Experimental Examination of Enhanced Nanoceramic-Based Self-Cleaning Sprays for High-Efficiency Hydrophobic Photovoltaic Panels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray Material and Samples
2.3. Characterization
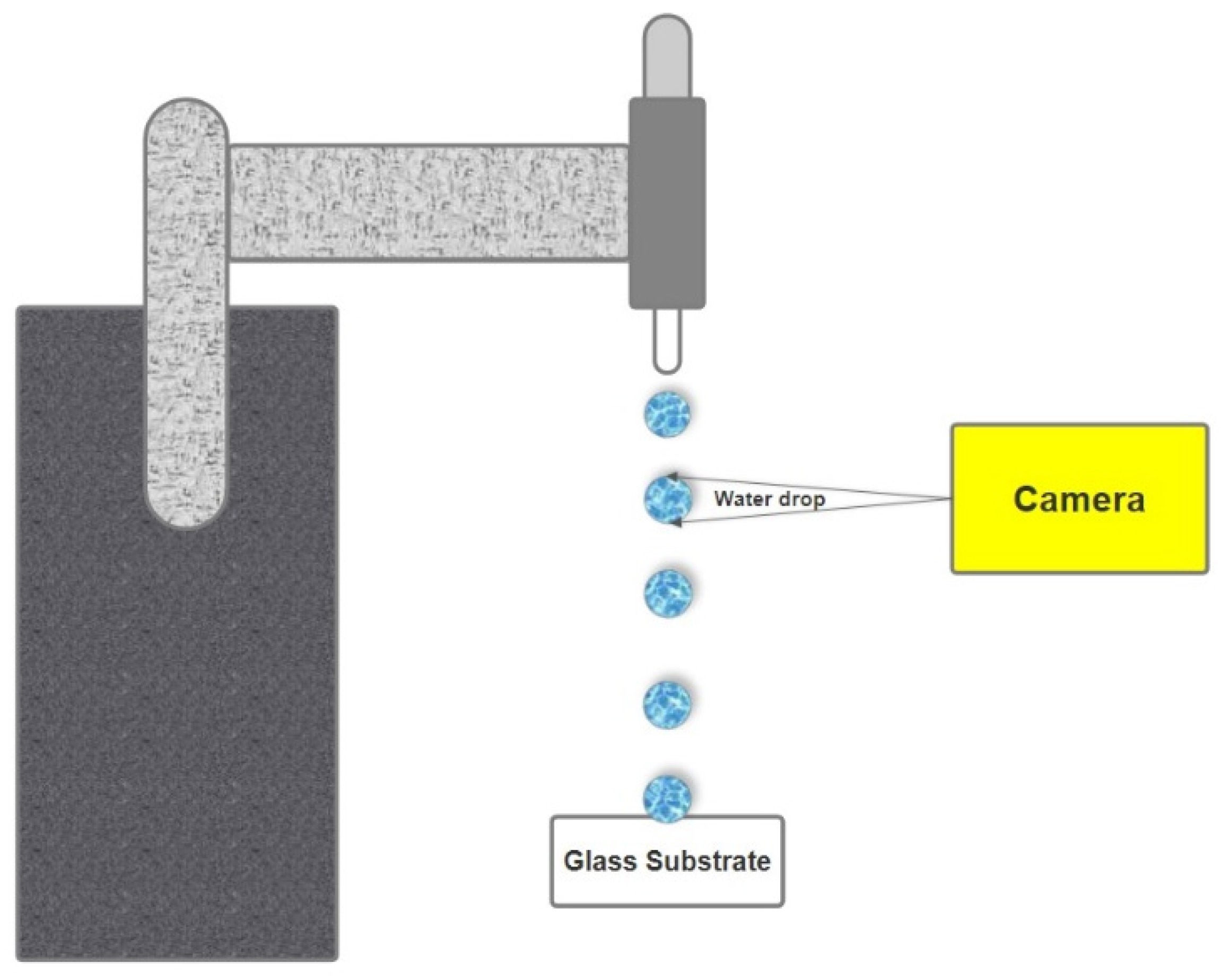
