Aging Effect of Plasma-Treated Carbon Fiber Surface: From an Engineering Point
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Morphology
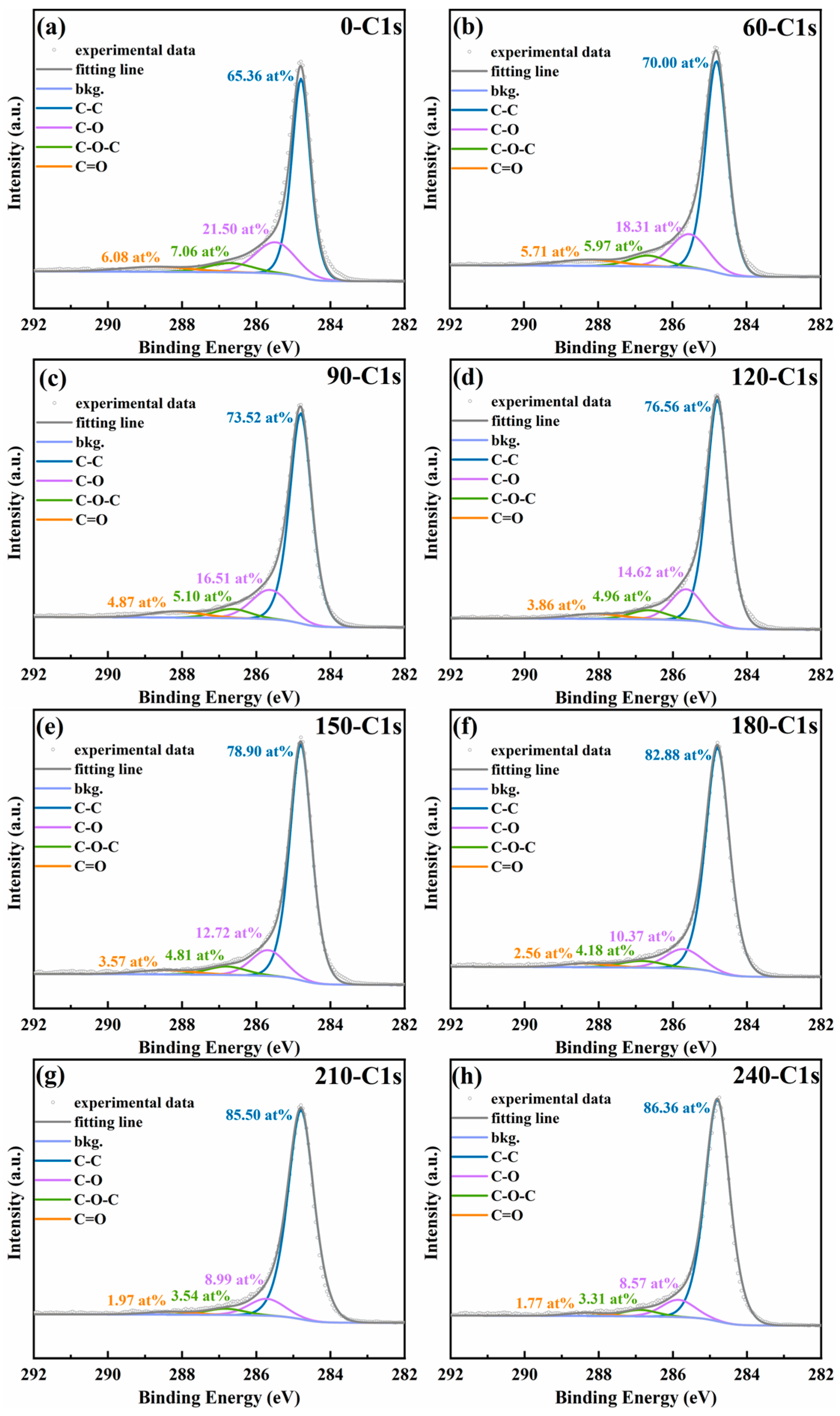
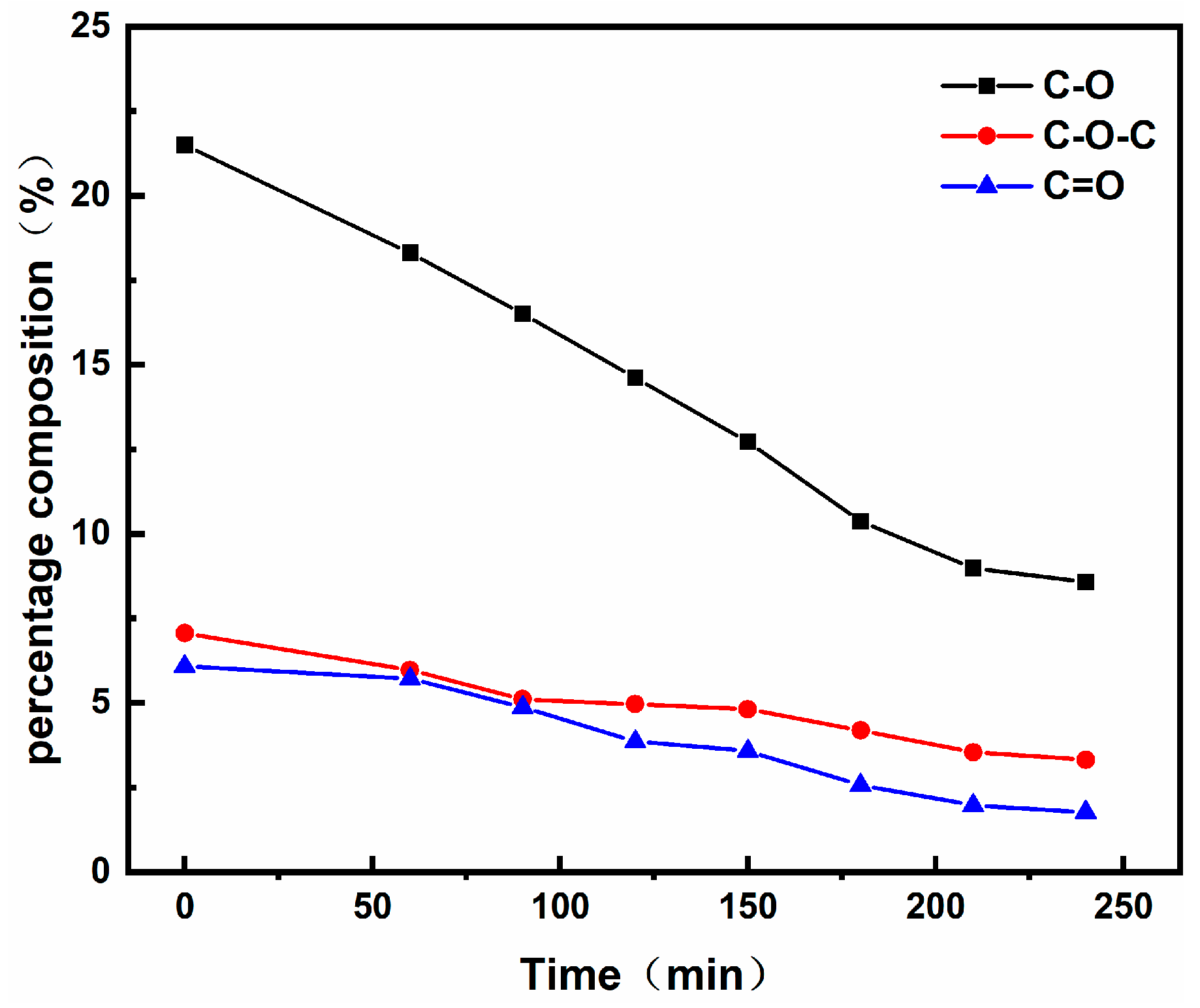
3.2. Surface Chemical Assessment
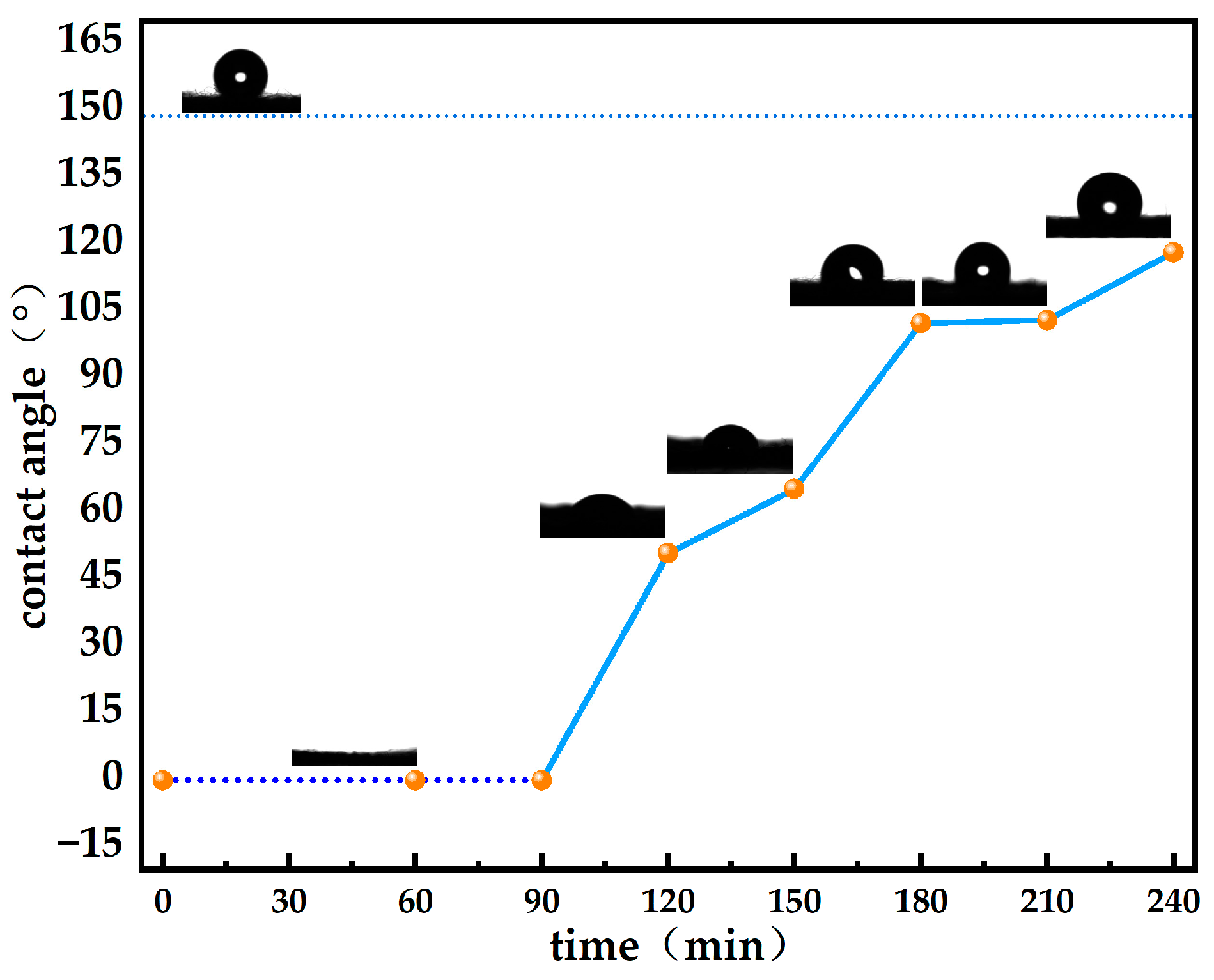
3.3. Contact Angle
3.4. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downs, W.B.; Baker, R.M. Novel Carbon Fiber-Carbon Filament Structures. Carbon 1991, 29, 1173–1179. [Google Scholar] [CrossRef]
- Suzuki, M. Activated Carbon Fiber: Fundamentals and Applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Carbon-Fiber Microelectrodes for in Vivo Applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ooi, C.H.; Othman, R.; Yeoh, F.Y. Activated carbon fiber-the hybrid of carbon fiber and activated carbon. Rev. Adv. Mater. Sci. 2014, 36, 118–136. [Google Scholar]
- Jin, Z.; Han, Z.; Chang, C.; Sun, S.; Fu, H. Review of Methods for Enhancing Interlaminar Mechanical Properties of Fiber-Reinforced Thermoplastic Composites: Interfacial Modification, Nano-Filling and Forming Technology. Compos. Sci. Technol. 2022, 228, 109660. [Google Scholar] [CrossRef]
- Yu, G.; Wei, H.; Li, D. Research progress of carbon fiber sizing agent and its effects on interface properties of composites. Eng. Plast. Appl. 2019, 47, 143–147. [Google Scholar]
- Hao, R.T.; Zhang, X.J.; Tian, Y.H. Research progress of heat-resistant thermoplastic sizing agents. Chem. Ind. Eng. Prog. 2018, 37 (Suppl. S1), 117–124. [Google Scholar]
