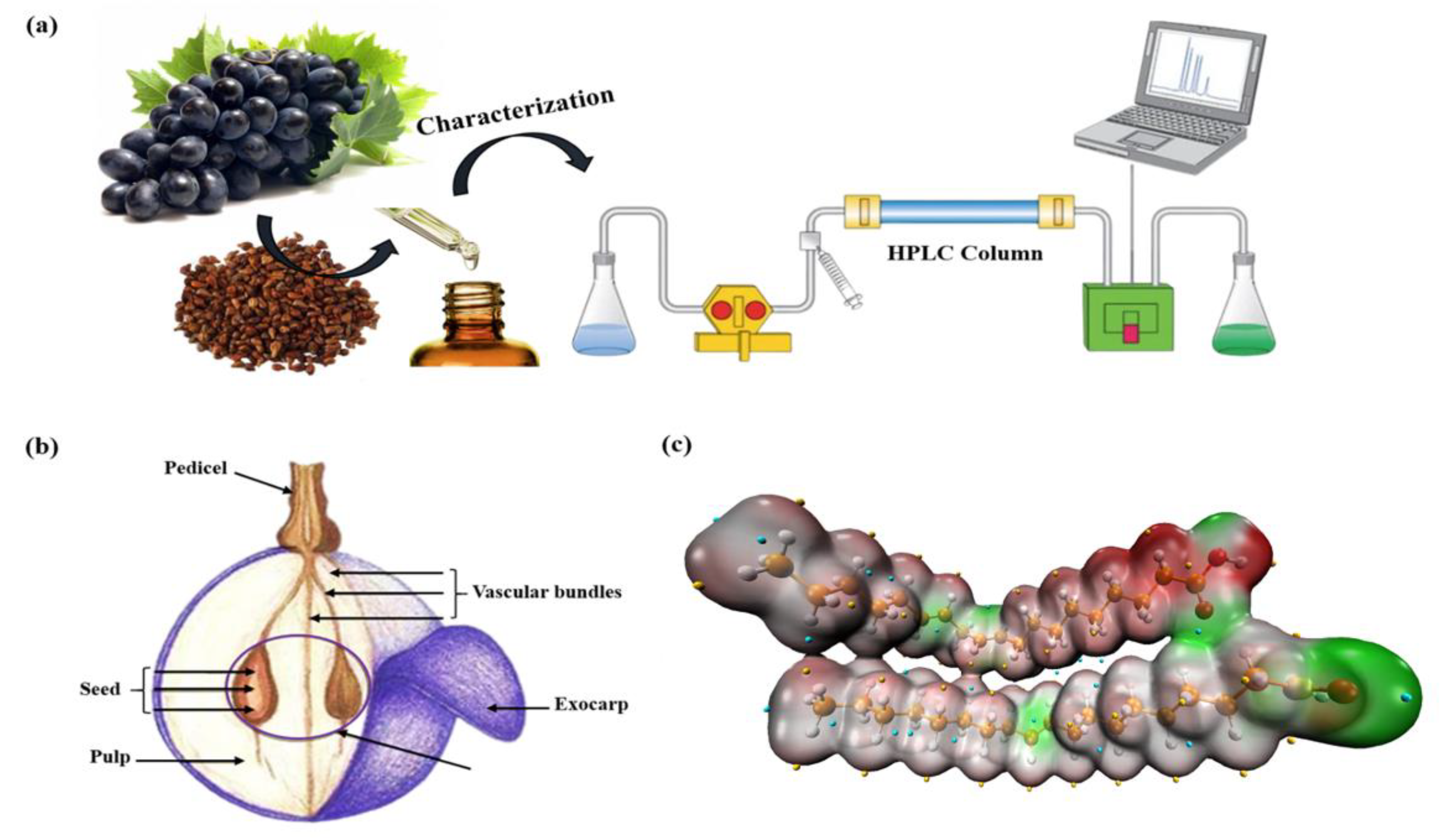
2.2. Plant Material and GSO Extraction
2.3. Weight Loss and Electrochemical Techniques
3. Results and Discussion
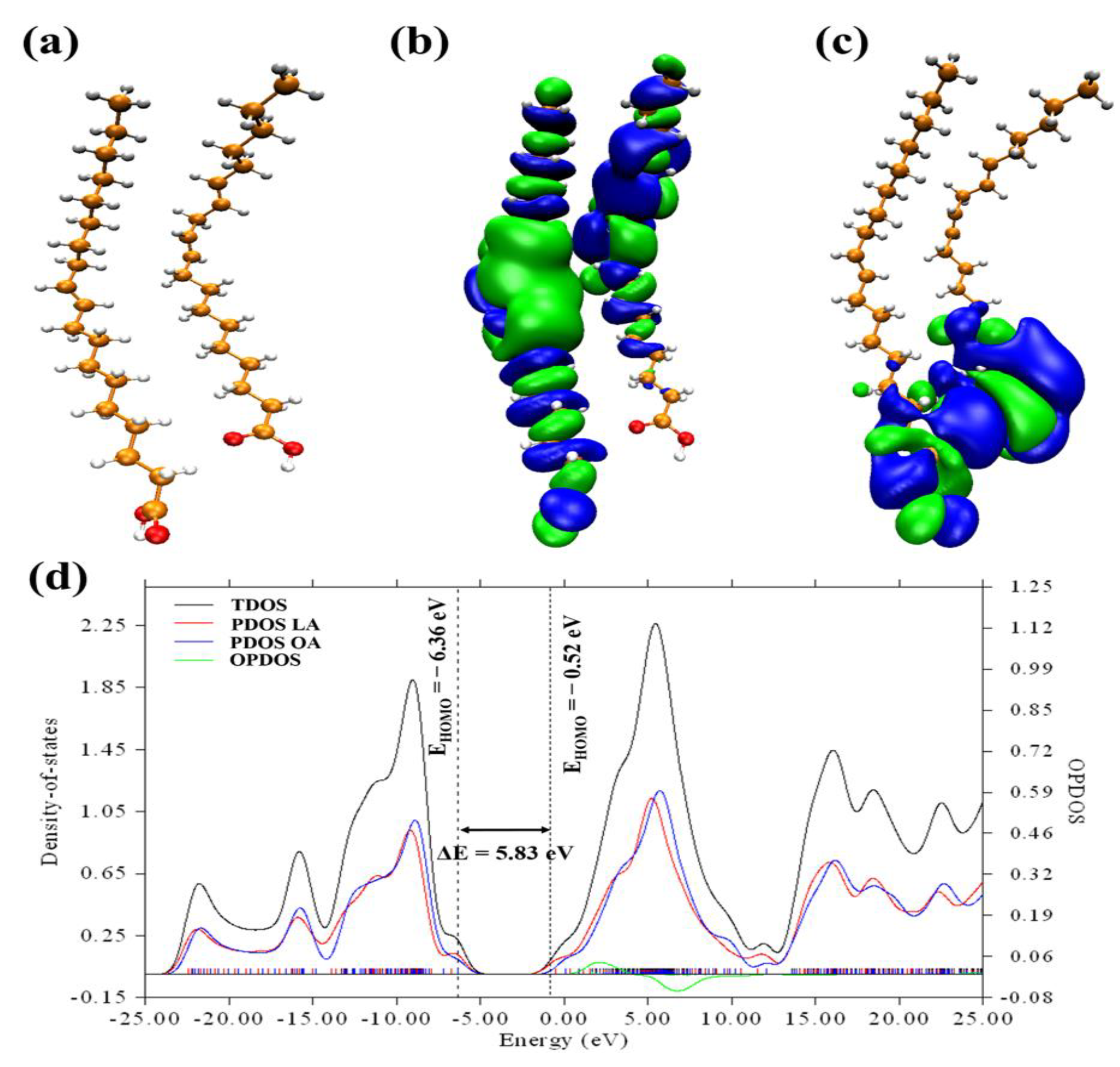
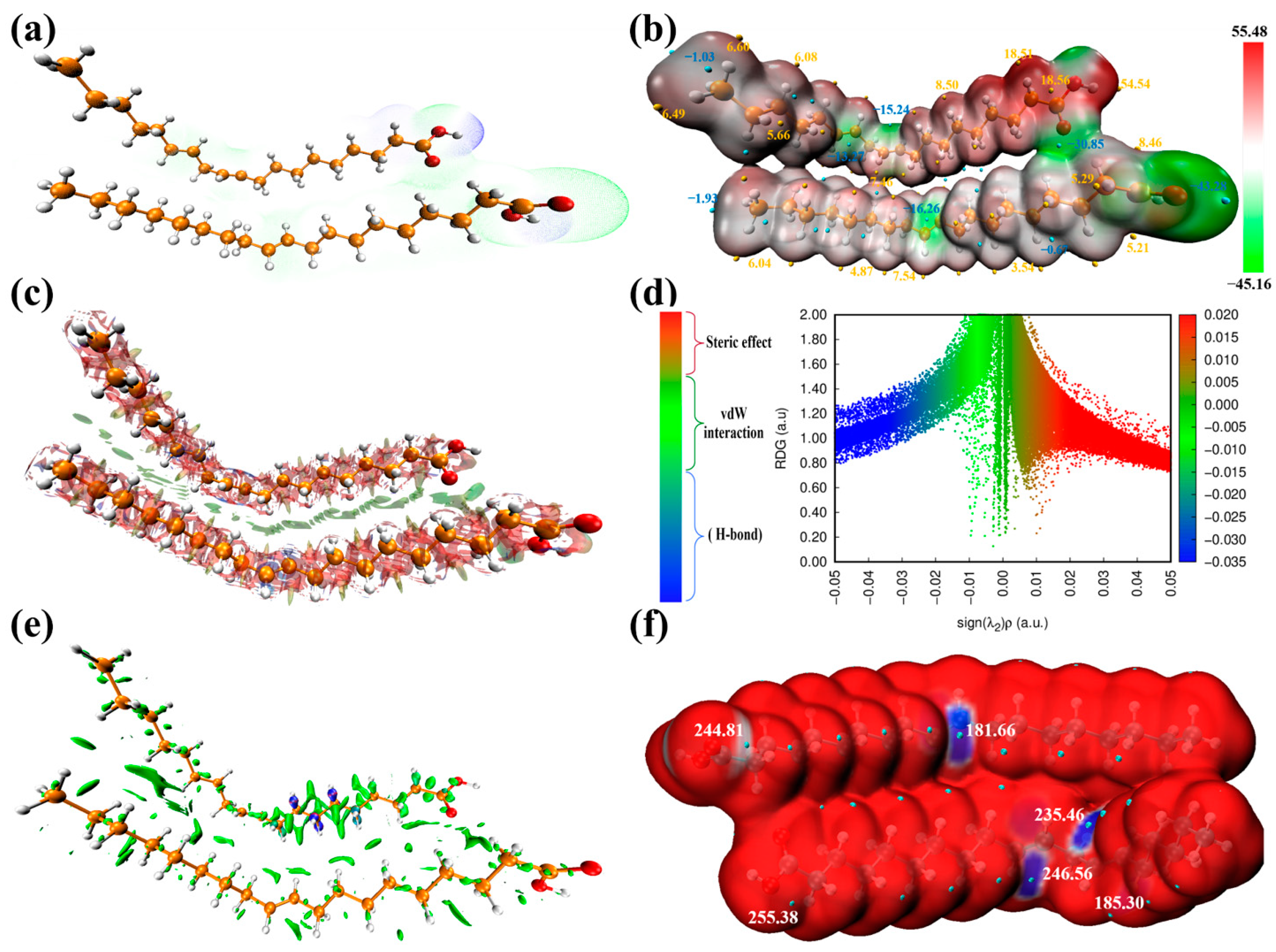
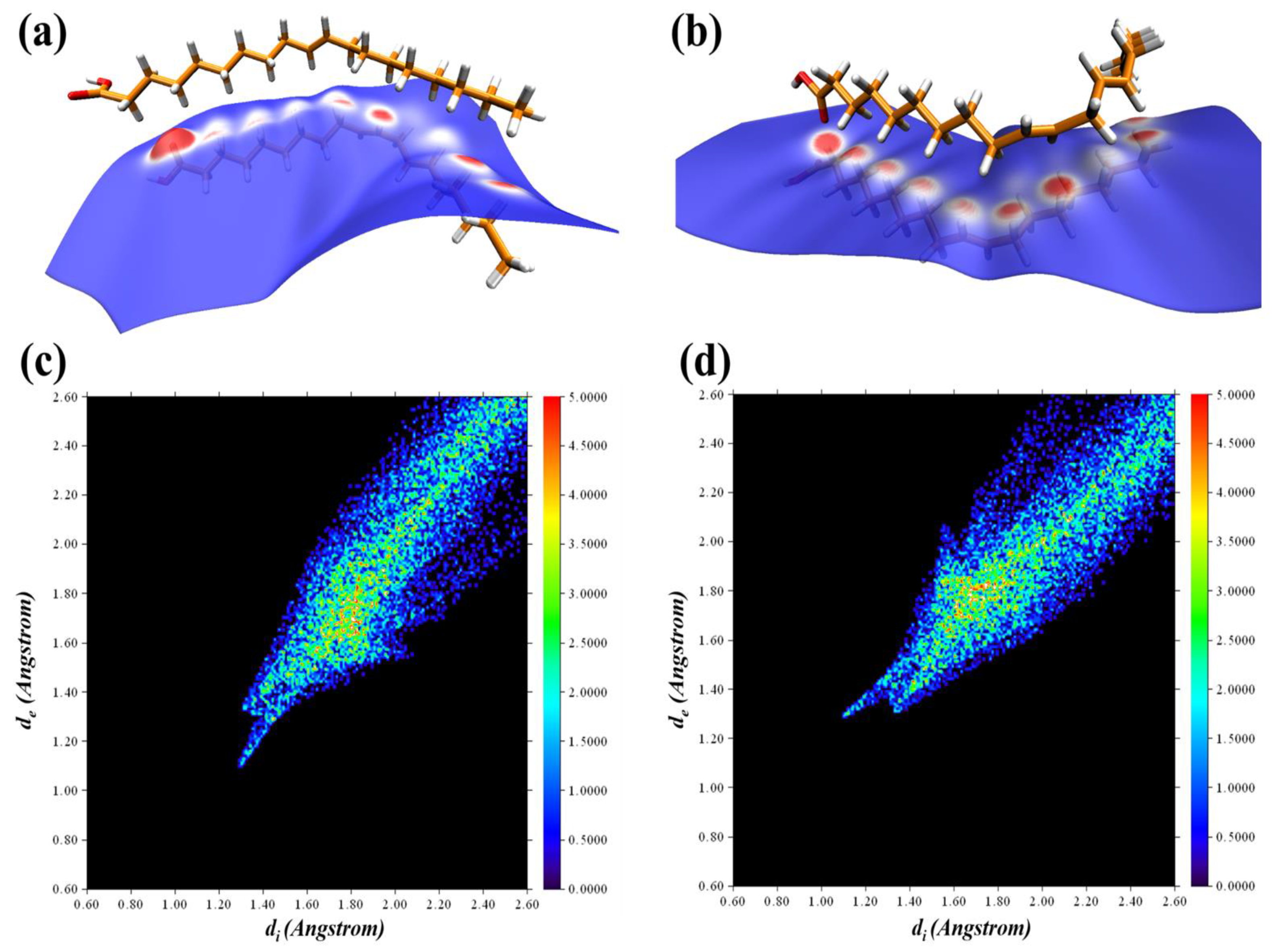
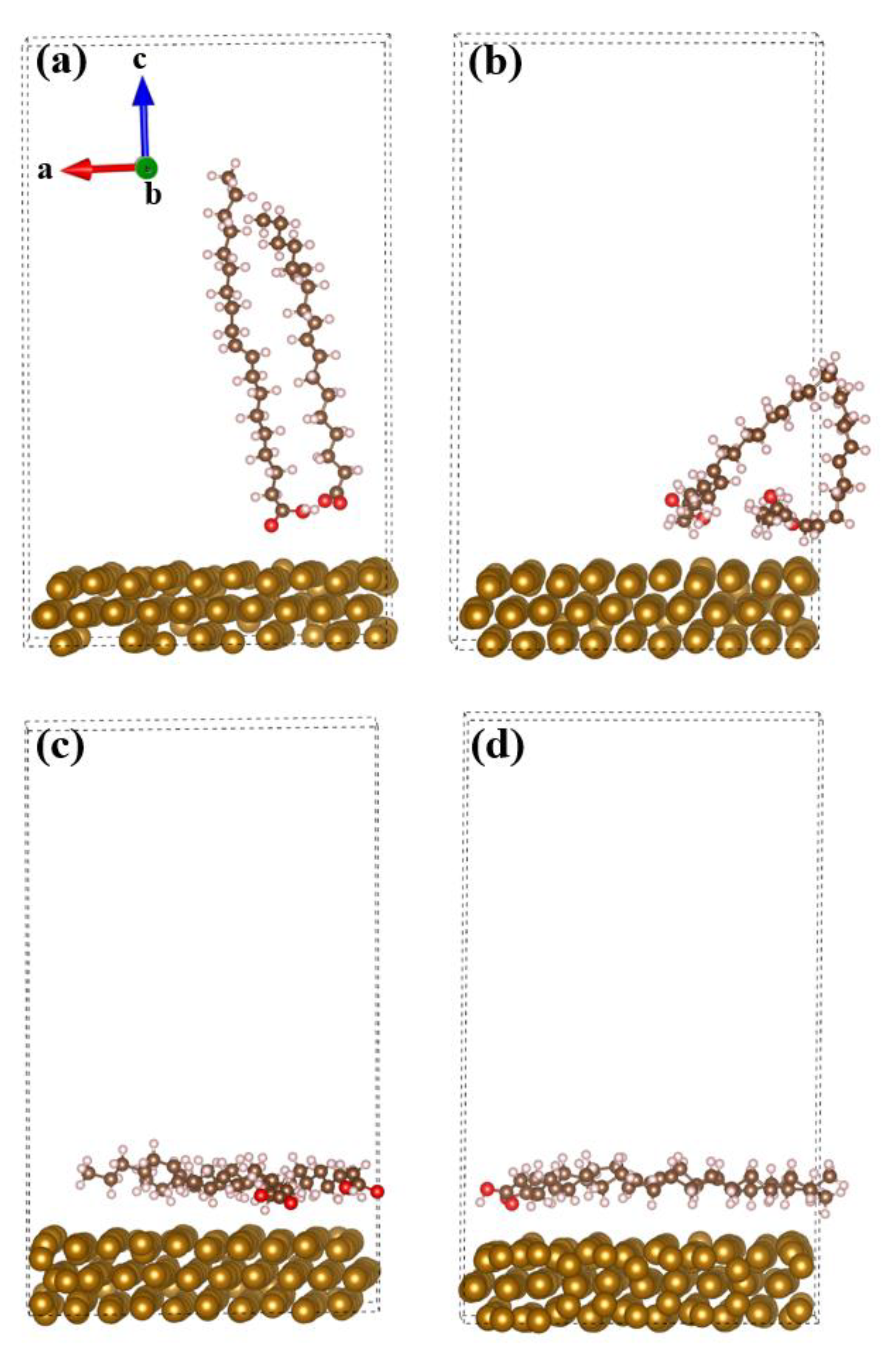
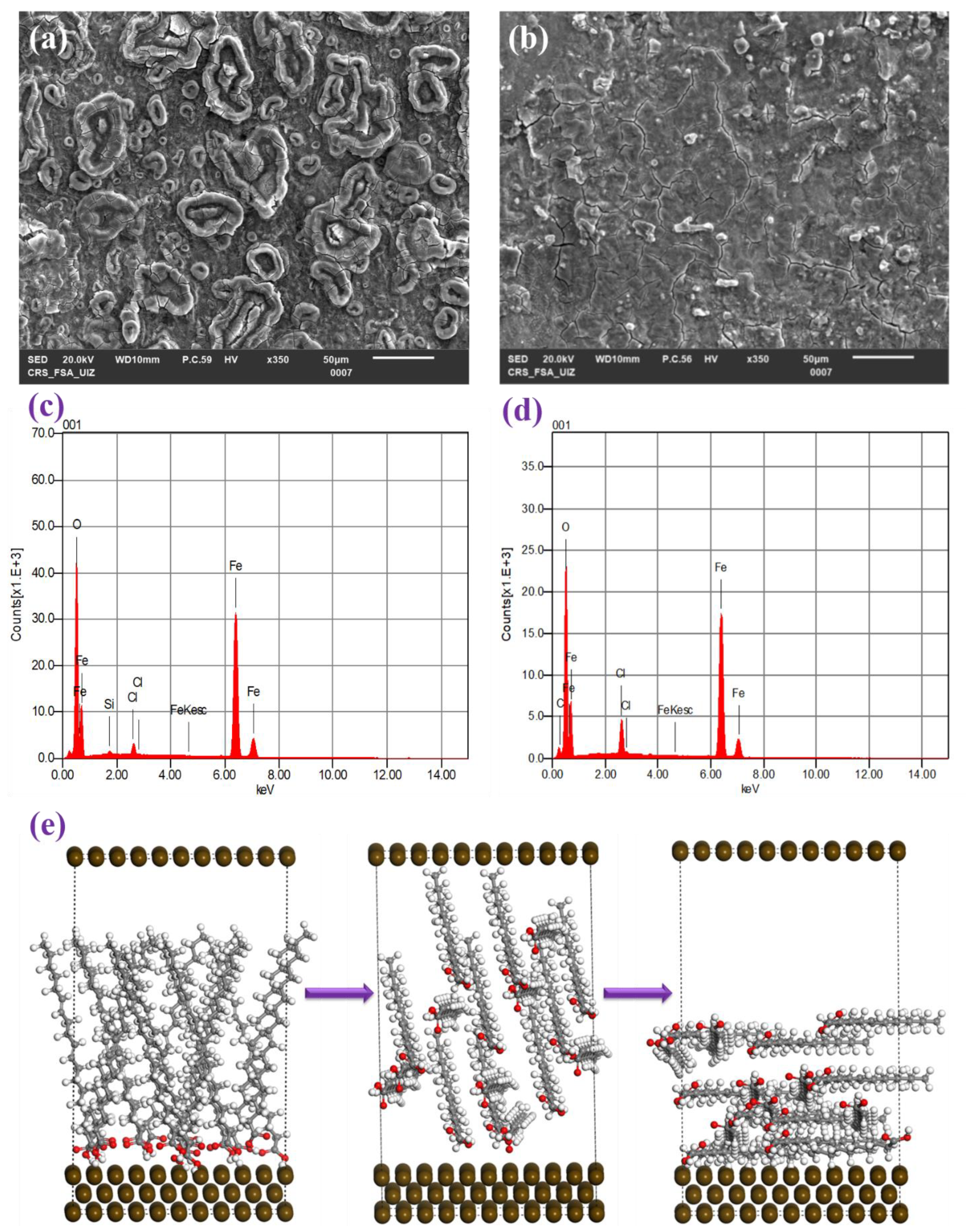
3.1. Revealing Nature’s Strength: Understanding the Corrosion Inhibition Mechanisms of GSO through Computational Perspectives
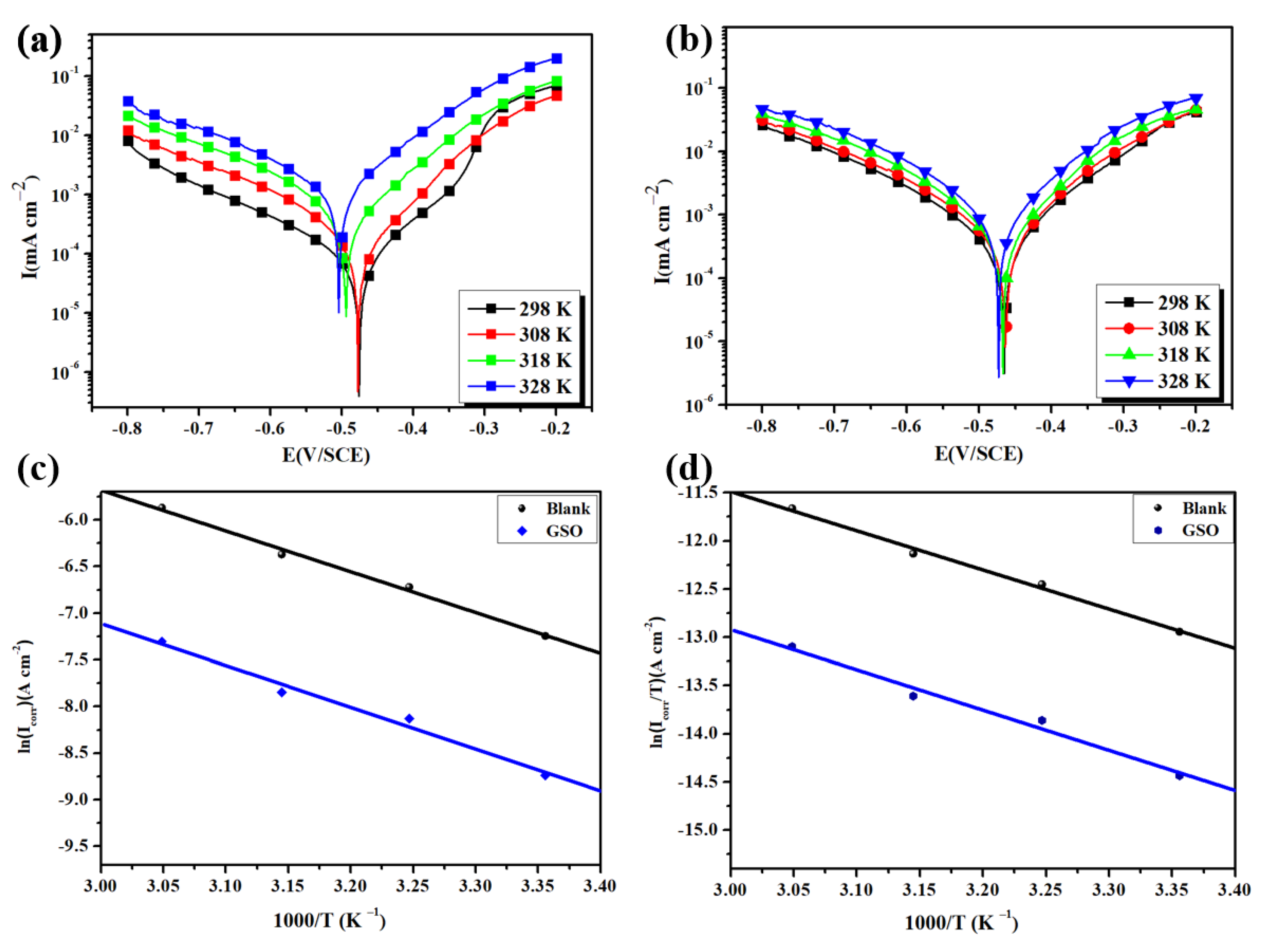
3.2. Anticorrosion Performance
3.3. Microstructural Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Wang, X.-X.; Jin, M.L.; He, P.; Zhang, S. Molecular Level Manipulation of Charge Density for Solid-Liquid TENG System by Proton Irradiation. Nano Energy 2022, 103, 107819. [Google Scholar] [CrossRef]
- Chen, X.-F. Periodic Density Functional Theory (PDFT) Simulating Crystal Structures with Microporous CHA Framework: An Accuracy and Efficiency Study. Inorganics 2023, 11, 215. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, H.; Li, H.; Zhu, L.; Tao, R.; Zhu, W.; Li, H.; Dai, S. Boric Acid-Based Ternary Deep Eutectic Solvent for Extraction and Oxidative Desulfurization of Diesel Fuel. Green Chem. 2019, 21, 3074–3080. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Yang, J.; Tsuru, T. Progress in Pervaporation Membranes for Dehydration of Acetic Acid. Sep. Purif. Technol. 2021, 262, 118338. [Google Scholar] [CrossRef]
- Olimov, B.; Gafurova, G.; Qudratov, O. Production and Properties of Corrosion Inhibitors in the Oil and Gas Industry. Universum 2022, 47–51. [Google Scholar] [CrossRef]
- Shetty, P. Schiff Bases: An Overview of Their Corrosion Inhibition Activity in Acid Media against Mild Steel. Chem. Eng. Commun. 2020, 207, 985–1029. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, C.; Qu, G.; Liu, S.; Ren, Y.; Chen, B.; Li, J.; Liu, L. A Critical Review of the Typical By-Product Clean Ecology Links in the Chinese Phosphorus Chemical Industry in China: Production Technologies, Fates and Future Directions. J. Environ. Chem. Eng. 2022, 10, 106685. [Google Scholar] [CrossRef]
- Haudi, H.; Wijoyo, H.; Cahyono, Y. Effect of Product Innovation and Marketing Strategy on Consumer Purchase Decisions in Indonesia’s Lightweight Roof Steel Industry. J. Crit. Rev. 2020, 7, 4147–4155. [Google Scholar]
