Camelina sativa Oilseed Cake as a Potential Source of Biopolymer Films: A Chemometric Approach to Synthesis, Characterization, and Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Biopolymer Film Preparation
2.4. Biopolymer Film Characterization
2.4.1. Film Thickness
2.4.2. Mechanical Properties
2.5. Barrier Properties
2.5.1. Water Vapor Permeability
2.5.2. Light Transmission
2.6. Physico-Chemical Properties
Moisture Content
2.7. Film Solubility
2.8. Film Morphology
2.9. Structural Properties—Fourier-Transform Infrared Spectroscopy (FTIR)
2.10. Antioxidative Activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.11. Antimicrobial Activity
Disc Diffusion Method
2.12. Statistical Analysis
Principal Component Analysis
2.13. Correlation Analysis
2.14. Standard Score
2.15. Artificial Neural Network Modeling
2.16. Data Manipulation
3. Results
3.1. Visual Appearance
3.2. Mechanical Properties
3.3. Barrier Properties
3.3.1. Water Vapor Permeability
3.3.2. Light Transmission
3.4. Physico-Chemical Properties
3.4.1. Moisture Content
3.4.2. Film Solubility
3.5. Film Morphology
3.6. Structural Properties—Fourier-Transform Infrared Spectroscopy (FTIR)
3.7. Antioxidative Activity—DPPH Assay
3.8. Antimicrobial Activity—Disc Diffusion Method
3.9. Statistical Analysis
3.9.1. Principal Component Analysis
3.9.2. Color Correlation Analysis
3.9.3. Standard Score Optimization
3.9.4. Modeling of the Predictive Artificial Neural Network
4. Practical Applications of CSoC in Food Packaging: Possibilities, Limitations, and Further Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamwal, V.; Mittal, A.; Dhaundiyal, A. Valorization of agro-industrial waste in composite films for sustainable packaging applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Klai, N.; Yadav, B.; El Hachimi, O.; Pandey, A.; Sellamuthu, B.; Tyagi, R.D. Chapter 18-Agro-Industrial Waste Valorization for Biopolymer Production and Life-Cycle Assessment Toward Circular Bioeconomy. In Biomass, Biofuels, Biochemical; Pandey, A., Tyagi, R.D., Varjani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–555. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Manoj Kumar, N.; Sivakumar, V.M.; Thirumarimurugan, M. Chapter 12—Food waste valorization for biopolymer production. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Gnansounou, E., Khanal, S.K., Raveendran, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 233–249. [Google Scholar] [CrossRef]
- Sani, I.K.; Masoudpour-Behabadi, M.; Sani, M.A.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.-W.; Mohammadi, F. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2023, 405 Pt B, 134964. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable Applications for the Valorization of Cereal Processing By-Products. Foods 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V. Chapter 26—Valorization of seafood processing by-products. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 515–536. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Paul, A.; Kumar, V.; Sar, T.; Kumar, D.; Sarsaiya, S.; Liu, H.; Zhang, Z.; Binod, P.; Sindhu, R.; et al. Recent trends and developments on integrated biochemical conversion process for valorization of dairy waste to value added bioproducts: A review. Bioresour. Technol. 2022, 344, 126193. [Google Scholar] [CrossRef]