2.4. Experimental Setup and Procedure
3. Results and Discussion
3.1. Apperance of PDMS-Based Coatings
3.2. Water Contact Angle (WCA)
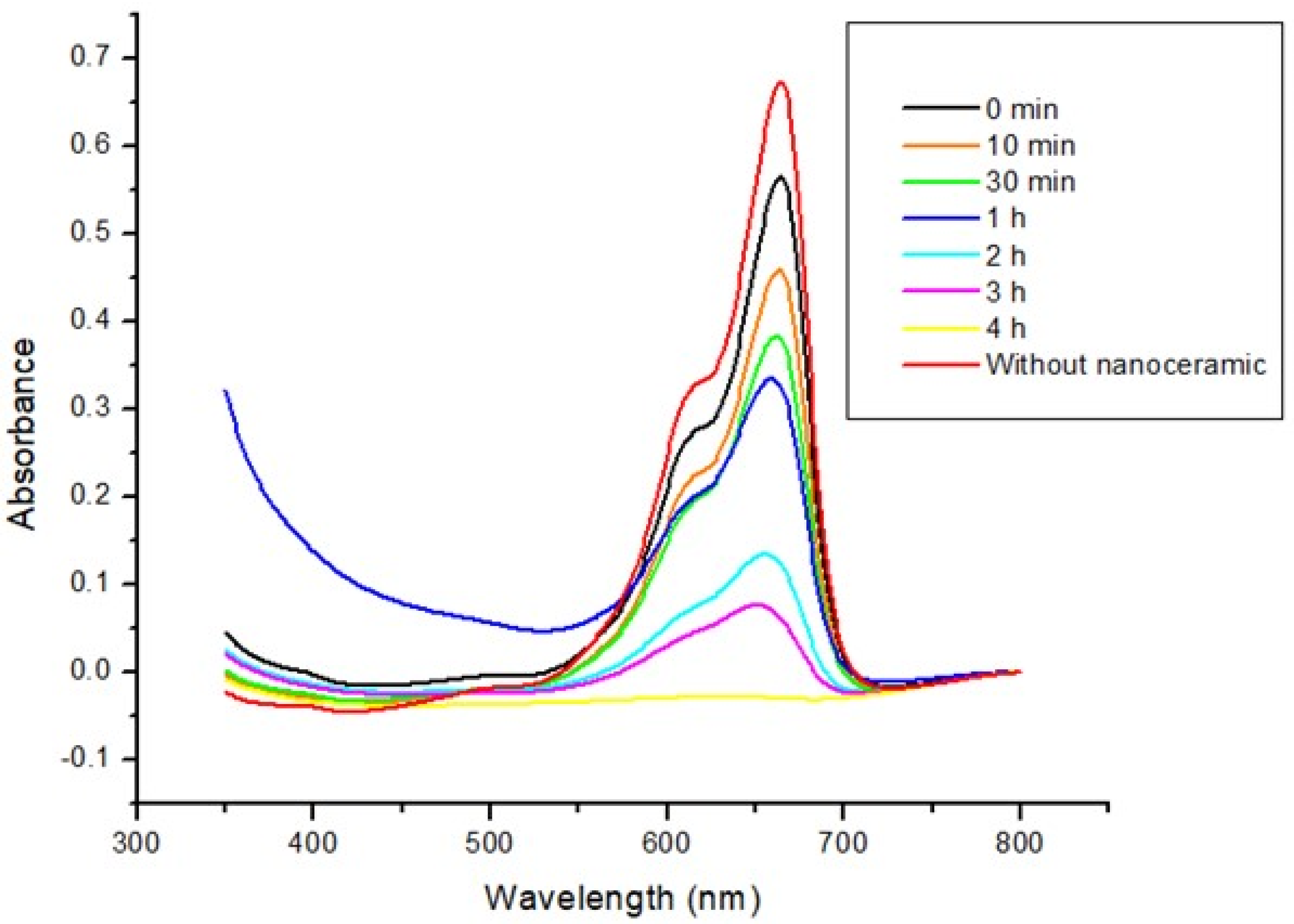
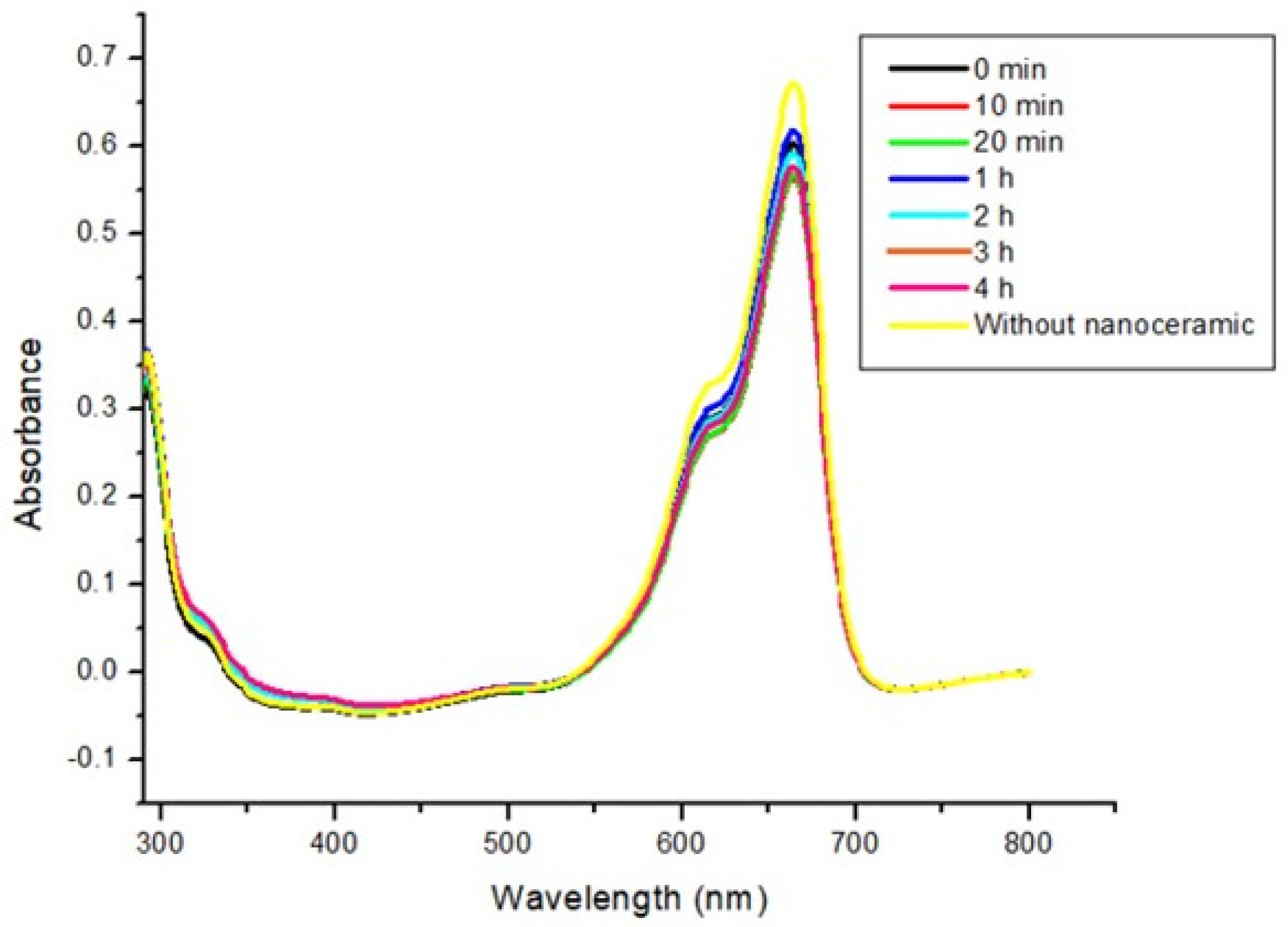
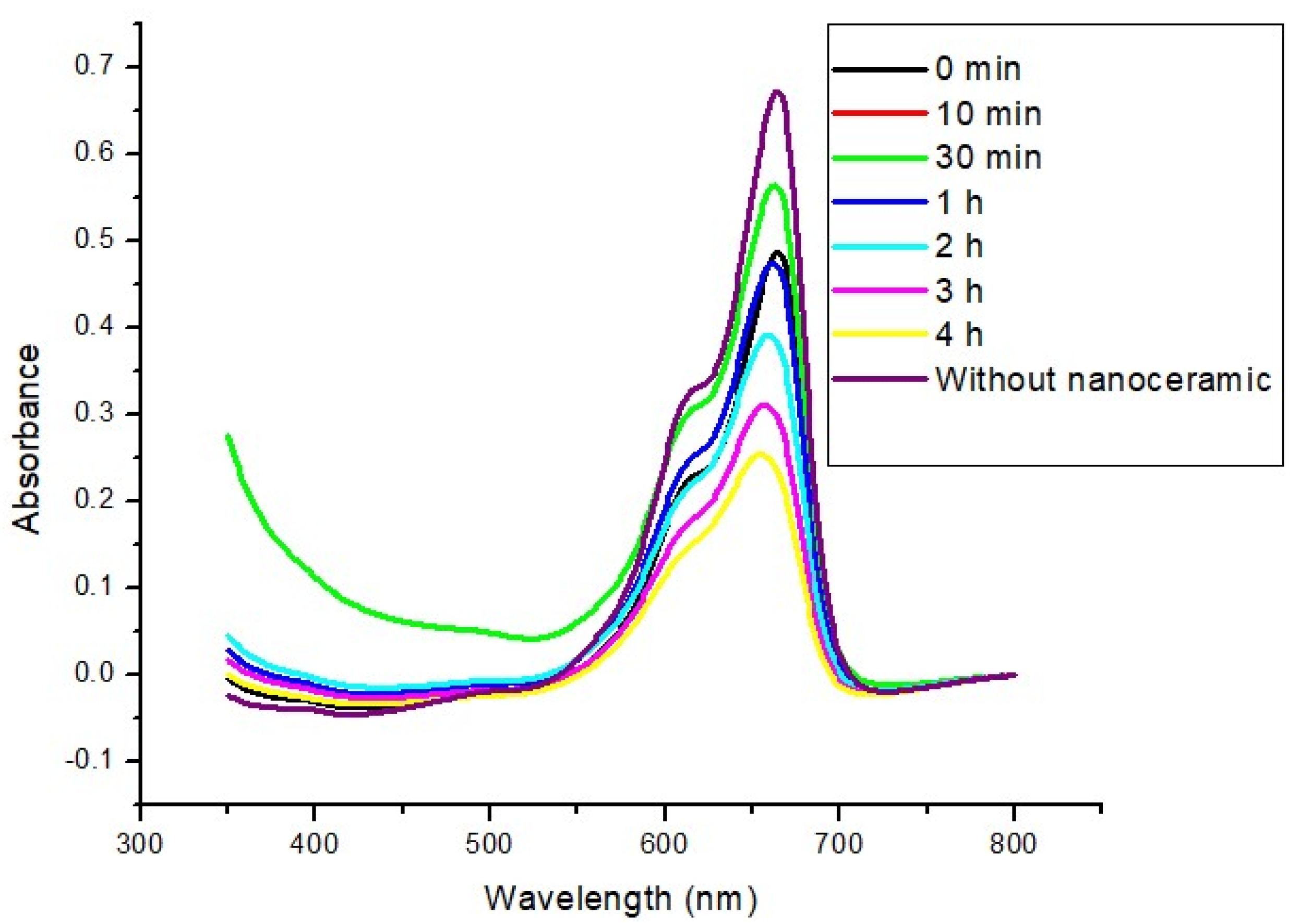
3.3. Photocatalysis
3.4. Zeta Potential
