The Preparation and Characterization of an Alginate–Chitosan-Active Bilayer Film Incorporated with Asparagus (Asparagus officinalis L.) Residue Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Preparation of Asparagus Residue Extract
2.4. Quantification of Total Phenols and Total Flavonoids
2.4.1. Quantification of Total Phenols
2.4.2. Total Flavonoid Quantification
2.5. Preparation of Bilayer Film
2.6. Bilayer Film Characterization
2.6.1. Thickness
2.6.2. Appearance and Color
2.6.3. Light Transmission and Opacity
2.6.4. Mechanical Properties of Films
2.6.5. Morphology of Film
2.6.6. Fourier Transform Infrared Spectroscopy (FTIR) of Films
2.6.7. Thermogravimetric Analysis (TGA)
2.6.8. Determination of Total Phenols of Films
2.6.9. Determination of Total Flavonoids of Films
2.7. Determination of the Antioxidant Activity of Films
2.7.1. ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Assay
2.7.2. DPPH Assay (2,2-diphenyl-1-picryl-hydrazyl)
2.7.3. Ferric-Reducing Power Assay (FRAP)
2.8. Determination of Antibacterial Activity of Films
2.8.1. Microbial Strains and Inoculum Preparation
2.8.2. Resazurin Cell Viability Assay
2.8.3. Microscopic Analysis
2.9. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Content of Total Phenols and Total Flavonoids from Asparagus Extract Residues
3.2. Bilayer Film Characterization
3.2.1. Thickness
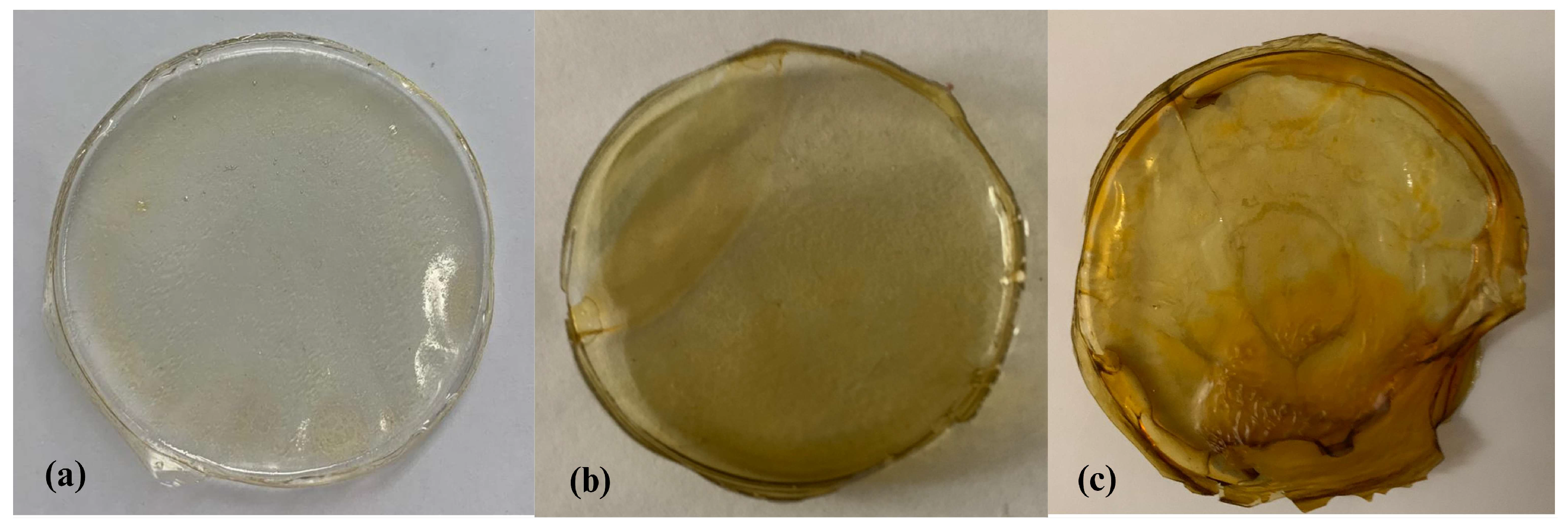