- Popović, S.; Hromiš, N.; Šuput, D.; Bulut, S.; Romanić, R.; Lazić, V. Chapter 3—Valorization of by-products from the production of pressed edible oils to produce biopolymer films. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 15–30. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial FoodWaste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- USDA. Oilseeds: World Markets and Trade. United States Department of Agriculture Foreign Agricultural Service. 2023. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 20 November 2023).
- Petraru, A.; Amariei, S.A. Novel Approach about Edible Packaging Materials Based on Oilcakes—A Review. Polymers 2023, 15, 3431. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Mariniello, L.; D’Agostino, A.; D’Agostino, M.; Cammarota, M.; Schiraldi, C.; Porta, R. Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications. Int. J. Mol. Sci. 2022, 23, 8478. [Google Scholar] [CrossRef]
- Arshad, M.; Mohanty, K.A.; Acker, R.V.; Riddle, R.; Todd, J.; Khalil, H.; Misra, M. Valorization of Camelina Oil to Biobased Materials and Biofuels for New Industrial Uses A Review. RSC Adv. 2022, 12, 27230–27245. [Google Scholar] [CrossRef]
- Neupane, D.; Lohaus, R.H.; Solomon, J.K.Q.; Cushman, J.C. Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate. Plants 2022, 11, 772. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Chen, C.; Afshar, R.K. Nutrient requirements of Camelina for biodiesel feedstock in Central Montana. Agron. J. 2017, 109, 309–316. [Google Scholar] [CrossRef]
- Bacenetti, J.; Restuccia, A.; Schillaci, G.; Failla, S. Biodiesel production from unconventional oilseed crops (Linum usitatissimum L. and Camelina sativa L.) in Mediterranean conditions: Environmental sustainability assessment. Renew. Energy 2017, 112, 444–456. [Google Scholar] [CrossRef]
- Juodka, R.; Nainiene, R.; Juškiene, V.; Juška, R.; Leikus, R.; Kadžiene, G.; Stankeviciene, D. Camelina (Camelina sativa (L.) Crantz) as Feedstuffs in Meat Type Poultry Diet: A Source of Protein and n-3 Fatty Acids. Animals 2022, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Sotin, H.; Rabesona, H.; Novales, B.; Le Quéré, J.-M.; Froissard, M.; Faure, J.-D.; Guyot, S.; Anton, M. Oil Bodies from Chia (Salvia hispanica L.) and Camelina (Camelina sativa L.) Seeds for Innovative Food Applications: Microstructure, Composition and Physical Stability. Foods 2023, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Kurasiak-Popowska, D.; Stuper-Szablewska, K. The phytochemical quality of Camelina sativa seed and oil. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef]