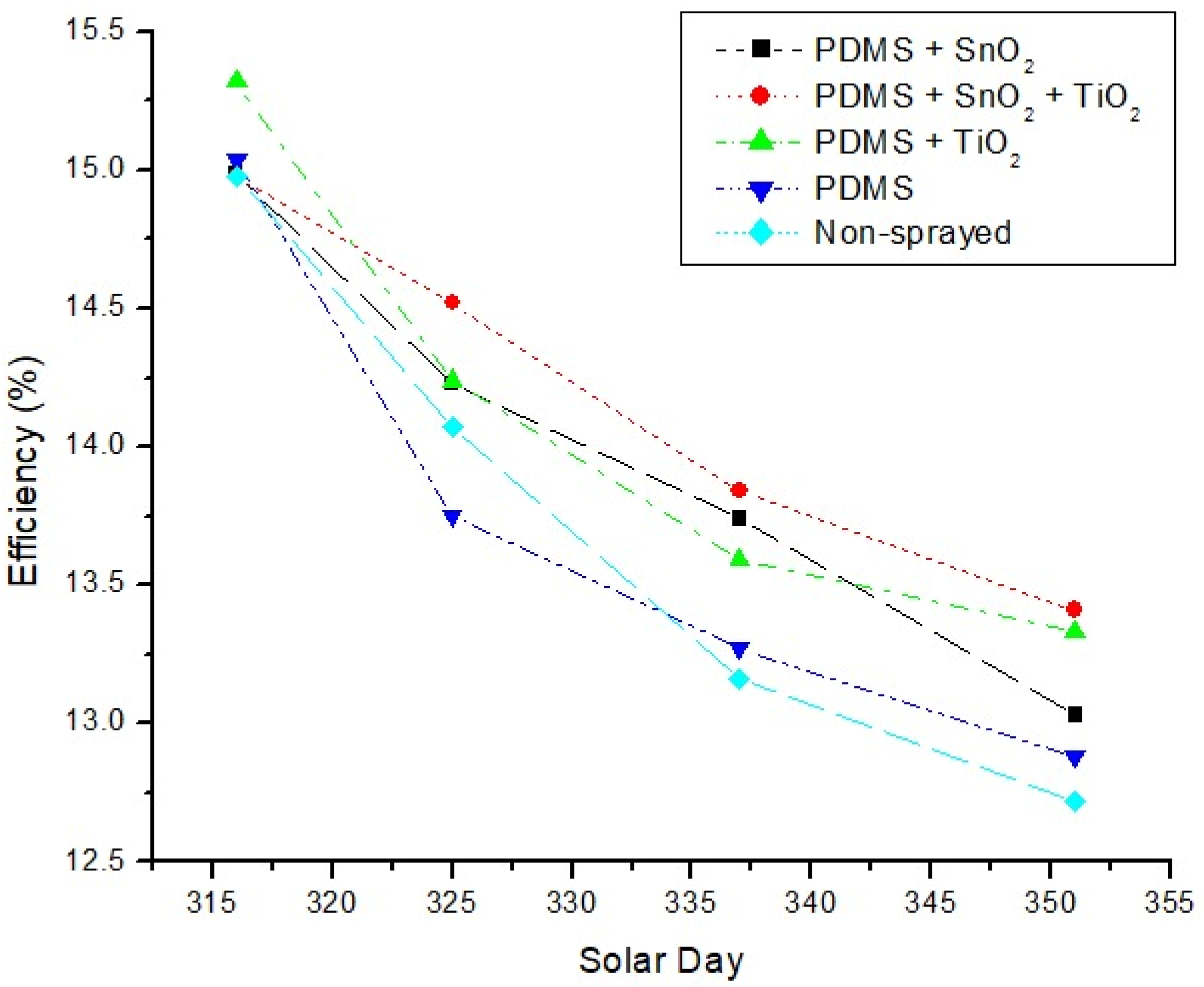
3.5. PV Efficiency
4. Conclusions
- (1)
- The addition of SnO2 and/or TiO2 nanoparticles to PDMS oil- spray increase the hydrophobicity of the sprayed surfaces, as evidenced by the increased (94–116) contact angles obtained. The spray with a mixture of both nano-TiO2 and nano-SnO2 resulted the highest energy efficiency and performance compared to sprays with individual types of nanoparticles. This contributes to the self-cleaning effect of the nanoparticle-based sprays, especially in highly dust-prone regions.