3.2.2. Appearance and Color of Films
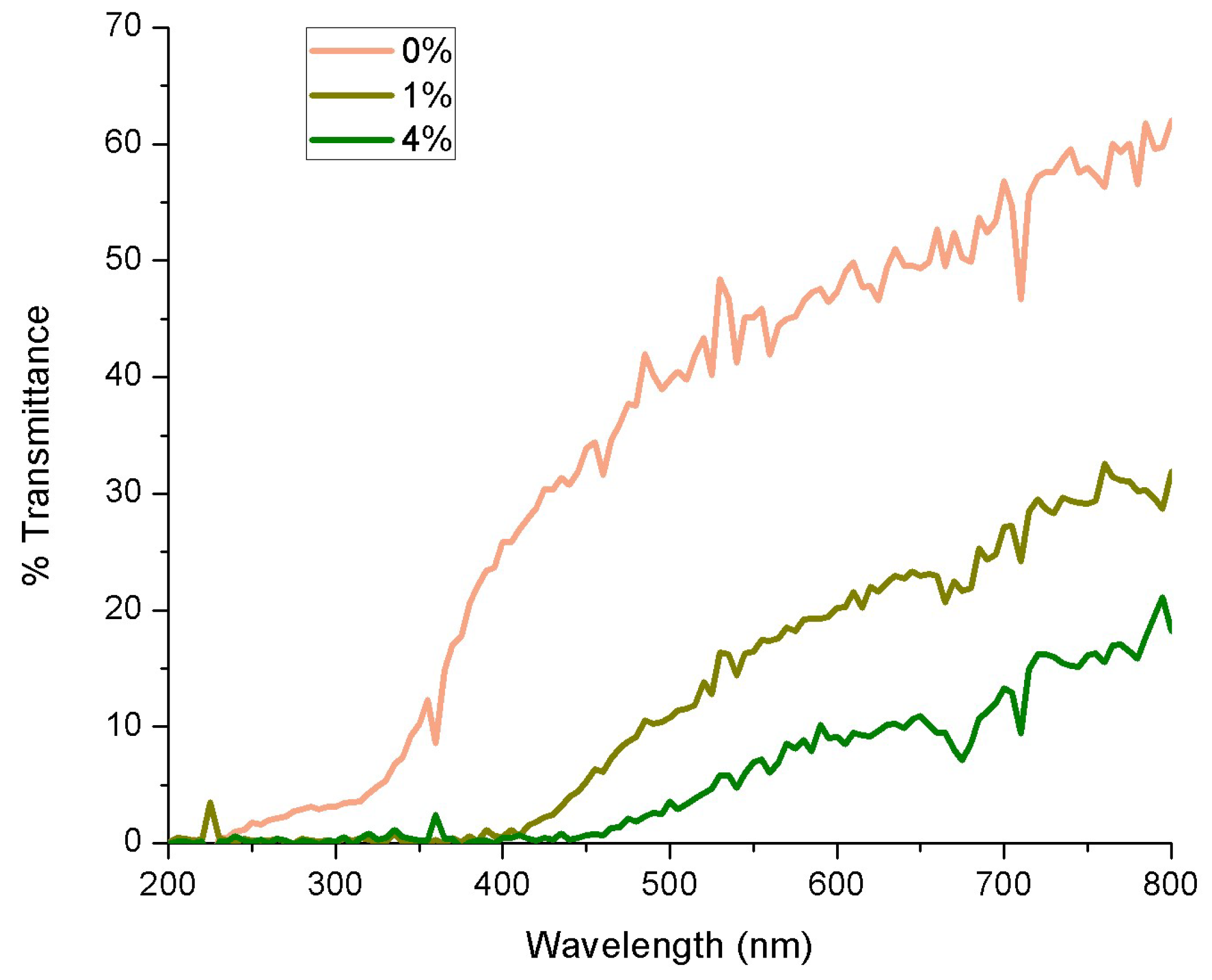
3.2.3. Light Transmission and Opacity
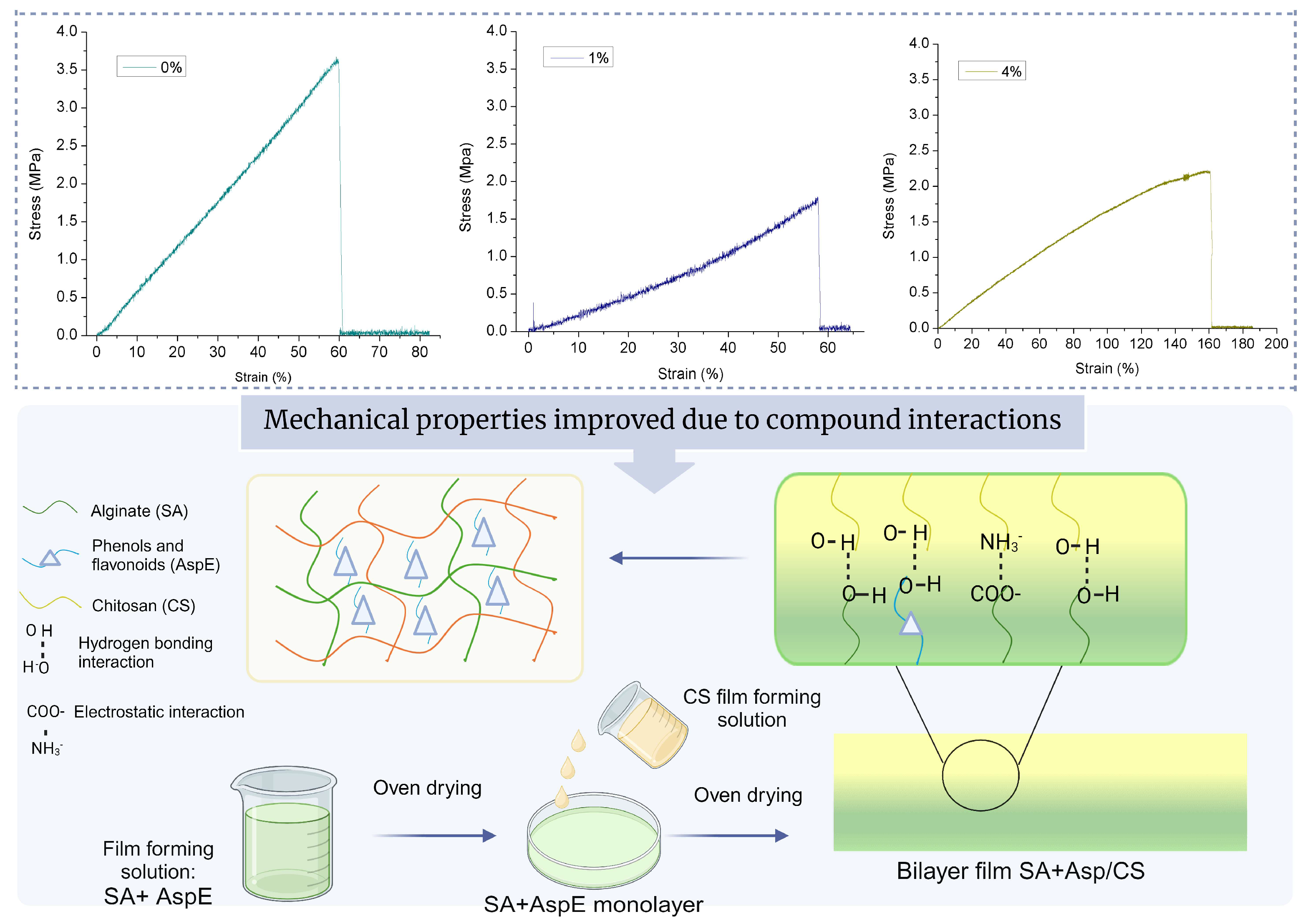
3.2.4. Mechanical Properties
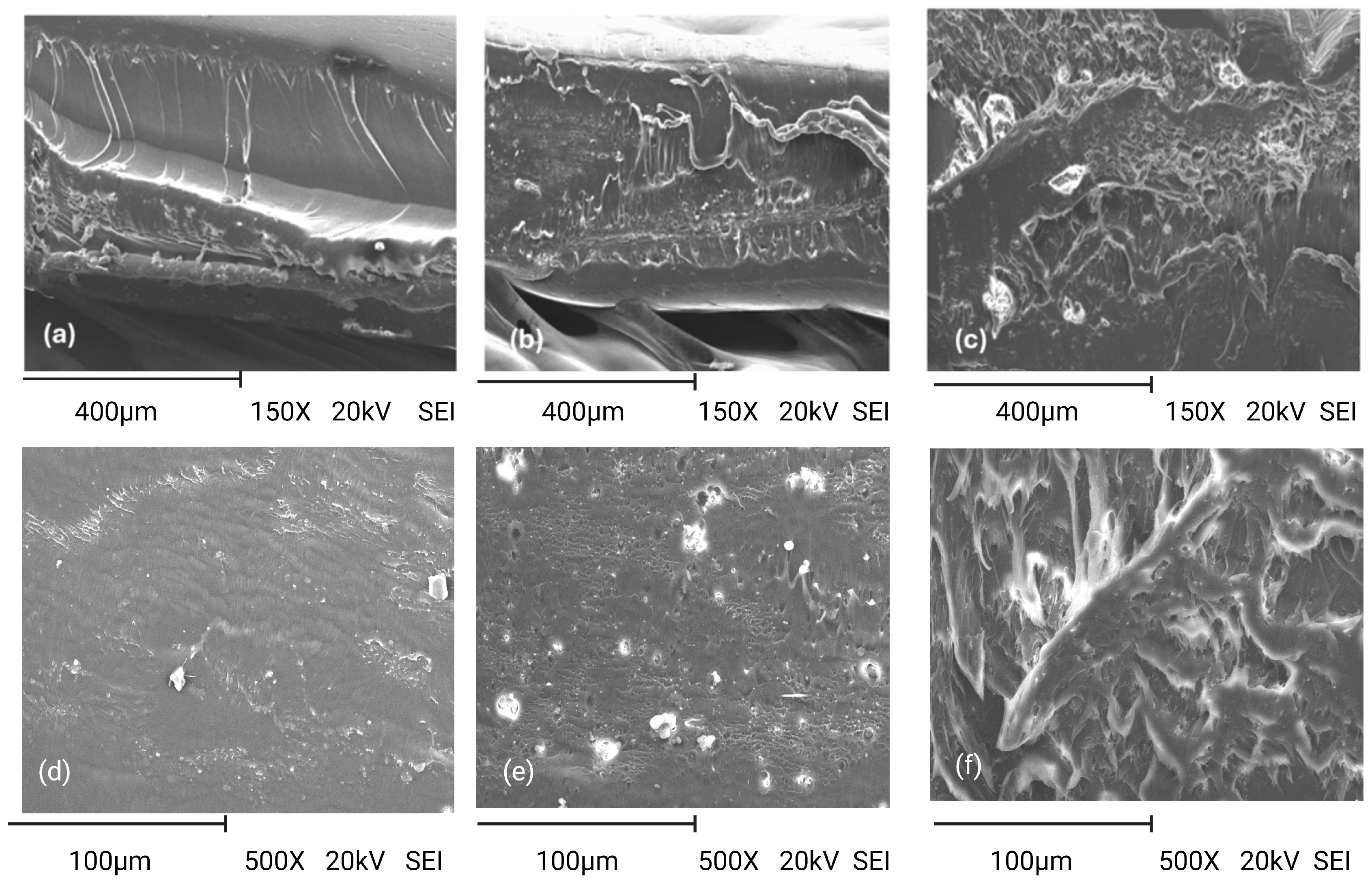
3.2.5. Morphology of Film
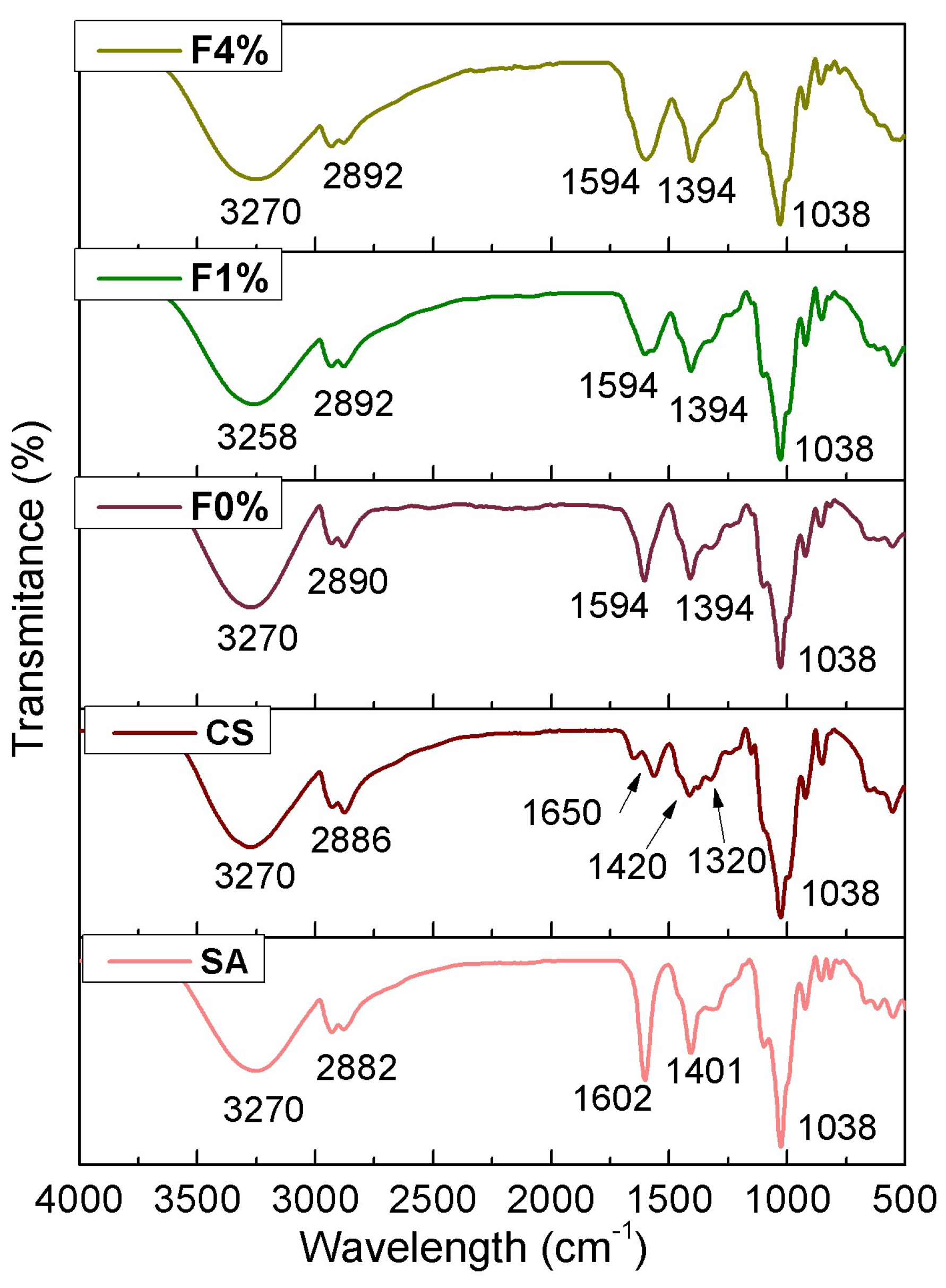
3.2.6. FTIR Analysis of Films
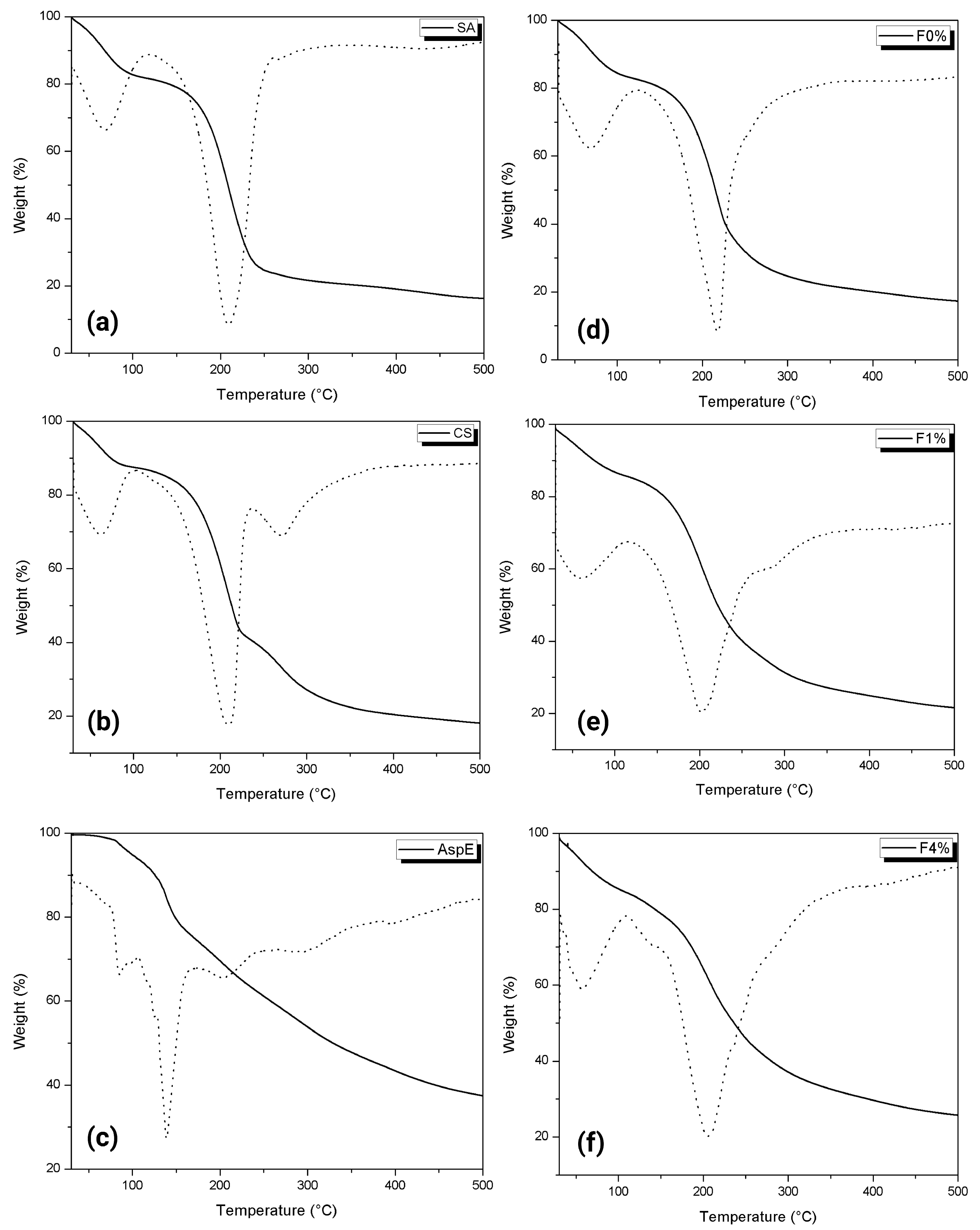
3.2.7. Thermogravimetric Analysis (TGA)
3.2.8. Determination of Total Phenols and Total Flavonoids of Films
3.2.9. Determination of the Antioxidant Activity of Films
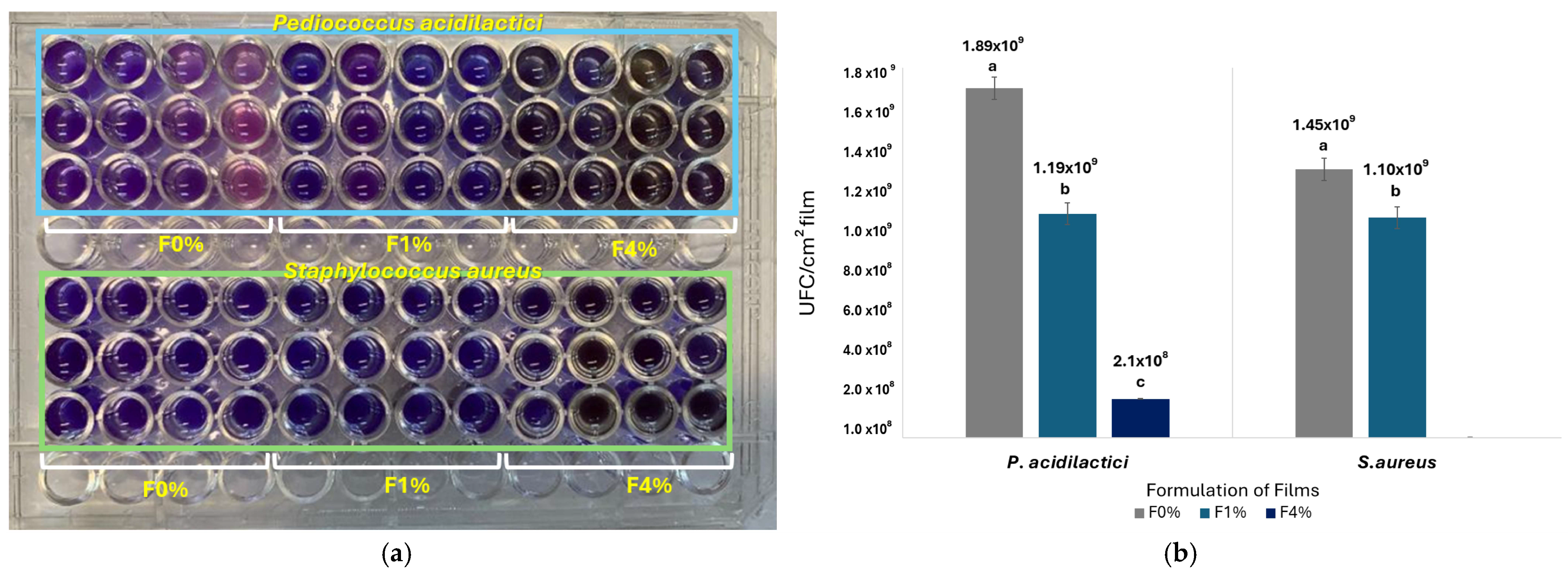
3.2.10. Determination of Antibacterial Activity by Cell Viability Analysis
Resazurin Cell Viability Assay
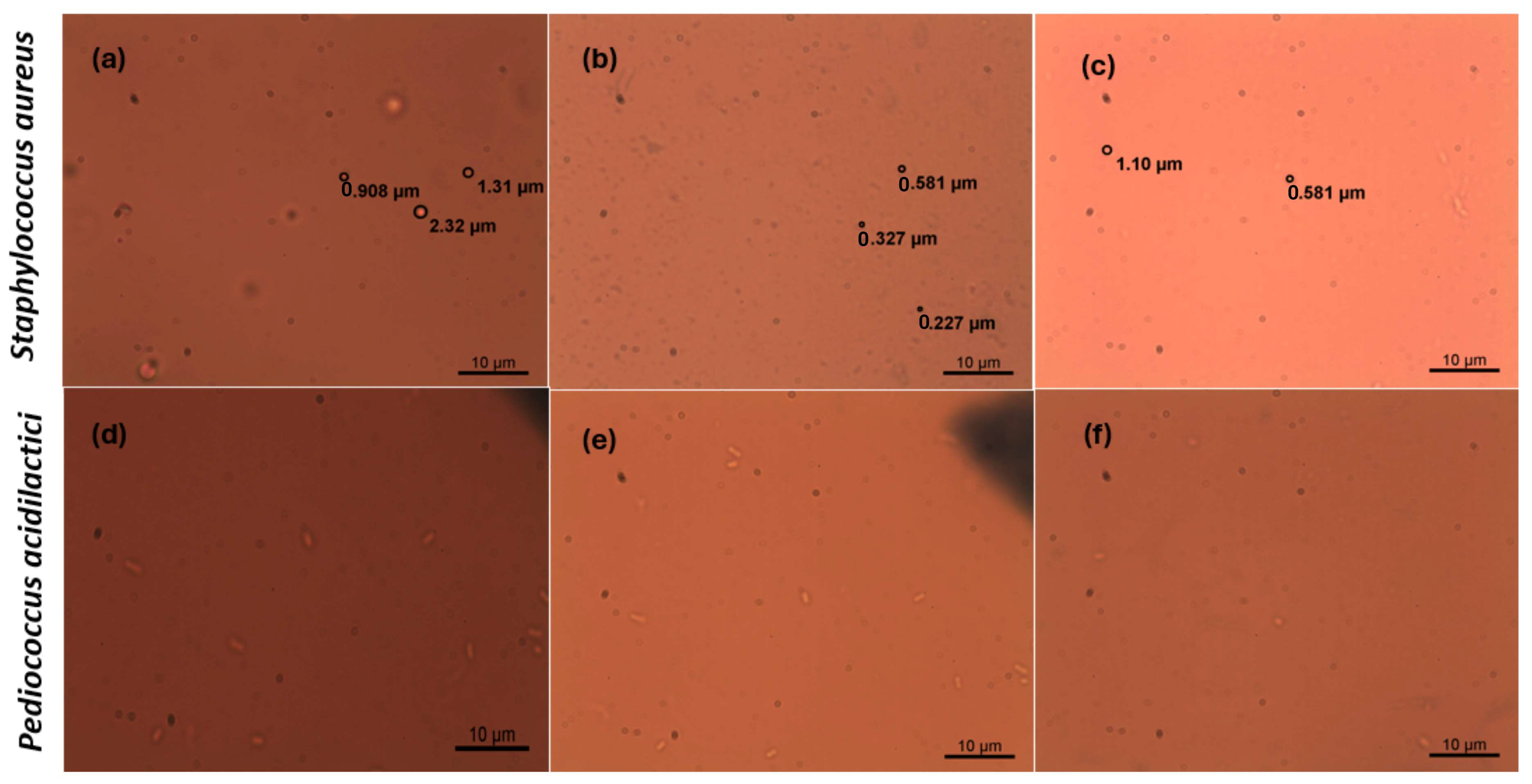
Microscopic Analysis
- Morphometric analysis
- Cell Membrane Integrity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Birch, J.; Xie, C.; Yang, H.; Dias, G.; Kong, L.; Bekhit, A.D. Optimization of extraction parameters of antioxidant activity of extracts from New Zealand and Chinese Asparagus officinalis L. root cultivars. Ind. Crops Prod. 2018, 119, 191–200. [Google Scholar] [CrossRef]
- Santiago, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Identifying the sustainability route of asparagus co-product extraction: From waste to bioactive compounds. Food Bioprod. Process. 2021, 129, 176–189. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Asparagus (Asparagus officinalis): Processing effect on nutritional and phyto-chemical composition of spear and hard-stem byproducts. Trends Food Sci. Technol. 2019, 93, 1–11. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M. Caracterización de Componentes Bioactivos del Espárrago Verde: Obtención de Ingredientes Funcionales a Partir de Los Subproductos Generados Durante su Transformación Industrial. Ph.D. Thesis, Universidad de Córdoba, Córdoba, Spain, 2009. [Google Scholar]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; Fité, J.L.B.; Paseiro-Losada, P.; Cruz, J.M. Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiang, K.; Ou, J.; Huang, C.; Liu, F.; Zheng, J.; Ou, S. Preparation of acrolein/resveratrol-grafted chitosan-sodium alginate bilayer films and their antibacterial and antioxidant activities. Food Hydrocoll. 2024, 149, 109601. [Google Scholar] [CrossRef]
- Oli, S.; Kumar Chauhan, H.; Kumar Bisht, A.; Agnihotri, S.; Dobhal, P. Bioactive compound, polyphenol content, and antioxidant activity of Asparagus racemosus Linn. root extract. Nat. Prod. Res. 2023, 1, 1–6. [Google Scholar] [CrossRef]
- Yan, J.; Li, M.; Wang, H.; Lian, X.; Fan, Y.; Xie, Z.; Niu, B.; Li, W. Preparation and property studies of chitosan-PVA biodegradable antibacterial multilayer films doped with Cu2O and nano-chitosan composites. Food Control 2021, 126, 108049. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Hamed, Y.S.; Kamel, R.M.; Shawir, S.M.S.; Sakr, H.; Ali, M.; Ammar, A.; Saleh, M.N.; El Fadly, E.; Salama, M.A.; et al. Enhanced physical properties, antioxidant and antibacterial activity of bio-composite films composed from carboxymethyl cellulose and polyvinyl alcohol incorporated with broccoli sprout seed extract for butter packaging. Int. J. Biol. Macromol. 2024, 255, 128346. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Yu, D.; Regenstein, J.M.; Dong, J.; Chen, W.; Xia, W. Chitosan/zein bilayer films with one-way water barrier characteristic: Physical, structural and thermal properties. Int. J. Biol. Macromol. 2022, 200, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Sharma, S.; Duffy, B.; Pathania, S.; Jaiswal, A.K.; Jaiswal, S. An active biodegradable layer-by-layer film based on chitosan-alginate-TiO2 for the enhanced shelf life of tomatoes. Food Packag. Shelf Life 2022, 34, 100971. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate-based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Gates, S.J.; Shukla, A. Layer-by-layer assembly of readily detachable chitosan and poly(acrylic acid) polyelectrolyte multilayer films. J. Polym. Sci. B Polym. Phys. 2016, 55, 127–131. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Beltran, S.; Sanz, M.T.; Garcia-Tojal, J.; Trigo-lopez, M. Designing bilayered composite films by direct agar/chitosan and citric acid-crosslinked PVA/agar layer-by-layer casting for packaging applications. Food Hydrocoll. 2023, 144, 108987. [Google Scholar] [CrossRef]