- Ilić, P.N.; Rakita, S.M.; Spasevski, N.J.; Đuragić, O.M.; Marjanović Jeromela, A.M.; Cvejić, S.; Zanetti, F. Nutritive value of serbian camelina genotypes as an alternative feed ingredient. Food Feed Res. 2022, 49, 209–221. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, V.K.; Du, Y.; Aghbashlo, M.; Show, P.L.; Pan, J.; Tabatabaei, M.; Rajaei, A. Potential application of polysaccharide mucilages as a substitute for emulsifiers: A review. Int. J. Biol. Macromol. 2023, 242, 124800. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Fabrication of bioaerogels from camelina seed mucilage for food applications. Food Hydrocoll. 2020, 102, 105597. [Google Scholar] [CrossRef]
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogozinski, T. Camelina sativa. Status quo and future perspectives. Ind. Crops Prod. 2022, 187, 115531. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035. [Google Scholar] [CrossRef]
- Šuput, D.Z.; Popović, S.Z.; Hromiš, N.M.; Rakita, S.M.; Spasevski, N.J.; Lončar, B.; Erceg, T.D.; Knežević, V.M. The influence of oil cake granulation and ultrasonic pretreatment on the properties of biopolymer films based on Camelina sativa oilseed cake. Food Feed Res. 2023, 2023, 61–75. [Google Scholar] [CrossRef]
- Saberi, B.; Thakur, R.; Bhuyan, D.J.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Development of edible blend films with good mechanical and barrier properties from pea starch and guar gum. Starch. Stärke 2017, 69, 1600227. [Google Scholar] [CrossRef]
- ISO 527-3:2018; Plastics-Determination of Tensile Properties. Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 2528:2017; Sheet Materials. Determination of Water Vapor Transmission Rate (WVTR). Gravimetric (Dish) Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ASTM D5576:00; Standard Practice for Determination of Structural Features in Polyolefins and Polyolefin Copolymers by Infrared Spectrophotometry (FT-IR). ASTM International: West Conshohocken, PA, USA, 2021.
- Razmavar, S.; Abdulla, M.A.; Ismail, S.B.; Hassandarvish, P. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. BioMed. Res. Int. 2014, 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, E.J.; Babič, J.; Pezo, L.; Banjac, V.; Čolović, R.; Kos, J.; Krulj, J.; Pavšič-Vrtač, K.; Jakovac-Strajn, B. Effects of extrusion process on Fusarium and Alternaria mycotoxins in whole grain triticale flour. LWT 2022, 155, 112926. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Ly, H.B.; Ho, L.S.; Al-Ansari, N.; Le, H.V.; Tran, V.Q.; Prakash, I.; Pham, B.T. Influence of data splitting on performance of machine learning models in prediction of shear strength of soil. Math. Probl. Eng. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Voća, N.; Pezo, L.; Jukić, Ž.; Lončar, B.; Šuput, D.; Krička, T. Estimation of the storage properties of rapeseeds using an artificial neural network. Ind. Crops Prod. 2022, 187, 115358. [Google Scholar] [CrossRef]
- Brandić, I.; Pezo, L.; Bilandžija, N.; Peter, A.; Šurić, J.; Voća, N. Comparison of Different Machine Learning Models for Modelling the Higher Heating Value of Biomass. Mathematics 2023, 11, 2098. [Google Scholar] [CrossRef]