- Saraswat, V.; Yadav, M.; Obot, I.B. Investigations on Eco-Friendly Corrosion Inhibitors for Mild Steel in Acid Environment: Electrochemical, DFT and Monte Carlo Simulation Approach. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124881. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, J.W. Experimental Evaluation of Vibration Response of External Post-Tensioned Tendons with Corrosion. KSCE J. Civ. Eng. 2019, 23, 2561–2572. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W.G. A Review on Corrosion Detection and Protection of Existing Reinforced Concrete (RC) Structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, W.G. Bayesian Network Model for Buried Gas Pipeline Failure Analysis Caused by Corrosion and External Interference. Reliab. Eng. Syst. Saf. 2020, 203, 107089. [Google Scholar] [CrossRef]
- Garavaglia, E.; Tedeschi, C. Analysis of the Mass and Deformation Variation Rates over Time and Their Influence on Long-Term Durability for Specimens of Porous Material. Sustain. Struct. 2022, 2, 000014. [Google Scholar] [CrossRef]
- Dauletbek, A.; Li, H.; Xiong, Z.; Lorenzo, R. A Review of Mechanical Behavior of Structural Laminated Bamboo Lumber. Sustain. Struct. 2021, 1, 000004. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Z.; Yang, C.; Dong, F.; Ren, Z.; Zhang, K. Spatial Distribution of Corrosion Products Influenced by the Initial Defects and Corrosion-Induced Cracking of the Concrete. J. Test. Eval. 2023, 51, 2582–2597. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, C.; Yu, Z.; Xiao, J.; Xu, Y. Impact of Defects in Steel-Concrete Interface on the Corrosion-Induced Cracking Propagation of the Reinforced Concrete. KSCE J. Civ. Eng. 2023, 27, 2621–2628. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya Leaves Extract as a Novel Eco-Friendly Corrosion Inhibitor for Cu in H2SO4 Medium. J. Colloid Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Tan, B.; Guo, L.; Li, H. Solvothermal Synthesis of Functionalized Carbon Dots from Amino Acid as an Eco-Friendly Corrosion Inhibitor for Copper in Sulfuric Acid Solution. J. Colloid Interface Sci. 2021, 604, 1–14. [Google Scholar] [CrossRef]
- da Mata, I.R.; Dal Bosco, S.M.; Garavaglia, J. Different Biological Activities (Antimicrobial, Antitumoral, and Antioxidant Activities) of Grape Seed Oil. In Multiple Biological Activities of Unconventional Seed Oils; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–227. [Google Scholar]