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Xiang, H.Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 2015, 52, 2690–2700. [Google Scholar] [CrossRef]
- Maksimović, Z.; Malencic, Ð.; Kovacevi, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Chen, C.; Mokhtar, R.A.M.; Sani, M.S.A.; Noor, N.Q.I.M. The Effect of Maturity and Extraction Solvents on Bioactive Compounds and Antioxidant Activity of Mulberry (Morus alba) Fruits and Leaves. Molecules 2022, 27, 2406. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Schimtz, R.; Brito, C.; Fuentes, R. Lactic acid bacteria in an alginate film inhibit Listeria monocytogenes growth on smoked salmon. Food Control 2011, 22, 485–489. [Google Scholar] [CrossRef]
- Mora-Sánchez, M.; Hernández-Carranza, P.; Ramírez-López, C.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Developing active chitosan-based edible film for extending the shelf life of guacamole. Front. Sustain. Food Syst. 2023, 7, 1254337. [Google Scholar] [CrossRef]
- Wessling, C.; Nielsen, T.; Leufvén, A. The influence of α-tocopherol concentration on the stability of linoleic acid and the properties of low-density polyethylene. Packag. Technol. Sci. 2000, 13, 19–28. [Google Scholar] [CrossRef]
- ASTM D1708-96; Standard Test Method for Tensile Properties of Plastic by Use of Microtensile Specimens. In Annual Book of ASTM Standards. American Society for Testing and Material: Philadelphia, PA, USA, 1992.
- Prior, R.L.; Wu, L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Arrua, D.; Strumia, M.C.; Nazareno, M.A. Immobilization of caffeic acid on a polypropylene film: Synthesis and antioxidant properties. J. Agric. Food Chem. 2010, 58, 9228–9234. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Tech. Metal. 2005, 40, 255–260. [Google Scholar]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, S0076–S6879. [Google Scholar]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; RodriguezFélix, F.; Castillo-Ortega, M.M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; García-Alonso, A.; Rodríguez-Arcos, R.; Castro, I.; Alba, C.; Rodríguez, J.M.; Goñi, I. Nutritional composition of green asparagus (Asparagus officinalis L.), edible part and by-products, and assessment of their effect on the growth of human gut-associated bacteria. Food Res. Int. 2023, 163, 112284. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.P.; Tang, J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007, 105, 101–106. [Google Scholar] [CrossRef]
- JIS Z 1707; General Rules of Plastic Films for Food Packaging. Japanese Standards Association: Minato-ku, Tokyo, Japan, 2019.