- Rajković, D.; Jeromela, A.M.; Pezo, L.; Lončar, B.; Grahovac, N.; Špika, A.K. Artificial neural network and random forest regression models for modelling fatty acid and tocopherol content in oil of winter rapeseed. J. Food Compos. Anal. 2023, 115, 105020. [Google Scholar] [CrossRef]
- Erdem, B.G.; Kaya, S. Characterization and application of novel composite films based on soy protein isolate and sunflower oil produced using freeze drying method. Food Chem. 2022, 366, 130709. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Atarés, L.; De Jesús, J.; Talens, P.; Chiralt, A. Characterization of SPI-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 99, 384–391. [Google Scholar] [CrossRef]
- Bulut, S. Research of Obtaining, Characterization and Optimization of Properties of Active, Biodegradable, Packaging Material Based on Pumpkin Oil Cake. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2021. [Google Scholar]
- Šuput, D.; Lazić, V.; Popović, S.; Hromiš, N.; Bulut, S.; Pezo, L.; Banićević, J. Effect of process parameters on biopolymer films based on sunflower oil cake. J. Process. Energy Agric. 2018, 22, 125–128. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Zannini, D.; Santagata, G.; Giosafatto, C.V.L. Cardoon seed oil cake proteins as substrate for microbial transglutaminase: Their application as matrix for bio-based packaging to extend the shelf-life of peanuts. Food Hydrocoll. 2024, 147, 109339. [Google Scholar] [CrossRef]
- Jang, S.-A.; Lim, G.-O.; Song, K.B. Preparation and Mechanical Properties of Edible Rapeseed Protein Films. J. Food Sci. 2011, 76, C218–C223. [Google Scholar] [CrossRef]
- Petraru, A.; Amariei, S. Sunflower Oilcake as a Potential Source for the Development of Edible Membranes. Membranes 2022, 12, 789. [Google Scholar] [CrossRef]
- Popović, S. The Study of Production and Characterization of Biodegradable, Composite Films Based on Plant Proteins. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2013. [Google Scholar]
- Bulut, S.; Popović, S.; Hromiš, N.; Šuput, D.; Adamović, D.; Lazić, V. Incorporation of essential oils into pumpkin oil cake-based materials in order to improve their properties and reduce water sensitivity. Hem. Ind. 2020, 74, 313–325. [Google Scholar] [CrossRef]
- Abdollahi, M.; Damirchi, S.; Shafafi, M.; Rezaei, M.; Ariaii, P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019, 126, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, E.J.; Chang, C.; Lam, R.S.H.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Barut Gok, S.; Yüksel, A.N.; Tekgül, Y.; Çalis¸kan Koç, G.; Kothakota, A. Evaluation of the impact of UV radiation on rheological and textural properties of food. J. Texture Stud. 2022, 53, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Kchaou, H.; Nasreddine, B.; Karbowiak, T. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocoll. 2018, 74, 176–186. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterization of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Riahi, Z.; Rhim, J.