- Lazcano-Silveira, R.; Jia, X.; Liu, K.; Liu, H.; Li, X.; Hui, M. Carbon 60 Dissolved in Grapeseed Oil Inhibits Dextran Sodium Sulfate-Induced Experimental Colitis. J. Inflamm. Res. 2022, 15, 4185–4198. [Google Scholar] [CrossRef]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan Film Functionalized with Grape Seed Oil—Preliminary Evaluation of Antimicrobial Activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; D’Amico, F.; Tammaro, L.; Gorrasi, G. Fabrication and Characterization of Bio-Nanocomposites Based on Halloysite-Encapsulating Grapefruit Seed Oil in a Pectin Matrix as a Novel Bio-Coating for Strawberry Protection. Nanomaterials 2022, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Zorlu, K.; Gümüş, E. Effect of Dietary Fish Oil Replacement with Grape Seed Oil on Growth Performance, Feed Utilization and Fatty Acid Profile of Mirror Carp, Cyprinus Carpio, Fingerlings. Aquac. Res. 2022, 53, 1755–1765. [Google Scholar] [CrossRef]
- Gaglieri, C.; Alarcon, R.T.; Magri, R.; North, M.; Bannach, G. Development of Renewable Thermosetting Polymers Based on Grape Seed Oil Derivatives. J. Appl. Polym. Sci. 2022, 139, e52990. [Google Scholar] [CrossRef]
- BaratianGhorghi, Z.; Faezian, A.; Yeganehzad, S.; Hesarinejad, M.A. Changes in Thermal, Textural, Color and Microstructure Properties of Oleogel Made from Beeswax with Grape Seed Oil under the Effect of Cooling Rate and Oleogelator Concentration. Res. Innov. Food Sci. Technol. 2022, 11, 43–54. [Google Scholar]
- Sun, L.; Wang, H.; Wei, J.; Xue, Y.; Lan, S.; Li, X.; Yu, D.; Wang, J. Extracting Oil from Grape Seed Using a Combined Wet Enzymatic Process and Pressing. Innov. Food Sci. Emerg. Technol. 2022, 77, 102941. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 (Rev: C.01); Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Ab Initio Study of Ionic Solutions by a Polarizable Continuum Dielectric Model. Chem. Phys. Lett. 1998, 286, 253–260. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Suhartono, T.; Hazmatulhaq, F.; Ko, Y.G. Interface Engineering of LDH-Based Material as Efficient Anti-Corrosive System via Synergetic Performance of Host, Interlayers, and Morphological Features of Nature-Mimic Architectures. Chem. Eng. J. 2023, 462, 142239. [Google Scholar] [CrossRef]
- Chaouiki, A.; Al Zoubi, W.; Ko, Y.G. Advanced Prediction of Organic–Metal Interactions through DFT Study and Electrochemical Displacement Approach. J. Magnes. Alloys 2023, 11, 301–316. [Google Scholar] [CrossRef]