- He, W.F.; Li, R.K.; Luo, S.H. Progress in plasma surface treatment on carbon fiber for composite material. Surf. Technol. 2020, 49, 76–89. [Google Scholar]
- Unterweger, C.; Duchoslav, J.; Stifter, D.; Fürst, C. Characterization of Carbon Fiber Surfaces and Their Impact on the Mechanical Properties of Short Carbon Fiber Reinforced Polypropylene Composites. Compos. Sci. Technol. 2015, 108, 41–47. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, Y.; Mi, Z.; Li, J.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. PEEK Composites with Polyimide Sizing SCF as Reinforcement: Preparation, Characterization, and Mechanical Properties. High Perform. Polym. 2019, 32, 383–393. [Google Scholar] [CrossRef]
- Saifutdinova, A.A.; Saifutdinov, A.I.; Gainullina, S.V.; Timerkaev, B.A. Modeling the Parameters of an Atmospheric Pressure Dielectric Barrier Discharge Controlled by the Shape of the Applied Voltage. IEEE Trans. Plasma Sci. 2022, 50, 1144–1156. [Google Scholar] [CrossRef]
- Xia, G.; Chen, Z.; Saifutdinov, A.I.; Eliseev, S.I.; Hu, Y.; Kudryavtsev, A.A. Longer Microwave Plasma Jet with Different Discharge Performances Originated by Plasma-Surface Interactions. IEEE Trans. Plasma Sci. 2014, 42, 2768–2769. [Google Scholar] [CrossRef]
- Hünnekens, B.; Krause, A.; Militz, H.; Viöl, W. Hydrophobic Recovery of Atmospheric Pressure Plasma Treated Surfaces of Wood-Polymer Composites (WPC). Eur. J. Wood Wood Prod. 2017, 75, 761–766. [Google Scholar] [CrossRef]