- (2)
- However, mixtures or dispersions of either nano-TiO2 or nano-SnO2 alone could be sufficient, providing acceptable performance and thus can be utilized in less dusty environments.
- (3)
- The strong photocatalytic characteristics of TiO2 played a significant role in achieving a self-cleaning effect, as observed from the energy efficiency results, thereby reducing PV efficiency degradation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsikalakis, A.; Tomtsi, T.; Hatziargyriou, N.; Poullikkas, A.; Malamatenios, C.; Giakoumelos, E.; Jaouad, O.C.; Chenak, A.; Fayek, A.; Matar, T.; et al. Review of best practices of solar electricity resources applications in selected Middle East and North Africa (MENA) countries. Renew. Sustain. Energy Rev. 2011, 15, 2838–2849. [Google Scholar] [CrossRef]
- Salah, S.I.; Eltaweel, M.; Abeykoon, C. Towards a sustainable energy future for Egypt: A systematic review of renewable energy sources, technologies, challenges, and recommendations. Clean. Eng. Technol. 2022, 8, 100497. [Google Scholar] [CrossRef]
- Goswami, Y. An Annual Review of Research and Development in Renewable Energy Technologies: Advances in Solar Energy; Taylor & Francis Group: Abingdon, UK, 2007; Volume 17. [Google Scholar]
- Abdeladim, K.; Bouchakour, S.; Arab, A.H.; Amrouche, S.O.; Yassaa, N. Promotion of renewable energy in some MENA region countries. IOP Conf. Ser. Earth Environ. Sci. 2018, 154, 012003. [Google Scholar] [CrossRef]
- Farag, M.M.; Bansal, R.C. Solar energy development in the GCC region—A review on recent progress and opportunities. Int. J. Model. Simul. 2022, 43, 579–599. [Google Scholar] [CrossRef]
- Hachicha, A.A.; Al-Sawafta, I.; Said, Z. Impact of dust on the performance of solar photovoltaic (PV) systems under United Arab Emirates weather conditions. Renew. Energy 2019, 141, 287–297. [Google Scholar] [CrossRef]
- Alami, A.H.; Rabaia, M.K.H.; Sayed, E.T.; Ramadan, M.; Abdelkareem, M.A.; Alasad, S.; Olabi, A.-G. Management of potential challenges of PV technology proliferation. Sustain. Energy Technol. Assess. 2022, 51, 101942. [Google Scholar] [CrossRef]
- El-Mahallawi, I.; Elshazly, E.; Ramadan, M.; Nasser, R.; Yasser, M.; El-Badry, S.; Elthakaby, M.; Oladinrin, O.T.; Rana, M.Q. Solar PV Panels-Self-Cleaning Coating Material for Egyptian Climatic Conditions. Sustainability 2022, 14, 11001. [Google Scholar] [CrossRef]
- Adak, D.; Bhattacharyya, R.; Barshilia, H.C. A state-of-the-art review on the multifunctional self-cleaning nanostructured coatings for PV panels, CSP mirrors and related solar devices. Renew. Sustain. Energy Rev. 2022, 159, 112145. [Google Scholar] [CrossRef]
- Adıgüzel, E.; Özer, E.; Akgündoğdu, A.; Yılmaz, A.E. Prediction of dust particle size effect on efficiency of photovoltaic modules with ANFIS: An experimental study in Aegean region, Turkey. Sol. Energy 2019, 177, 690–702. [Google Scholar] [CrossRef]
- El-Sybaee, I.M.; El-Keway, A.A.; Elmeadawy, M.I.; Abdel-Maksoud, M.A. Study the effect of dust deposition on solar photovoltaic and solar radiation. Misr J. Agric. Eng. 2018, 35, 1397–1408. [Google Scholar] [CrossRef]
- Tanesab, J.; Parlevliet, D.; Whale, J.; Urmee, T.; Pryor, T. The contribution of dust to performance degradation of PV modules in a temperate climate zone. Sol. Energy 2015, 120, 147–157. [Google Scholar] [CrossRef]
- Mostefaoui, M.; Ziane, A.; Bouraiou, A.; Khelifi, S. Effect of sand dust accumulation on photovoltaic performance in the Saharan environment: Southern Algeria (Adrar). Environ. Sci. Pollut. Res. 2019, 26, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Styszko, K.; Jaszczur, M.; Teneta, J.; Hassan, Q.; Burzyńska, P.; Marcinek, E.; Łopian, N.; Samek, L. An analysis of the dust deposition on solar photovoltaic modules. Environ. Sci. Pollut. Res. 2019, 26, 8393–8401. [Google Scholar] [CrossRef]