- Batah, A.; Chaouiki, A.; El Mouden, O.I.; Belkhaouda, M.; Bammou, L.; Salghi, R. Almond Waste Extract as an Efficient Organic Compound for Corrosion Inhibition of Carbon Steel (C38) in HCl Solution. Sustain. Chem. Pharm. 2022, 27, 100677. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Ko, Y.G. Nature-Inspired Architecture Combining Organic–Inorganic Frameworks: Unique Structure and Active Sites toward a Stable Anti-Corrosion Coating. Appl. Mater. Today 2023, 32, 101852. [Google Scholar] [CrossRef]
- Ahchouch, H.; Chaouiki, A.; Talhajt, S.A.; Bammou, L.; Belkhaouda, M.; Salghi, R.; Ko, Y.G. Inter-and Intra-Molecular Synergism in Designing MgO-MCC Composite-Based Coating: An Efficient Inhibitor for Excellent Anticorrosion Performance. Process Saf. Environ. Prot. 2023, 177, 1461–1476. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Al-Moubaraki, A.H.; Bakhouch, M.; El Yazidi, M.; Ko, Y.G. Electrochemical Behavior and Interfacial Bonding Mechanism of New Synthesized Carbocyclic Inhibitor for Exceptional Corrosion Resistance of Steel Alloy: DFTB, MD and Experimental Approaches. Arab. J. Chem. 2022, 15, 104323. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Rosales, E.P.; Raffaini, G.; Ganazzoli, F.; Brenna, A.; Pedeferri, M.; Ormellese, M. Molecular Modelling and Electrochemical Evaluation of Organic Inhibitors in Concrete. Corros. Sci. 2015, 100, 231–241. [Google Scholar] [CrossRef]
- Raffaini, G.; Catauro, M.; Ganazzoli, F.; Bolzoni, F.; Ormellese, M. Organic Inhibitors to Prevent Chloride-Induced Corrosion in Concrete: Atomistic Simulations of Triethylenetetramine-Based Inhibitor Film. Macromol. Symp. 2021, 395, 2000231. [Google Scholar] [CrossRef]
- Ormellese, M.; Pérez, E.A.; Raffaini, G.; Ganazzoli, F.; Lazzari, L. Inhibition Mechanism in Concrete by Organic Substances: An Experimental and Theoretical Study. In Proceedings of the NACE CORROSION, Atlanta, Georgia, 22–26 March 2009; p. NACE-09221. [Google Scholar]
- Raffaini, G.; Bolzoni, F.; Ormellese, M. Benzoate-Based Inhibitor Film to Prevent Chloride-Induced Corrosion: Simulation Study of Efficiency of Dry or Hydrated Film. Macromol. Symp. 2023, 411, 2200165. [Google Scholar] [CrossRef]
- Raffaini, G.; Catauro, M.; Ganazzoli, F.; Bolzoni, F.; Ormellese, M. Hydration of Triethylenetetramine Based Inhibitor Film Accelerate the Chloride-Induced Corrosion in Concrete: A Molecular Dynamics Study. Macromol. Symp. 2022, 404, 2100296. [Google Scholar] [CrossRef]