- Pykönen, M.; Sundqvist, H.; Järnström, J.; Kaukoniemi, O.-V.; Tuominen, M.; Lahti, J.; Peltonen, J.; Fardim, P.; Toivakka, M. Effects of Atmospheric Plasma Activation on Surface Properties of Pigment-Coated and Surface-Sized Papers. Appl. Surf. Sci. 2008, 255, 3217–3229. [Google Scholar] [CrossRef]
- Mendez-Linan, J.I.; Ortiz-Ortega, E.; Moreno, M.F.J.; Palma, M.I.M.; Martínez-Guerra, E.; Aguirre-Tostado, F.S.; Martínez-Chapa, S.O.; Hosseini, S.; Madou, M. Aging Effect of Plasma-Treated Carbon Surfaces: An Overlooked Phenomenon. Carbon 2020, 169, 32–44. [Google Scholar] [CrossRef]
- Min, S.-J.; Kim, J.; Park, C.; Jin, J.-H.; Min, N.K. Long-Term Stability of Superhydrophilic Oxygen Plasma-Modified Single-Walled Carbon Nanotube Network Surfaces and the Influence on Ammonia Gas Detection. Appl. Surf. Sci. 2017, 410, 105–110. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.; Park, C.H. Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging. Polymers 2020, 12, 2756. [Google Scholar] [CrossRef]
- Egghe, T.; Ghobeira, R.; Morent, R.; Hoogenboom, R.; De Geyter, N. Comparative Study of the Aging Behavior of Plasma Activated Hexamethyldisiloxane-Based Plasma Polymers and Silicone Elastomer Thin Films. Prog. Org. Coat. 2022, 172, 107091. [Google Scholar] [CrossRef]
- Mei, T.; Gao, M.; Liu, D.; Wang, Y.; Huang, Y. Enhanced Electrocatalytic Activity of Carbon Cloth by Synergetic Effect of Plasma and Acid Treatment. Plasma Sci. Technol. 2021, 23, 025504. [Google Scholar] [CrossRef]
- Rani, K.V.; Chandwani, N.; Kikani, P.; Nema, S.K.; Sarma, A.; Sarma, B. Optimization and Surface Modification of Silk Fabric Using DBD Air Plasma for Improving Wicking Properties. J. Text. Inst. 2017, 109, 368–375. [Google Scholar] [CrossRef]