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 961–967. [Google Scholar] [CrossRef]
- Alfonso-Arce, C. Caracterización de Películas Comestibles de Quitosano y la Afectación de las Propiedades por Aplicación de Aceites Esenciales. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. [Google Scholar]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/sodium alginate multilayer composite film cross-linked with green tea extract for active food packaging application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131013. [Google Scholar] [CrossRef]
- Zamudio-Flores, P.B.; Ochoa-Reyes, E.; Ornelas-Paz, J.d.J.; Tirado-Gallegos, J.M.; Bello-Pérez, L.A.; Rubio-Ríos, A.; Cárdenas-Felix, R.G. Caracterización fisicoquímica, mecánica y estructural de películas de almidones oxidados de avena y plátano adicionadas con betalaínas. Agrociencia 2015, 49, 483–498. [Google Scholar]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Ramirez-Pimentel, J.G.; Raya-Pérez, J.C.; Jímenez-Alvarado, R.; Hernández-Fuentes, A.D. Evaluación del color y propiedades antioxidantes de películas comestibles de gelatina incorporadas con aceite rico en licopeno y β-caroteno. Investig. Y Desarro. En. Cienc. Y Tecnol. De. Aliment. 2018, 3, 411–416. [Google Scholar]
- Corsello, F.A. Preparación y Caracterización de Películas de Quitosano Modificadas con Nanocristales de Celulosa. Master’s Thesis, Universidad Nacional de la Plata, Buenos aires, Argentina, 2015. [Google Scholar]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Yao, Y.; Tao, J.; Zou, J.; Zhang, B.; Li, T.; Dai, J.; Zhu, M.; Wang, S.; Fu, K.K.; Henderson, D.; et al. Light management in plastic-paper hybrid substrate towards high-performance optoelectronics. Energy Environ. Sci. 2016, 9, 2278–2285. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz, Ł. Recent progress on UV-light barrier food packaging films—A systematic review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- Hernández-Gómez, E.; Madera-Santana, T.J.; Quintana-Owen, P.; Ortiz-Vázquez, E.; Ramón Sierra, J.M.; Vargas y Vargas, M.L. Propiedades fisicoquímicas y bioactivas de películas comestibles basadas en alginato y miel Melipona. Biotecnica 2024, 1, 16–24. [Google Scholar]
- Bierhalz, A.C.K.; Westin, C.B.; Moraes, A.M. Comparison of the properties of membranes produced with alginate and chitosan from mushroom and from shrimp. Int. J. Biol. Macromol. 2016, 91, 496–504. [Google Scholar] [CrossRef]
- McHugh, T.H.; Aujard, J.F.; Krochta, J.M. Plasticized Whey Protein Edible Films: Water Vapor Permeability Properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Agostini de Moraes, M.; Cocenza, D.S.; Cruz Vasconcellos, F.d.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar] [CrossRef]
- Parris, T.; Coffin, D.R.; Joubran, R.F.; Pessen, H. Composition factors affecting the water vapor permeability and tensile properties of hydrophilic films. J. Agric. Food Chem. 1995, 43, 1432–1435. [Google Scholar] [CrossRef]
- Queirós, L.; Sousa, S.; Duarte, A.; Domingues, F.; Ramos, A. Development of carboxymethylxylan films with functional properties. J. Food Sci. Technol. 2017, 54, 9–17. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef]
- Rodriguez, M.; Oses, J.; Ziani, K.; Mate, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- Balasubramaniam, M.P.; Murugan, P.; Chenthamara, D.; Ramakrishnan, S.G.; Salim, A.; Lin, F.H.; Robert, B.; Subramaniam, S. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater. Today Commun. 2020, 25, 101510. [Google Scholar] [CrossRef]
- Arifin, H.R.; Djali, M.; Nurhadi, B.; Azlin-Hasim, S.; Masruchin, N.; Vania, P.A.; Hilmi, A. Corn Starch-Based Bionanocomposite Film Reinforced With ZnO Nanoparticles and Different Types of Plasticizers. Front. Sustain. Food Syst. 2022, 6, 886219. [Google Scholar] [CrossRef]
- Santoso, B.; Hazirah, R.; Priyanto, G.; Hermanto, D.; Sugito, D. Utilization of Uncaria gambir Roxb filtrate in the formation of bioactive edible films based on corn starch. Food Sci. Technol. Int. 2019, 39, 837–842. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.J.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Cira-Chávez, L.A.; Del-Toro-Sánchez, C.L.; Shirai, K. Physicochemical and Antioxidant Properties of Chitosan Films Incorporated with Cinnamon Oil. Int. J. Polym. Sci. 2015, 1, 8. [Google Scholar] [CrossRef]
- Aziman, N.; Kian, L.K.; Jawaid, M.; Sanny, M.; Alamery, S. Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers 2021, 13, 391. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, Modification and Application of Titanium Dioxide nanoparticles: A Review. Nanoscale 2022, 18, 14. [Google Scholar] [CrossRef]
- Paulino, L.G.S.; Avila, L.B.; Moraes, C.C.; Khan, M.R.; Manoharadas, S.; Reis, G.S.D.; Dotto, G.L.; Da Rosa, G.S. Double-Layer Membranes of Chitosan and Sodium Alginate Added to Natural Olive Leaf Extract for Potential Use in Skin Lesions. Resources 2023, 12, 97. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Mojerlou, F.; Zare, E.N. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions. Carbohydr. Polymers. 2014, 106, 34–41. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożnaa, M.; Filipkowskab, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from aqueous solutions by chitosan immobilized in alginate beads. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Elmotasem, H. Chitosan—Alginate blend films for the transdermal delivery of meloxicam. Asian J. Pharm. Sci. 2008, 3, 12–29. [Google Scholar]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef]
- Kloster, G.A.; Marcovich, N.E.; Mosiewicki, M.A. Composite films based on chitosan and nanomagnetite. Eur. Polym. J. 2015, 66, 386–396. [Google Scholar] [CrossRef]
- Waldi, J. Production of Bioplastic Poly-ß-Hydroxyalkanoate (PHA) Produced by Rastonia eutropha Using Hydrolyzed Sago Starch Substrate with Isopropyl Palmitate as Plasticizer. Undergraduate Thesis, Bogor Agricultural University, Jawa Barat, Indonicia, 2007. [Google Scholar]
- Xu, Y.; Liu, X.; Jiang, Q.; Yu, D.; Xu, Y.; Wang, B.; Xia, W. Development and properties of bacterial cellulose, curcumin, and chitosan composite biodegradable films for active packaging materials. Carbohydr. Polym. 2021, 260, 117778. [Google Scholar] [CrossRef]