- Li, Q.; Han, L.; Luo, Q.; Liu, X.; Yi, J. Towards Understanding the Corrosion Behavior of Zinc-Metal Anode in Aqueous Systems: From Fundamentals to Strategies. Batter. Supercaps 2022, 5, e202100417. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.; Wang, Z.; Liu, H.; Meng, G.; Liu, H. Corrosion Inhibition of Deposit-Covered X80 Pipeline Steel in Seawater Containing Pseudomonas Stutzeri. Bioelectrochemistry 2023, 149, 108279. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, X.; Chen, P.; Xiao, P.; Zhou, W.; Li, Y. Current Research Art of Rare Earth Compound Modified SiC-CMCs for Enhanced Wet-Oxygen Corrosion Resistance. Ceram. Int. 2022, 48, 24131–24143. [Google Scholar] [CrossRef]
- Nagay, B.E.; Cordeiro, J.M.; Barao, V.A. Insight into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials. Curr. Oral Health Rep. 2022, 9, 7–21. [Google Scholar] [CrossRef]
- Shozib, I.A.; Ahmad, A.; Abdul-Rani, A.M.; Beheshti, M.; Aliyu, A.A. A Review on the Corrosion Resistance of Electroless Ni-P Based Composite Coatings and Electrochemical Corrosion Testing Methods. Corros. Rev. 2022, 40, 1–37. [Google Scholar] [CrossRef]
- Wei, X.X.; Zhang, B.; Wu, B.; Wang, Y.J.; Tian, X.H.; Yang, L.X.; Oguzie, E.E.; Ma, X.L. Enhanced Corrosion Resistance by Engineering Crystallography on Metals. Nat. Commun. 2022, 13, 726. [Google Scholar] [CrossRef]
- Teshaboyev, A.M.; Meliboyev, I.A. Types and Applications of Corrosion-Resistant Metals. Cent. Asian J. Theor. Appl. Sci. 2022, 3, 15–22. [Google Scholar]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Electronic Effect vs. Molecular Size Effect: Experimental and Computational Based Designing of Potential Corrosion Inhibitors. Chem. Eng. J. 2022, 430, 132645. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Eliboyev, I.; Berdimuradov, K.; Kholikov, A.; Akbarov, K.; Dagdag, O.; Rbaa, M.; El Ibrahimi, B.; Verma, D.K.; Haldhar, R. Green β-Cyclodextrin-Based Corrosion Inhibitors: Recent Developments, Innovations and Future Opportunities. Carbohydr. Polym. 2022, 292, 119719. [Google Scholar] [CrossRef]
- Farhadian, A.; Zhao, Y.; Naeiji, P.; Rahimi, A.; Berisha, A.; Zhang, L.; Rizi, Z.T.; Iravani, D.; Zhao, J. Simultaneous Inhibition of Natural Gas Hydrate Formation and CO2/H2S Corrosion for Flow Assurance inside the Oil and Gas Pipelines. Energy 2023, 269, 126797. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D.; Wu, J.; Ou, J. How Surface Orientation Affects Coalescence-Induced Droplet Jumping Behavior and Subsequent Atmospheric Corrosion Resistance of a Superhydrophobic Surface? Corros. Sci. 2022, 197, 110082. [Google Scholar] [CrossRef]