- Owen, M.J.; Gentle, T.M.; Orbeck, T.; Williams, D.E. Dynamic wettability of hydrophobic polymers. In Polymer Surface Dynamics; Springer US: New York, NY, USA, 1988; pp. 101–110. [Google Scholar]
- Morra, M.; Occhiello, E.; Gila, L.; Garbassi, F. Surface Dynamics vs. Adhesion in Oxygen Plasma Treated Polyolefins. J. Adhes. 1990, 33, 77–88. [Google Scholar] [CrossRef]
- Riedl, B.; Angel, C.; Prégent, J.; Blanchet, P.; Stafford, L. Effect of Wood Surface Modification by Atmospheric-Pressure Plasma on Waterborne Coating Adhesion. Bioresources 2014, 9, 4908–4923. [Google Scholar] [CrossRef]
- Vizireanu, S.; Ionita, M.D.; Ionita, R.E.; Stoica, S.D.; Teodorescu, C.M.; Husanu, M.-A.; Apostol, N.G.; Baibarac, M.; Panaitescu, D.M.; Dinescu, G. Aging Phenomena and Wettability Control of Plasma Deposited Carbon Nanowall Layers. Plasma Process. Polym. 2017, 14, 1700023. [Google Scholar] [CrossRef]
- Cao, Y.; Hua, H.; Yang, P.; Chen, M.; Chen, W.; Wang, S. Investigation into the Reaction Mechanism Underlying the Atmospheric Low-Temperature Plasma-Induced Oxidation of Cellulose. Carbohydr. Polym. 2020, 233, 115632. [Google Scholar] [CrossRef]
- Ortiz-Ortega, E.; Hosseini, S.; Martínez-Chapa, S.O.; Madou, M. Aging of Plasma-Activated Carbon Surfaces: Challenges and Opportunities. Appl. Surf. Sci. 2021, 565, 150362. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent Approaches to Reduce Aging Phenomena in Oxygen- and Nitrogen-Containing Plasma Polymer Films: An Overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Bachmann, S.; Schulze, M.; Morasch, J.; Hesse, S.; Hussein, L.; Krell, L.; Schnagl, J.; Stark, R.W.; Narayan, S. Aging of oxygen and hydrogen plasma discharge treated aC: H and ta-C coatings. Appl. Surf. Sci. 2016, 371, 613–623. [Google Scholar] [CrossRef]
- Guo, W.; Chen, B.; Lam, V.T.; Brink, G.H.T.; Kooi, B.J.; Svetovoy, V.B.; Palasantzas, G. Effect of Airborne Hydrocarbons on the Wettability of Phase Change Nanoparticle Decorated Surfaces. ACS Nano 2019, 13, 13430–13438. [Google Scholar] [CrossRef]
- Sahoo, G.; Polaki, S.R.; Ghosh, S.; Krishna, N.G.; Kamruddin, M. Temporal-Stability of Plasma Functionalized Vertical Graphene Electrodes for Charge Storage. J. Power Sources 2018, 401, 37–48. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, Y.; Gao, M.; Huang, Y. Aging Effect of Plasma-Treated Carbon Fiber Surface: From an Engineering Point. Coatings 2024, 14, 80. https://doi.org/10.3390/coatings14010080
Wang S, Wang Y, Gao M, Huang Y. Aging Effect of Plasma-Treated Carbon Fiber Surface: From an Engineering Point. Coatings. 2024; 14(1):80. https://doi.org/10.3390/coatings14010080
Chicago/Turabian StyleWang, Shiwen, Yu Wang, Ming Gao, and Yifan Huang. 2024. "Aging Effect of Plasma-Treated Carbon Fiber Surface: From an Engineering Point" Coatings 14, no. 1: 80. https://doi.org/10.3390/coatings14010080
APA StyleWang, S., Wang, Y., Gao, M., & Huang, Y. (2024). Aging Effect of Plasma-Treated Carbon Fiber Surface: From an Engineering Point. Coatings, 14(1), 80. https://doi.org/10.3390/coatings14010080