- Chen, J.; Pan, G.; Ouyang, J.; Ma, J.; Fu, L.; Zhang, L. Study on impacts of dust accumulation and rainfall on PV power reduction in East China. Energy 2020, 194, 116915. [Google Scholar] [CrossRef]
- Ammari, N.; Mehdi, M.; Merrouni, A.A.; El Gallassi, H.; Chaabelasri, E.; Ghennioui, A. Experimental study on the impact of soiling on the modules temperature and performance of two different PV technologies under hot arid climate. Heliyon 2022, 8, e11395. [Google Scholar] [CrossRef]
- Chaudhary, A.S.; Chaturvedi, D. Thermal Image Analysis and Segmentation to Study Temperature Effects of Cement and Bird Deposition on Surface of Solar Panels. Int. J. Image Graph. Signal Process. 2017, 9, 12–22. [Google Scholar] [CrossRef]
- Solar Resource Maps and GIS Data for 200+ Countries|Solargis. Available online: https://solargis.com/maps-and-gis-data/download/egypt (accessed on 21 January 2023).
- IRENA. Renwable Energy Outlook: Egypt. 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Oct/IRENA_Outlook_Egypt_2018_En.pdf?rev=6c21d902a8d943798abe082a58ec2ce8 (accessed on 21 January 2023).
- Deb, D.; Brahmbhatt, N.L. Review of yield increase of solar panels through soiling prevention, and a proposed water-free automated cleaning solution. Renew. Sustain. Energy Rev. 2018, 82, 3306–3313. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, M.; Pachauri, R.K.; Babu, K.D. Comprehensive review on effect of dust on solar photovoltaic system and mitigation techniques. Sol. Energy 2019, 191, 596–622. [Google Scholar] [CrossRef]
- Syafiq, A.; Pandey, A.; Adzman, N.; Rahim, N.A. Advances in approaches and methods for self-cleaning of solar photovoltaic panels. Sol. Energy 2018, 162, 597–619. [Google Scholar] [CrossRef]
- Vyas, B.K.; Adhwaryu, A.; Bhaskar, K. Planning and developing large solar power plants: A case study of 750 MW Rewa Solar Park in India. Clean. Eng. Technol. 2022, 6, 100396. [Google Scholar] [CrossRef]
- Maghrabie, H.M.; Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Building-integrated photovoltaic/thermal (BIPVT) systems: Applications and challenges. Sustain. Energy Technol. Assess. 2021, 45, 101151. [Google Scholar] [CrossRef]
- Alamri, H.R.; Rezk, H.; Abd-Elbary, H.; Ziedan, H.A.; Elnozahy, A. Experimental investigation to improve the energy efficiency of solar PV panels using hydrophobic SiO2 nanomaterial. Coatings 2020, 10, 503. [Google Scholar] [CrossRef]
- El-Shimy, M. Viability analysis of PV power plants in Egypt. Renew. Energy 2009, 34, 2187–2196. [Google Scholar] [CrossRef]
- Boghdady, T.A.; Alamer, A.J.; Yousef, M.M.; Elshafee, A.M.; Hassan, M.A.M.; Seif, A.M. Technical and economic study of powering poultry farm in Egypt using PV-biomass on-grid energy generation system: Case study. WSEAS Trans. Power Syst. 2021, 16, 67–77. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, L.; Liu, X.; Li, J. Self-cleaning mechanisms and laws of hydrophilic or hydrophobic surfaces of solar photovoltaic glass. Chem. Eng. Res. Des. 2022, 188, 364–377. [Google Scholar] [CrossRef]
- Al-Badra, M.; Abd-Elhady, M.; Kandil, H. A novel technique for cleaning PV panels using antistatic coating with a mechanical vibrator. Energy Rep. 2020, 6, 1633–1637. [Google Scholar] [CrossRef]
- Soklič, A.; Tasbihi, M.; Kete, M.; Štangar, U.L. Deposition and possible influence of a self-cleaning thin TiO2/SiO2 film on a photovoltaic module efficiency. Catal. Today 2015, 252, 54–60. [Google Scholar] [CrossRef]