- Huang, H.; Niu, J.; Xing, X.; Lin, Q.; Chen, H.; Qiao, Y. Effects of the Shot Peening Process on Corrosion Resistance of Aluminum Alloy: A Review. Coatings 2022, 12, 629. [Google Scholar] [CrossRef]
- Rajamohan, N.; Al Shibli, F.S.Z.S.; Rajasimman, M.; Vasseghian, Y. Eco-Friendly Biomass from Ziziphus Spina-Christi for Protection of Carbon Steel in Acidic Conditions–Parameter Effects and Corrosion Mechanism Studies. Chemosphere 2022, 291, 132756. [Google Scholar] [CrossRef] [PubMed]
- Caihong, Y.; Singh, A.; Ansari, K.R.; Ali, I.H.; Kumar, R. Novel Nitrogen Based Heterocyclic Compound as Q235 Steel Corrosion Inhibitor in 15% HCl under Dynamic Condition: A Detailed Experimental and Surface Analysis. J. Mol. Liq. 2022, 362, 119720. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Yang, C.; Han, X. Study on Hydrogen Embrittlement and Reversibility of Hot-Stamped Aluminized 22MnB5 Steel. Mater. Sci. Eng. A 2022, 848, 143411. [Google Scholar] [CrossRef]
- Moustafa, A.H.E.; Abdel-Rahman, H.H.; Awad, M.K.; Naby, A.A.N.A.; Seleim, S.M. Molecular Dynamic Simulation Studies and Surface Characterization of Carbon Steel Corrosion with Changing Green Inhibitors Concentrations and Temperatures. Alex. Eng. J. 2022, 61, 2492–2519. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Q.; Zhang, Y.; Yang, Z.; Wu, P.; Huang, D.; Li, H.; Wang, H.; Tang, Y. Synergistic Regulating the Aluminum Corrosion by Ellagic Acid and Sodium Stannate Hybrid Additives for Advanced Aluminum-Air Battery. Electrochim. Acta 2022, 417, 140311. [Google Scholar] [CrossRef]
- Khan, M.; Chu, S.H.; Deng, X.W.; Wang, Y. Protection of Steel Tube against Corrosion Using Self-Prestressing UHPC Prepared with Expansive Agent and Steel Fibers. Structures 2022, 37, 95–108. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion Inhibitors: Physisorbed or Chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Öztürk, S.; Alptekin, F.; Önal, S.; Sünbül, S.E.; Şahin, Ö.; İçin, K. Effect of Titanium Addition on the Corrosion Behavior of CoCuFeNiMn High Entropy Alloy. J. Alloys Compd. 2022, 903, 163867. [Google Scholar] [CrossRef]