- Dias, M.R.; Leão, R.C.; De Souza Costa, h. obtenção e caracterização de microesferas de alginato/vidro bioativo. Rev. Eletrônica Perspect. Da Ciência E Tecnol. 2020, 12, 122–137. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, X.; Wang, S.; Yin, C.; Gao, X.; Xiong, G.; Xu, X.; Qi, J.; Mei, L. Emulsified blend film based on konjac glucomannan/carrageenan/ camellia oil: Physical, structural, and water barrier properties. Carbohydr. Polym. 2021, 251, 117100. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Pérez-Larios, A.; Ruvalcaba-Gómez, J.M.; Sánchez-Burgos, J.A.; Romero-Toledo, R.; Montalvo-González, E. Funcionalización de los recubrimientos a base de quitosano para la conservación postcosecha de frutas y hortalizas. TIP. Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–14. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Kim, S.W. Free radical scavenging activities of different deacetylated chitosans using an ESR spectrometer. Carbohydr. Polym. 2004, 55, 17–22. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Mugnaini, G.; Bonini, M.; Gentile, L.; Panza, O.; Del Nobile, M.A.; Conte, A.; Esposito, R.; D’Errico, G.; Moccia, F.; Panzella, L. Effect of design and molecular interactions on the food preserving properties of alginate/pullulan edible films loaded with grape pomace extract. J. Food Eng. 2024, 361, 111716. [Google Scholar] [CrossRef]
- Kanniah, P.; Chelliah, P.; Thangapandi, J.; Gnanadhas, G.; Mahendran, V.; Robert, M. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver based chitosan nanocomposite. Int. J. Biol. Macromol. 2021, 189, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Njoku, D.I.; Guo, Q.; Dai, W.; Chen, J.; Mao, G.; Sun, Q.; Sun, H.; Peng, Y.K. The multipurpose application of resazurin in micro-analytical techniques: Trends from the microbial, catalysis and single molecule detection assays. Trends Anal. Chem. 2023, 167, 117288. [Google Scholar] [CrossRef]
- Filipiak, Y.; Larocca, C. Utilización del azul tripán para diferenciar ovocitos bovinos vivos y muertos en fertilización in vitro. Arch. Zootec. 2012, 61, 309–312. [Google Scholar] [CrossRef][Green Version]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutson, A.; Vasilets, V.; Friedman, G. Floating Electrode Dielectric Barrier Discharge Plasma in Air Promoting Apoptotic Behavior in Melanoma Skin Cancer Cell Lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Hernández-Abril, P.A.; López-Meneses, A.K.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M.; Luque-Alcaraz, A.G. Cellular Internalization and Toxicity of Chitosan Nanoparticles Loaded with Nobiletin in Eukaryotic Cell Models (Saccharomyces cerevisiae and Candida albicans). Materials 2024, 17, 1525. [Google Scholar] [CrossRef]
- Lewis-Luján, L.M.; Rosas-Burgos, E.C.; Ezquerra-Brauer, J.M.; Burboa-Zazueta, M.G.; Assanga, S.B.I.; Del Castillo-Castro, T.; Penton, G.; Plascencia-Jatomea, M. Inhibition of Pathogenic Bacteria and Fungi by Natural Phenoxazinone from Octopus Ommochrome Pigments. J. Microbiol. Biotechnol. 2022, 32, 989–1002. [Google Scholar] [CrossRef]

| Film | |||
|---|---|---|---|
| F0% | F1% | F4% | |
| Thickness (mm) | 0.14 ± 0.009 a | 0.15 ± 0.006 b | 0.25 ± 0.606 c |
| L* | 86.78 ± 1.01 a | 76.04 ± 0.43 b | 63.04 ± 3.02 c |
| a* | −0.65 ± 0.17 c | −3.12 ± 0.53 b | 7.53 ± 1.8 a |
| b* | 6.69 ± 1.2 c | 41.47 ± 0.4 b | 53.68 ± 9.7 a |
| °Hue | 95.38 ± 1.2 a | 94.33 ± 0.8 a | 81.24 ± 4.2 b |
| C* | 6.72 ± 2.2 c | 41.59 ± 1.5 b | 54.29 ± 12.8 a |
| Opacity (mm−1) | 0.96 ± 0.01 a | 1.56 ± 0.006 b | 1.95 ± 0.17 c |
| Film | Elongation (%) | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| F0% | 44.9 ± 0.31 b | 1.83 ± 0.09 a | 4.43 ± 0.07 a |
| F1% | 30.7 ± 2.09 b | 0.76 ± 0.08 b | 2.02 ± 0.01 b |
| F4% | 92.9 ± 1.00 a | 1.12 ± 0.00 ab | 1.43 ± 0.00 b |
| Film | Determination | |
|---|---|---|
| Flavonoids | Phenols | |
| - | (mg QE/g film) | (mg GAE/g film) |
| F0% | 27.97 ± 1.00 c | 3.90 ± 1.00 c |
| F1% | 88.40 ± 5.01 b | 26.32 ± 4.00 b |
| F4% | 108.77 ± 10.00 a | 30.12 ± 2.00 a |
| Film | Antioxidant Activity | ||
|---|---|---|---|
| ABTS | DPPH | FRAP | |
| - | (% ARA) | (mg TE/g film) | |
| F0% | 7.84 c | 6.98 c | 0.12 c |
| F1% | 43.96 b | 51.05 b | 154.6 b |
| F4% | 48.76 a | 61.58 a | 207.4 a |
| - | Film | ||
|---|---|---|---|
| Bacteria | F0% | F1% | F4% |
| - | (µm2) | - | |
| Staphylococcus aureus | 2.07 ± 0.41 a | 1.27 ± 0.043 b | 1.23 ± 0.37 c |
| Pediococcus acidilactici | 1.81 ± 0.48 a | 1.88 ± 0.54 a | 1.96 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña-Pacheco, L.V.; Moreno-Robles, A.L.; Plascencia-Jatomea, M.; Del Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Tapia-Hernández, J.A.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z. The Preparation and Characterization of an Alginate–Chitosan-Active Bilayer Film Incorporated with Asparagus (Asparagus officinalis L.) Residue Extract. Coatings 2024, 14, 1232. https://doi.org/10.3390/coatings14101232
Acuña-Pacheco LV, Moreno-Robles AL, Plascencia-Jatomea M, Del Toro-Sánchez CL, Ayala-Zavala JF, Tapia-Hernández JA, Moreno-Vásquez MJ, Graciano-Verdugo AZ. The Preparation and Characterization of an Alginate–Chitosan-Active Bilayer Film Incorporated with Asparagus (Asparagus officinalis L.) Residue Extract. Coatings. 2024; 14(10):1232. https://doi.org/10.3390/coatings14101232
Chicago/Turabian StyleAcuña-Pacheco, Leslie V., Ana L. Moreno-Robles, Maribel Plascencia-Jatomea, Carmen L. Del Toro-Sánchez, Jesús F. Ayala-Zavala, José A. Tapia-Hernández, María J. Moreno-Vásquez, and Abril Z. Graciano-Verdugo. 2024. "The Preparation and Characterization of an Alginate–Chitosan-Active Bilayer Film Incorporated with Asparagus (Asparagus officinalis L.) Residue Extract" Coatings 14, no. 10: 1232. https://doi.org/10.3390/coatings14101232
APA StyleAcuña-Pacheco, L. V., Moreno-Robles, A. L., Plascencia-Jatomea, M., Del Toro-Sánchez, C. L., Ayala-Zavala, J. F., Tapia-Hernández, J. A., Moreno-Vásquez, M. J., & Graciano-Verdugo, A. Z. (2024). The Preparation and Characterization of an Alginate–Chitosan-Active Bilayer Film Incorporated with Asparagus (Asparagus officinalis L.) Residue Extract. Coatings, 14(10), 1232. https://doi.org/10.3390/coatings14101232