- Appasamy, J.S.; Kurnia, J.C.; Assadi, M.K. Synthesis and evaluation of nitrogen-doped titanium dioxide/single walled carbon nanotube-based hydrophilic self-cleaning coating layer for solar photovoltaic panel surface. Sol. Energy 2020, 196, 80–91. [Google Scholar] [CrossRef]
- Seifi, A.; Salari, D.; Khataee, A.; Çoşut, B.; Arslan, L.; Niaei, A. Enhanced photocatalytic activity of highly transparent superhydrophilic doped TiO2 thin films for improving the self-cleaning property of solar panel covers. Ceram. Int. 2022, 49, 1678–1689. [Google Scholar] [CrossRef]
- Chungsiriporn, J.; Pongyeela, P.; Chairerk, N. Sol-gel self cleaning superhydrophobic nanocoating for glass surface of solar cell. Songklanakarin J. Sci. Technol. 2020, 42, 923–927. [Google Scholar]
- Syafiq, A.; Pandey, A.K.; Rahim, N.A.; Vengadaesvaran, B.; Shahabuddin, S. Self-cleaning and weather resistance of nano-SnO2/modified silicone oil coating for photovoltaic (PV) glass applications. J. Mater. Sci. Mater. Electron. 2019, 30, 12584–12596. [Google Scholar] [CrossRef]
- Sathya, R.A.; Ponraj, C. Superhydrophobic route of fabricating antireflective, self-cleaning, and durable coatings for solar cell applications. J. Coat. Technol. Res. 2024, 21, 1–30. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, J.; Yan, X.; Tan, P.; Wang, M.; Zheng, Y.; Shen, Y.; Chen, J.; Nie, Y.; Liu, S. A three-layer superhydrophobic coatings inspired by human scalp structure with excellent anti-reflection and durable effects for photovoltaic applications. J. Clean. Prod. 2023, 414, 137605. [Google Scholar] [CrossRef]
- Syafiq, A.; Rahim, N.A.; Balakrishnan, V.; Pandey, A. Development of self-cleaning polydimethylsiloxane/nano-calcium carbonate-titanium dioxide coating with fog-resistance response for building glass. Pigment. Resin Technol. 2022, 53, 249–260. [Google Scholar] [CrossRef]
- McShea, L.; Kambo, H.S.; Maclean, M.; E Sandison, M. Metal oxide-doped elastomeric materials for amplifying visible light-based antimicrobial activity. Mater. Res. Express 2022, 9, 085402. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Er, A.O.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and electronic properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Ali, M.S.; Dhoble, S.J.; Rahim, N.A.; Omar, A.; Abu Bakar, A.H. Application of transparent self-cleaning coating for photovoltaic panel: A review. Curr. Opin. Chem. Eng. 2022, 36, 100801. [Google Scholar] [CrossRef]
- Olkowicz, K.; Buczko, Z.; Nasiłowska, B.; Kowalczyk, K.; Czwartos, J. Superhydrophobic Coating Based on Porous Aluminum Oxide Modified by Polydimethylsiloxane (PDMS). Materials 2022, 15, 1042. [Google Scholar] [CrossRef]
- Elkaseer, A.; Schneider, S.; Deng, Y.; Scholz, S.G. Effect of Process Parameters on the Performance of Drop-On-Demand 3D Inkjet Printing: Geometrical-Based Modeling and Experimental Validation. Polymers 2022, 14, 2557. [Google Scholar] [CrossRef]
- Khannyra, S.; Luna, M.; Gil, M.A.; Addou, M.; Mosquera, M.J. Self-cleaning durability assessment of TiO2/SiO2 photocatalysts coated concrete: Effect of indoor and outdoor conditions on the photocatalytic activity. Build. Environ. 2022, 211, 108743. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chang, G.; Wu, S.; Yang, T.; Zhou, B.; Tang, J.; Liu, L.; Guan, R.; Zhang, G.; Wang, J.; et al. Highly transparent, superhydrophobic, and durable silica/resin self-cleaning coatings for photovoltaic panels. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 133983. [Google Scholar] [CrossRef]