- Paksoy, A.H.; Martins, J.P.; Cao, H.; Chen, Y.; Gibson, G.; Xiao, P. Influence of Alumina Addition on Steam Corrosion Behaviour of Ytterbium Disilicates for Environmental Barrier Coating Applications. Corros. Sci. 2022, 207, 110555. [Google Scholar] [CrossRef]
- Ayoola, A.A.; Babalola, R.; Durodola, B.M.; Alagbe, E.E.; Agboola, O.; Adegbile, E.O. Corrosion Inhibition of A36 Mild Steel in 0.5 M Acid Medium Using Waste Citrus Limonum Peels. Results Eng. 2022, 15, 100490. [Google Scholar] [CrossRef]
- Idris, I.A.; Bello, A.U.; Usman, B. Experimental and Theoretical Evaluation Of Corrosion Inhibition of Honeycomb Propolis Extract On Mild Steel In Acidic Media. J. Mater. Environ. Sci. 2022, 13, 576–598. [Google Scholar]
- Alharthi, N.H.; El-Hashemy, M.A.; Derafa, W.M.; Althobaiti, I.O.; Altaleb, H.A. Corrosion Inhibition of Mild Steel by Highly Stable Polydentate Schiff Base Derived from 1, 3-Propanediamine in Aqueous Acidic Solution. J. Saudi Chem. Soc. 2022, 26, 101501. [Google Scholar] [CrossRef]
- Leng, Z.; Li, T.; Wang, X.; Zhang, S.; Zhou, J. Effect of Graphite Content on the Conductivity, Wear Behavior, and Corrosion Resistance of the Organic Layer on Magnesium Alloy MAO Coatings. Coatings 2022, 12, 434. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite Organic Coatings for Corrosion Protection of Metals: A Review of Recent Advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Jiang, X.; Wan, W.; Wang, B.; Zhang, L.; Yin, L.; Van Bui, H.; Xie, J.; Zhang, L.; Lu, H.; Deng, L. Enhanced Anti-Corrosion and Microwave Absorption Performance with Carbonyl Iron Modified by Organic Fluorinated Chemicals. Appl. Surf. Sci. 2022, 572, 151320. [Google Scholar] [CrossRef]



| Name | Composition (%) |
|---|---|
| PA | 7.33 ± 0.02 |
| PAA | 0.098 ± 0.05 |
| HA | 0.06 ± 0.01 |
| SA | 4.52 ± 0.05 |
| OA | 19.02 ± 0.10 |
| LA | 67.11 ± 0.12 |
| α-LA | 0.21 ± 0.02 |
| AC | 0.19 ± 0.02 |
| GA | 0.15 ± 0.02 |
| BA | 0.10 ± 0.03 |
| Inhibitor | Concentration (g/L) | −Ecorr (mV/SCE) | −βc (mV dec−1) | βa (mV dec−1) | Icorr (μA cm−2) | IEI (%) |
|---|---|---|---|---|---|---|
| 1 M HCl | - | 463.1 ± 0.5 | 168.2 ± 0.5 | 123.4 ± 0.6 | 636.1 ± 0.4 | - |
| GSO | 0.5 | 465.2 ± 0.2 | 191.7 ± 0.2 | 76.3 ± 0.7 | 147.1 ± 0.3 | 76.98 |
| 0.3 | 464.1 ± 0.1 | 196.8 ± 0.6 | 73.8 ± 0.2 | 238.2 ± 0.7 | 62.67 | |
| 0.1 | 470.0 ± 0.3 | 195.4 ± 0.1 | 94.4 ± 0.4 | 287.0 ± 0.5 | 54.97 | |
| 0.05 | 470.5 ± 0.2 | 194.6 ± 0.8 | 80.1 ± 0.3 | 328.1 ± 0.2 | 48.42 |
| Inhibitor | Concentration (g/L) | Rct (Ω cm2) | Cdl (μF/cm2) | Ect (%) |