-W. Antioxidant pectin/pullulan edible coating incorporated with Vitis vinifera grape seed extract for extending the shelf life of peanuts. Postharvest Biol. Technol. 2022, 183, 111740. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Exploration of the antioxidant and antimicrobial capacity of two sunflower protein concentrate films with naturally present phenolic compounds. Food Hydrocoll. 2012, 29, 374–381. [Google Scholar] [CrossRef]
- Hager, A.-S.; Vallons, K.J.R.; Arendt, E.K. Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J. Agric. Food Chem. 2012, 60, 6157–6163. [Google Scholar] [CrossRef]
- Chambi, H.N.M.; Lacerda, R.S.; Makishi, G.L.A.; Bittante, A.M.Q.B.; Gomide, C.A.; Sobral, P.J.A. Protein extracted from castor bean (Ricinus communis L.) cake in high pH results in films with improved physical properties. Ind. Crops Prod. 2014, 61, 217–224. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium alginate-based green packaging films functionalized by guava leaf extracts and their bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, K. Xanthoceras sorbifolium Bunge leaf extract activated chia seeds mucilage/chitosan composite film: Structure, performance, bioactivity, and molecular dynamics perspectives. Food Hydrocoll. 2023, 144, 109050. [Google Scholar] [CrossRef]
- Rai, S.; Poonia, A. Formulation and characterization of edible films from pea starch and casein. J. Pharmacogn. Phytochem. 2019, 8, 317–321. [Google Scholar]
- Šuput, D. Synthesis, Characterization, Optimization of Properties and Application of Edible Active Packaging Material Based on Starch. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2016. [Google Scholar]
- Ağçeli, G.K. A new approach to nanocomposite carbohydrate polymer films: Levan and chia seed mucilage. Int. J. Biol. Macromol. 2022, 218, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Lun, L.W.; Anas, A.; Gunny, N.; Kasim, F.H. Fourier Transform Infrared Spectroscopy (FTIR) analysis of Paddy Straw Pulp treated using Deep Eutectic Solvent. AIP Conf. Proc. 2017, 1835, 20049. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Q.; Yu, L.; Xie, F. Thermomechanically processed chitosan:gelatin films being transparent, mechanically robust and less hygroscopic. Carbohydr. Polym. 2021, 272, 118522. [Google Scholar] [CrossRef] [PubMed]
- Riyanta, A.B.; Riyanto, S.; Lukitaningsih, E.; Rohman, A. The employment of Fourier Transform Infrared Spectroscopy (FTIR) and chemometrics for analysis of candlenut oil in binary mixture with grape seed oil. Food Res. 2020, 4, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wachirattanapongmetee, K.; Katekaew, S.; Weerapreeyakul, N.; Thawornchinsombut, S. Differentiation of protein types extracted from tilapia byproducts by FTIR spectroscopy combined with chemometric analysis and their antioxidant protein hydrolysates. Food Chem. 2024, 437, 137862. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-calculated IR spectrum amide I, II, and III band contributions of N-Methylacetamide fine components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Pradini, D.; Juwono, H.; Madurani, K.A.; Kurniawan, F. A preliminary study of identification halal gelatin using quartz crystal microbalance (QCM) sensor. Mal. J. Fund. Appl. Sci. 2018, 14, 325–330. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous Crystallization and Multiple Melting Behavior of Poly(l-lactide): Molecular Weight Dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Mieriņa, I.; Adere, L.; Krasauska, K.; Zoltnere, E.; Skrastiņa, D.Z.; Jure, M. Antioxidant Properties of Oil and Press-Cakes. Proc. Latv. Acad. Sci. B Nat. Exact Appl. Sci. 2017, 6, 515–521. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Makuc, D.; Poklar Ulrih, N.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Jin, D.; Liu, X.; Zheng, X.; Wang, X.; He, J. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Z.; Wei, Y.; Zhang, L.; Ning, E.; Yu, L.; Zhu, J.; Wang, X.; Ma, Y.; Fan, Y. Qualitative and quantitative analysis of polyphenols in camelina seed and their antioxidant activities. Nat. Prod. Res. 2023, 37, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Tomičić, Z.; Čabarkapa, I.; Čolović, R.; Đuragić, O.; Tomičić, R. Salmonella in the feed industry: Problems and potential solutions. J. Agron. Technol. Eng. Manag. 2019, 2, 130–137. [Google Scholar]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Chiavari, C.; Benevelli, M.; Grazia, L.; Lanciotti, R. Survival of spoilage and pathogenic microorganisms on cardboard and plastic packaging materials. Front. Microbiol. 2017, 8, 2606. [Google Scholar] [CrossRef]
- Tomičić, R.; Tomičić, Z.; Thaler, N.; Humar, M.; Raspor, P. Factors influencing adhesion of bacteria Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and yeast Pichia membranifaciens to wooden surfaces. Wood Sci. Technol. 2020, 54, 1663–1676. [Google Scholar] [CrossRef]
- Răducu, A.L.; Popa, A.; Sicuia, O.; Boiu-Sicuia, O.A.; Israel-Roming, F.; Cornea, C.P.; Jurcoane, S. Antimicrobial activity of camelina oil and hydroalcoholic seed extracts. Rom. Biotecnol. Lett. 2021, 26, 2355–2360. [Google Scholar] [CrossRef]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol. Med. Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Ćurčić, L.; Lončar, B.; Pezo, L.; Stojić, N.; Prokić, D.; Filipović, V.; Pucarević, M. Chemometric Approach to Pesticide Residue Analysis in Surface Water. Water 2022, 14, 4089. [Google Scholar] [CrossRef]
- Ring, M.; Wunderlich, S.; Scheuring, D.; Landes, D.; Hotho, A. A survey of network-based intrusion detection data sets. Comput. Secur. 2019, 86, 147–167. [Google Scholar] [CrossRef]
- Sharma, R.; Kamble, S.S.; Gunasekaran, A.; Kumar, V.; Kumar, A. A systematic literature review on machine learning applications for sustainable agriculture supply chain performance. Comput. Oper. Res. 2020, 119, 104926. [Google Scholar] [CrossRef]
- Kollo, T.; von Rosen, D. Chapter 4—Multivariate Linear Models. In Advanced Multivariate Statistics with Matrices; Hazewinkel, M., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 355–472. [Google Scholar] [CrossRef]