- Syafiq, A.; Vengadaesvaran, B.; Rahim, N.A.; Pandey, A.; Bushroa, A.; Ramesh, K.; Ramesh, S. Transparent self-cleaning coating of modified polydimethylsiloxane (PDMS) for real outdoor application. Prog. Org. Coat. 2019, 131, 232–239. [Google Scholar] [CrossRef]
- Tayel, S.A.; Abu El-Maaty, A.E.; Mostafa, E.M.; Elsaadawi, Y.F. Enhance the performance of photovoltaic solar panels by a self-cleaning and hydrophobic nanocoating. Sci. Rep. 2022, 12, 21236. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Hanif, M.; Umar, M.; Furqan, M.Q.; Younas, M.; Mehmood, U. Development of Polydimethylsiloxane (PDMS)-Based Hydrophobic Coating for Self-clean Solar Panels. Arab. J. Sci. Eng. 2024, 49, 8079–8086. [Google Scholar] [CrossRef]
- Saharudin, K.; Sreekantan, S.; Basiron, N.; Endut, N. Improved Hydrophobicity of Polydimethylsiloxane (PDMS) Coating with ZnO Nanostructures. J. Phys. Conf. Ser. 2018, 1082, 012106. [Google Scholar] [CrossRef]
- Sutar, R.S.; Shi, B.; Kanchankoti, S.S.; Ingole, S.S.; Jamadar, W.S.; Sayyad, A.J.; Khot, P.B.; Sadasivuni, K.K.; Latthe, S.S.; Liu, S.; et al. Development of self-cleaning superhydrophobic cotton fabric through silica/PDMS composite coating. Surf. Topogr. Metrol. Prop. 2023, 11, 045004. [Google Scholar] [CrossRef]
- Alshammari, A.A.; Salilih, E.M.; Almatrafi, E.; Rady, M. Polymeric coatings for passive radiative cooling of PV modules in hot and humid weather: Design, optimization, and performance evaluation. Case Stud. Therm. Eng. 2021, 57, 104341. [Google Scholar] [CrossRef]
- Elshazly, E.; El-Rehim, A.A.; El-Mahallawi, I. Comparison of dust and high-temperature effects on mono and poly photovoltaic panels. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1172, 012019. [Google Scholar] [CrossRef]
- Elshazly, E.; El-Rehim, A.A.A.; Kader, A.A.; El-Mahallawi, I. Effect of Dust and High Temperature on Photovoltaics Performance in the New Capital Area. WSEAS Trans. Environ. Dev. 2021, 17, 360–370. [Google Scholar] [CrossRef]
| Substrate No. | Materials in Spray | Initial Diameter (mm) | Equilibrium Diameter (mm) | Calculated Contact Angle (°) |
|---|---|---|---|---|
| 1 | PDMS + SnO2 | 4.44 | 4.29 | 115.9 |
| 2 | PDMS + SnO2 + TiO2 | 4.5 | 5.14 | 100.6 |
| 3 | PDMS + TiO2 | 4.36 | 5.29 | 94.23 |
| 4 | PDMS | 4.29 | 5.81 | 81.45 |
| 5 | None | 4.67 | 12.63 | 15.26 |
| Type of Nanoceramic in Sample | Zeta Potential Value (mV) |
|---|---|
| 5% SnO2 | +0.626 |
| 2.5% SnO2 + 2.5% TiO2 | +0.366 |
| 5% TiO2 | +1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdrabo, M.; Elkaseer, A.; Elshazly, E.; El-Deab, M.S.; El-Mahallawi, I. Experimental Examination of Enhanced Nanoceramic-Based Self-Cleaning Sprays for High-Efficiency Hydrophobic Photovoltaic Panels. Coatings 2024, 14, 1239. https://doi.org/10.3390/coatings14101239
Abdrabo M, Elkaseer A, Elshazly E, El-Deab MS, El-Mahallawi I. Experimental Examination of Enhanced Nanoceramic-Based Self-Cleaning Sprays for High-Efficiency Hydrophobic Photovoltaic Panels. Coatings. 2024; 14(10):1239. https://doi.org/10.3390/coatings14101239
Chicago/Turabian StyleAbdrabo, Merna, Ahmed Elkaseer, Engy Elshazly, Mohamed S. El-Deab, and Iman El-Mahallawi. 2024. "Experimental Examination of Enhanced Nanoceramic-Based Self-Cleaning Sprays for High-Efficiency Hydrophobic Photovoltaic Panels" Coatings 14, no. 10: 1239. https://doi.org/10.3390/coatings14101239
APA StyleAbdrabo, M., Elkaseer, A., Elshazly, E., El-Deab, M. S., & El-Mahallawi, I. (2024). Experimental Examination of Enhanced Nanoceramic-Based Self-Cleaning Sprays for High-Efficiency Hydrophobic Photovoltaic Panels. Coatings, 14(10), 1239. https://doi.org/10.3390/coatings14101239