|---|---|---|---|---|
| 1 M HCl | - | 18.1 ± 1.5 | 221.16 ± 2.05 | - |
| GSO | 0.5 | 80.2 ± 0.6 | 132.69 ± 1.15 | 77.5 |
| 0.3 | 50.1 ± 0.8 | 212.31 ± 1.98 | 64.0 | |
| 0.1 | 40.2 ± 0.7 | 265.39 ± 0.87 | 55.1 | |
| 0.05 | 35.1 ± 0.9 | 303.30 ± 1.26 | 48.57 |
| Inhibitor | Kads (L/g) | R2 | (kJ/mol) |
|---|---|---|---|
| GSO | 2.16 | 0.99042 | −11.85 |
| Medium | Temperature (K) | −Ecorr (mV/SCE) | Icorr (µA/cm2) | −βc (mV/dec) | EI (%) |
|---|---|---|---|---|---|
| 1 M HCl | 298 ± 1 | 463.1 ± 0.5 | 636.1 ± 0.4 | 168.2 ± 0.5 | - |
| 308 ± 1 | 467.2 ± 0.2 | 896.4 ± 0.2 | 165.1 ± 0.1 | - | |
| 318 ± 1 | 470.1 ± 0.6 | 3428.1 ± 0.7 | 167.4 ± 0.6 | - | |
| 328 ± 1 | 477.0 ± 0.8 | 6720.2 ± 0.7 | 164.3 ± 0.8 | - | |
| 1 M HCl + GSO | 298 ± 1 | 465.2 ± 0.2 | 147.1 ± 0.3 | 191.7 ± 0.2 | 76.98 |
| 308 ± 1 | 477.4 ± 0.3 | 221.3 ± 0.3 | 189.1 ± 0.4 | 75.33 | |
| 318 ± 1 | 493.6 ± 0.5 | 798.5 ± 0.6 | 188.5 ± 0.5 | 76.72 | |
| 328 ± 1 | 504.3 ± 0.6 | 1613.6 ± 0.7 | 190.8 ± 0.6 | 75.99 |
| Medium | ∆H* (kJ/mol) | ∆S* (J/mol K) | Ea (kJ/mol) |
|---|---|---|---|
| 1 M HCl | 33.79 | −191.53 | 36.38 |
| 1 M HCl + GSO | 34.64 | −200.87 | 37.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batah, A.; Al-Moubaraki, A.H.; Noor, E.A.; Al-Ahmari, J.M.; Al-Ghamdi, A.A.; Id El Mouden, O.; Salghi, R.; Chafiq, M.; Chaouiki, A.; Ko, Y.G. Environmentally Benign Grape Seed Oil for Corrosion Inhibition: Cutting-Edge Computational Modeling Techniques Revealing the Intermolecular and Intramolecular Synergistic Inhibition Action. Coatings 2024, 14, 77. https://doi.org/10.3390/coatings14010077
Batah A, Al-Moubaraki AH, Noor EA, Al-Ahmari JM, Al-Ghamdi AA, Id El Mouden O, Salghi R, Chafiq M, Chaouiki A, Ko YG. Environmentally Benign Grape Seed Oil for Corrosion Inhibition: Cutting-Edge Computational Modeling Techniques Revealing the Intermolecular and Intramolecular Synergistic Inhibition Action. Coatings. 2024; 14(1):77. https://doi.org/10.3390/coatings14010077
Chicago/Turabian StyleBatah, Ahmed, Aisha H. Al-Moubaraki, Ehteram A. Noor, Jamilah M. Al-Ahmari, Azza A. Al-Ghamdi, Omar Id El Mouden, Rachid Salghi, Maryam Chafiq, Abdelkarim Chaouiki, and Young Gun Ko. 2024. "Environmentally Benign Grape Seed Oil for Corrosion Inhibition: Cutting-Edge Computational Modeling Techniques Revealing the Intermolecular and Intramolecular Synergistic Inhibition Action" Coatings 14, no. 1: 77. https://doi.org/10.3390/coatings14010077