- Doumpos, M.; Zopounidis, C. Preference disaggregation and statistical learning for multicriteria decision support: A review. Eur. J. Oper. Res. 2011, 209, 203–214. [Google Scholar] [CrossRef]
- Turányi, T.; Tomlin, A.S. Analysis of Kinetic Reaction Mechanisms; Springer: Berlin/Heidelberg, Germany, 2014; Volume 20, pp. 5–359. [Google Scholar]

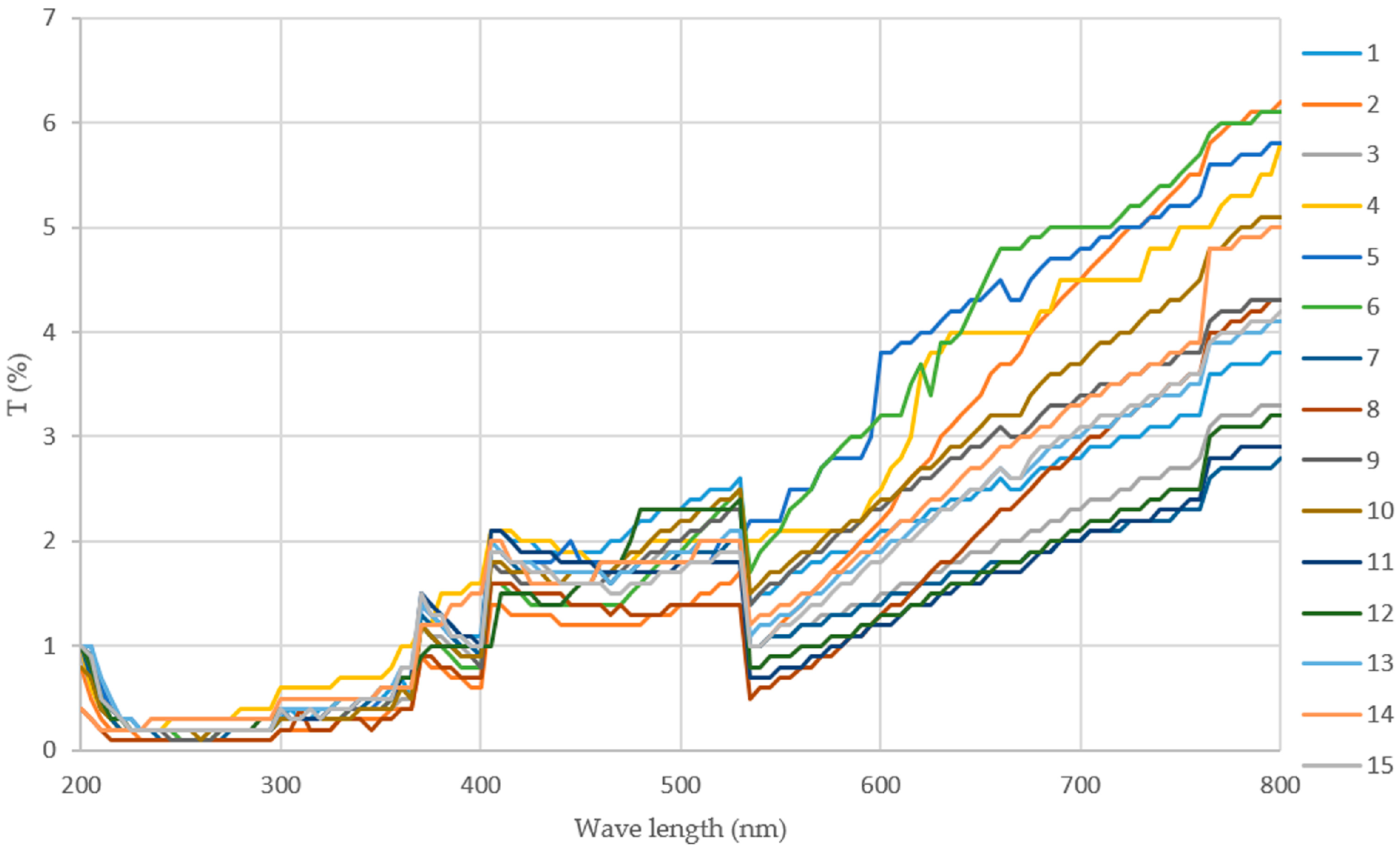

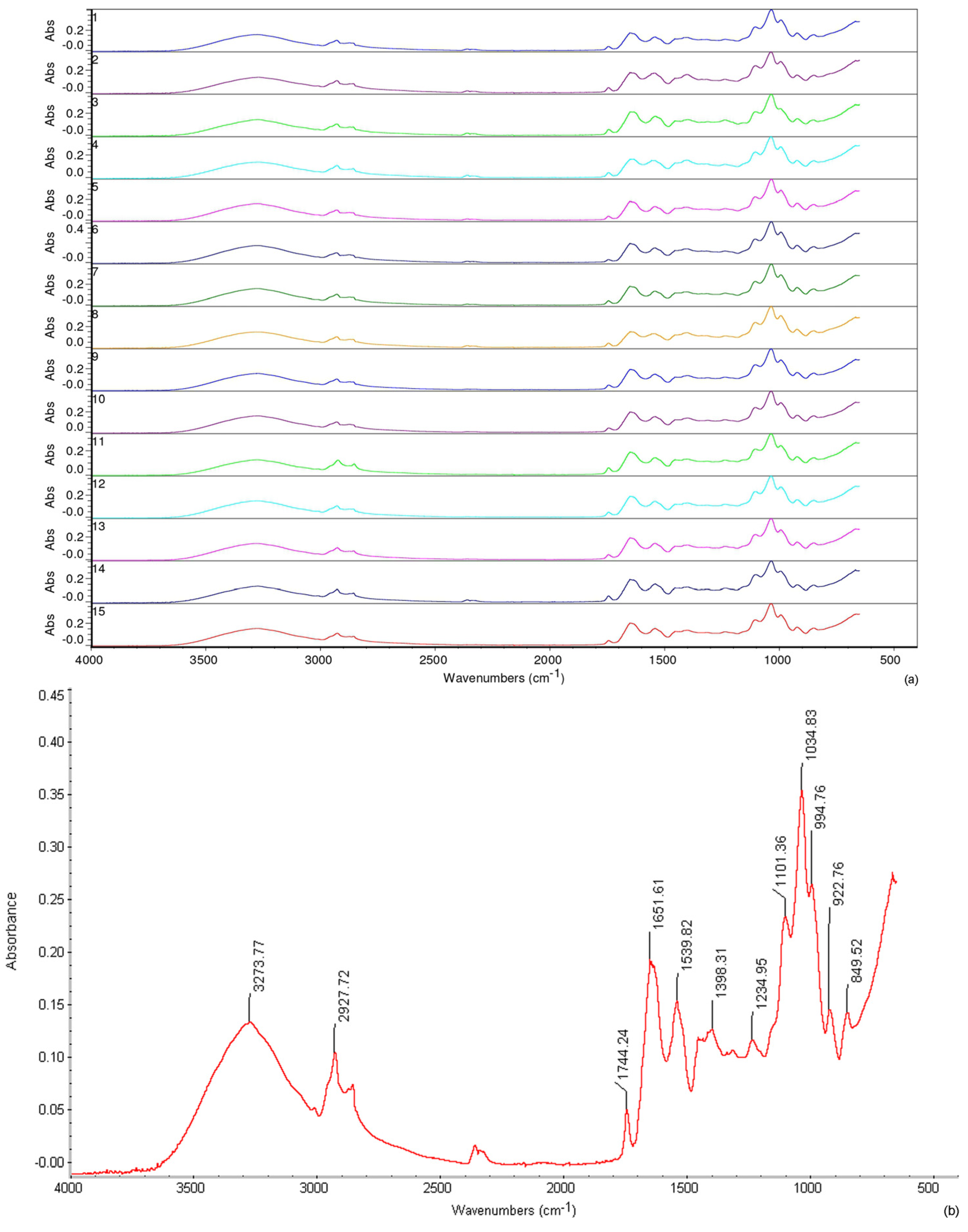
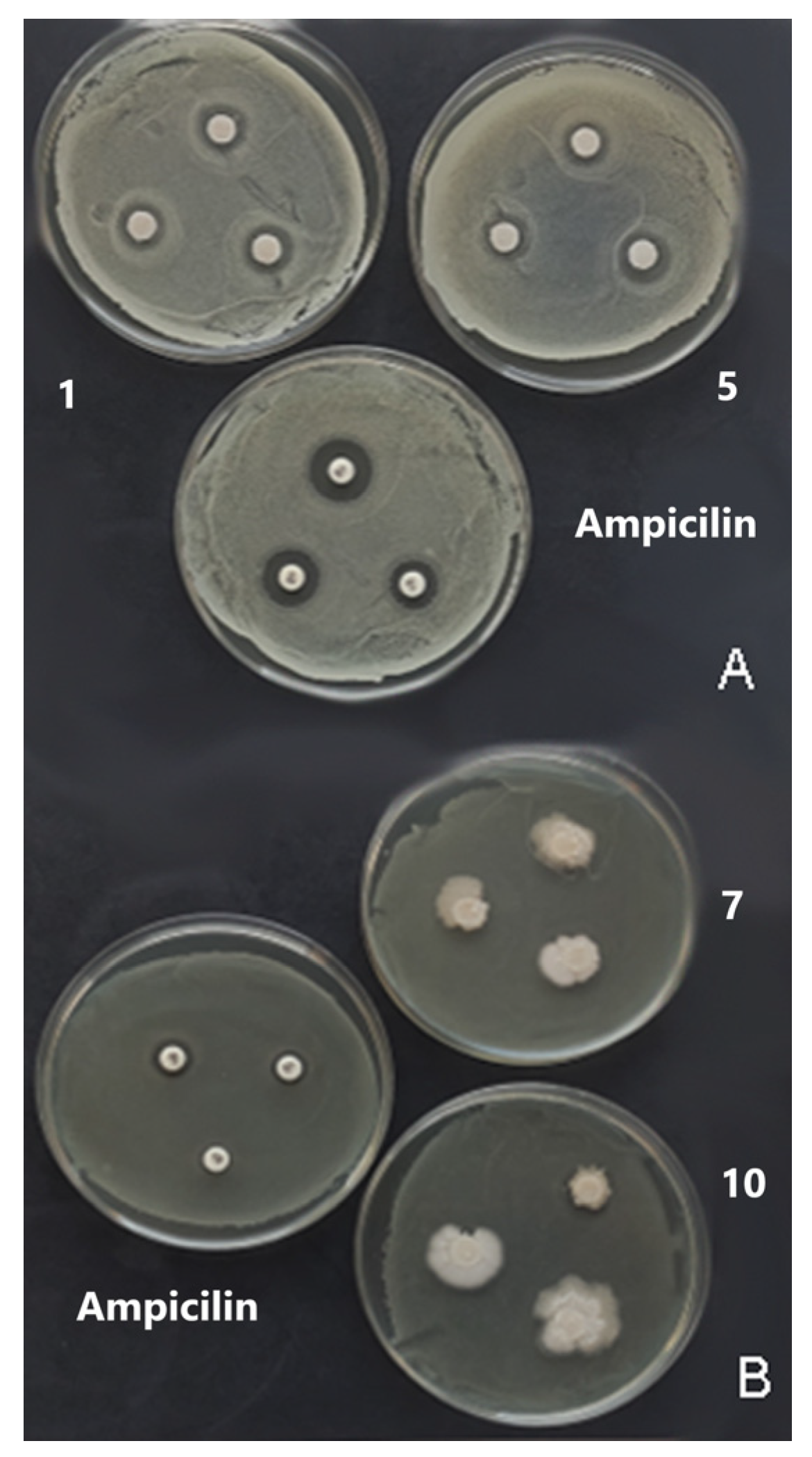
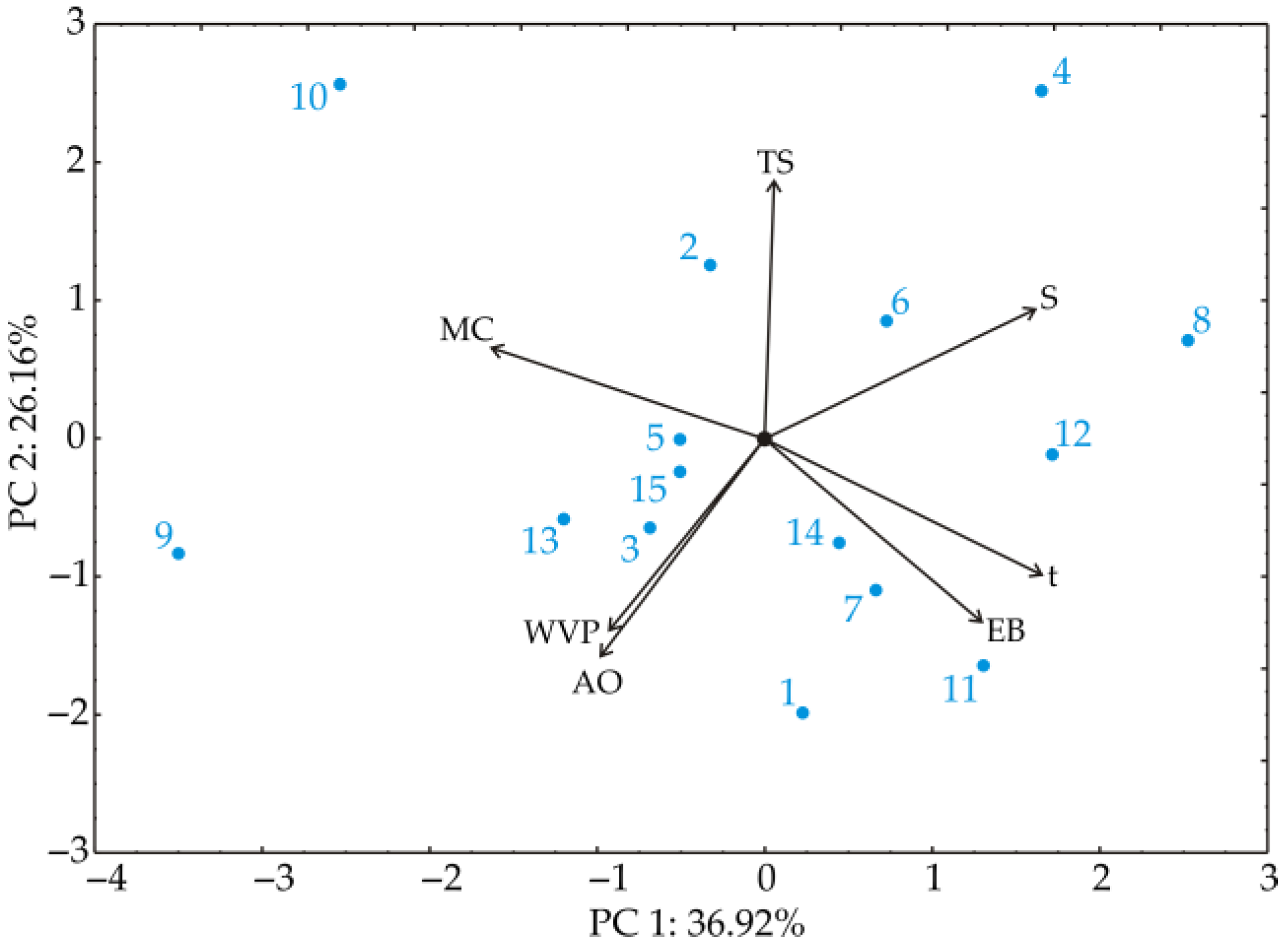


| RUN | pH | t° | c | MC | S | t | TS | EB | WVP | AO |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 60 | 4 | 25.93 ± 1.27 def | 33.02 ± 0.15 de | 228.33 ± 1.53 c | 0.54 ± 0.01 ab | 16.66 ± 1.5 f | 9.40 ± 0.09 e | 76.86 ± 5.21 e |
| 2 | 12 | 60 | 4 | 31.70 ± 0.09 gh | 40.92 ± 0.57 f | 221.33 ± 4.04 bc | 0.59 ± 0.02 bc | 5.75 ± 0.75 ab | 7.92 ± 0.03 cd | 29.13 ± 1.53 b |
| 3 | 8 | 100 | 4 | 24.69 ± 1.14 cde | 25.91 ± 2.85 bc | 238.67 ± 3.79 d | 0.96 ± 0.03 ef | 7.40 ± 2.15 abc | 8.21 ± 0.07 cde | 77.32 ± 3.45 e |
| 4 | 12 | 100 | 4 | 23.69 ± 2.03 cd | 44.57 ± 3.21 f | 246.67 ± 3.05 de | 1.85 ± 0.07 j | 9.85 ± 1.06 cde | 6.46 ± 0.05 b | 23.47 ± 1.89 ab |
| 5 | 8 | 80 | 3 | 29.12 ± 1.08 fg | 26.02 ± 1.67 bc | 245.33 ± 2.89 de | 0.93 ± 0.02 e | 9.69 ± 0.68 cde | 6.21 ± 0.05 ab | 70.55 ± 4.28 de |
| 6 | 12 | 80 | 3 | 28.25 ± 2.25 efg | 42.53 ± 0.32 f | 215.33 ± 3.51 ab | 0.53 ± 0.01 ab | 10.54 ± 1.31 cde | 7.84 ± 0.11 cd | 16.44 ± 1.02 a |
| 7 | 8 | 80 | 5 | 27.60 ± 1.30 defg | 29.48 ± 1.11 cde | 292.33 ± 3.51 g | 0.70 ± 0.01 d | 12.52 ± 0.57 de | 6.45 ± 0.38 b | 75.35 ± 3.62 e |
| 8 | 12 | 80 | 5 | 21.44 ± 2.00 bc | 41.42 ± 1.11 f | 294.67 ± 3.51 gh | 1.24 ± 0.02 h | 12.88 ± 0.63 e | 6.94 ± 0.06 bc | 23.60 ± 0.89 ab |
| 9 | 10 | 60 | 3 | 37.21 ± 1.53 i | 20.58 ± 0.45 a | 209.67 ± 2.52 a | 0.62 ± 0.01 c | 5.77 ± 0.80 ab | 12.26 ± 0.82 f | 69.56 ± 2.12 de |
| 10 | 10 | 100 | 3 | 34.28 ± 0.80 hi | 21.85 ± 1.52 ab | 270.33 ± 3.21 f | 1.76 ± 0.03 i | 4.34 ± 0.15 a | 5.10 ± 0.53 a | 69.25 ± 2.08 de |
| 11 | 10 | 60 | 5 | 17.35 ± 1.38 ab | 32.83 ± 1.78 de | 302.33 ± 4.51 h | 0.49 ± 0.02 a | 10.60 ± 0.98 cde | 8.02 ± 0.73 cd | 69.89 ± 3.05 de |
| 12 | 10 | 100 | 5 | 13.86 ± 1.53 a | 34.52 ± 3.05 e | 292.67 ± 2.52 gh | 1.03 ± 0.01 fg | 8.83 ± 0.56 cb | 5.86 ± 0.30 ab | 66.01 ± 3.56 d |
| 13 | 10 | 80 | 4 | 28.69 ± 0.98 efg | 24.82 ± 1.77 abc | 244.33 ± 4.51 de | 1.07 ± 0.02 g | 8.76 ± 1.16 cb | 9.10 ± 0.78 de | 78.04 ± 1.69 e |
| 14 | 10 | 80 | 4 | 14.60 ± 0.73 a | 26.62 ± 0.74 bc | 245.67 ± 2.52 de | 0.94 ± 0.02 ef | 10.30 ± 1.87 cde | 9.03 ± 0.65 de | 52.47 ± 1.98 c |
| 15 | 10 | 80 | 4 | 28.17 ± 0.39 efg | 28.10 ± 1.91 cd | 251.00 ± 2.00 d | 0.97 ± 0.01 ef | 9.32 ± 0.66 cd | 8.96 ± 0.42 de | 55.16 ± 3.11 c |
| Zone of Inhibition (mm) against Bacteria | ||||
|---|---|---|---|---|
| Gram-Negative Bacteria | Gram-Positive Bacteria | |||
| Sample Number | Escherichia coli ATCC 10536 | Salmonella typhimurium ATCC 14028 | Staphylococcus aureus ATCC 25923 | Listeria monocytogenes ATCC 19111 |
| 1. | - | - | 10 | - |
| 2. | - | - | - | - |
| 3. | - | - | 12 | - |
| 4. | - | - | - | - |
| 5. | - | - | 10 | - |
| 6. | - | - | - | - |
| 7. | - | - | 8 | - |
| 8. | - | - | - | - |
| 9. | - | - | - | - |
| 10. | - | - | - | - |
| 11. | - | - | 9 | - |
| 12. | - | - | - | - |
| 13. | - | 9 | 10 | - |
| 14. | - | 9 | 10 | - |
| 15. | - | 8 | 9 | - |
| Antibiotic: | ||||
| Ampicilin 10 mcg | 9 | 9 | 15 | 10 |
| Parameters | No. of Neurons in Hidden Layer | Performance | Error | Train. Algorithm (BFGS) | Hidden Activation | Output Activation | ||
|---|---|---|---|---|---|---|---|---|
| Train. | Test | Train. | Train. | |||||
| MC | 5 | 0.951 | 0.559 | 0.715 | 27.243 | 10 | Tanh | Tanh |
| S | 9 | 1.000 | 0.992 | 0.243 | 0.661 | 218 | Tanh | Tanh |
| t | 3 | 1.000 | 0.744 | 2.167 | 176.711 | 11 | Exp. | Exp. |
| TS | 10 | 0.999 | 0.924 | 0.000 | 0.005 | 51 | Log. | Exp. |
| EB | 6 | 0.971 | 0.898 | 0.581 | 0.851 | 32 | Exp. | Exp. |
| WVP | 4 | 0.997 | 0.982 | 0.029 | 0.089 | 37 | Exp. | Log. |
| AO | 3 | 0.999 | 0.686 | 30.478 | 78.930 | 5 | Log. | Log. |
| Response | χ2 | RMSE | MBE | MPE | SSE | AARD | r2 |
|---|---|---|---|---|---|---|---|
| MC | 16.691 | 3.947 | −1.527 | 11.625 | 233.670 | 11.625 | 0.688 |
| S | 0.760 | 0.842 | 0.240 | 1.457 | 10.638 | 1.457 | 0.989 |
| t | 278.927 | 16.135 | 0.600 | 4.967 | 3.9 × 103 | 4.967 | 0.809 |
| TS | 0.008 | 0.089 | 0.000 | 9.639 | 0.118 | 9.639 | 0.952 |
| EB | 0.242 | 0.475 | 0.009 | 2.961 | 3.389 | 2.961 | 0.976 |
| WVP | 0.096 | 0.299 | 0.113 | 3.107 | 1.345 | 3.107 | 0.977 |
| AO | 141.448 | 11.490 | −1.321 | 24.116 | 1980.275 | 24.116 | 0.727 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuput, D.; Pezo, L.; Rakita, S.; Spasevski, N.; Tomičić, R.; Hromiš, N.; Popović, S. Camelina sativa Oilseed Cake as a Potential Source of Biopolymer Films: A Chemometric Approach to Synthesis, Characterization, and Optimization. Coatings 2024, 14, 95. https://doi.org/10.3390/coatings14010095
Šuput D, Pezo L, Rakita S, Spasevski N, Tomičić R, Hromiš N, Popović S. Camelina sativa Oilseed Cake as a Potential Source of Biopolymer Films: A Chemometric Approach to Synthesis, Characterization, and Optimization. Coatings. 2024; 14(1):95. https://doi.org/10.3390/coatings14010095
Chicago/Turabian StyleŠuput, Danijela, Lato Pezo, Slađana Rakita, Nedeljka Spasevski, Ružica Tomičić, Nevena Hromiš, and Senka Popović. 2024. "Camelina sativa Oilseed Cake as a Potential Source of Biopolymer Films: A Chemometric Approach to Synthesis, Characterization, and Optimization" Coatings 14, no. 1: 95. https://doi.org/10.3390/coatings14010095
APA StyleŠuput, D., Pezo, L., Rakita, S., Spasevski, N., Tomičić, R., Hromiš, N., & Popović, S. (2024). Camelina sativa Oilseed Cake as a Potential Source of Biopolymer Films: A Chemometric Approach to Synthesis, Characterization, and Optimization. Coatings, 14(1), 95. https://doi.org/10.3390/coatings14010095







