Superhydrophobic Corrosion-Resistant Coating of AZ91D Magnesium Alloy: Preparation and Performance
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Pretreatment
2.3. Preparation
2.4. Characterization
2.5. Self-Cleaning Performance and Chemical Stability Test
2.6. Corrosion Performance
3. Results and Discussion
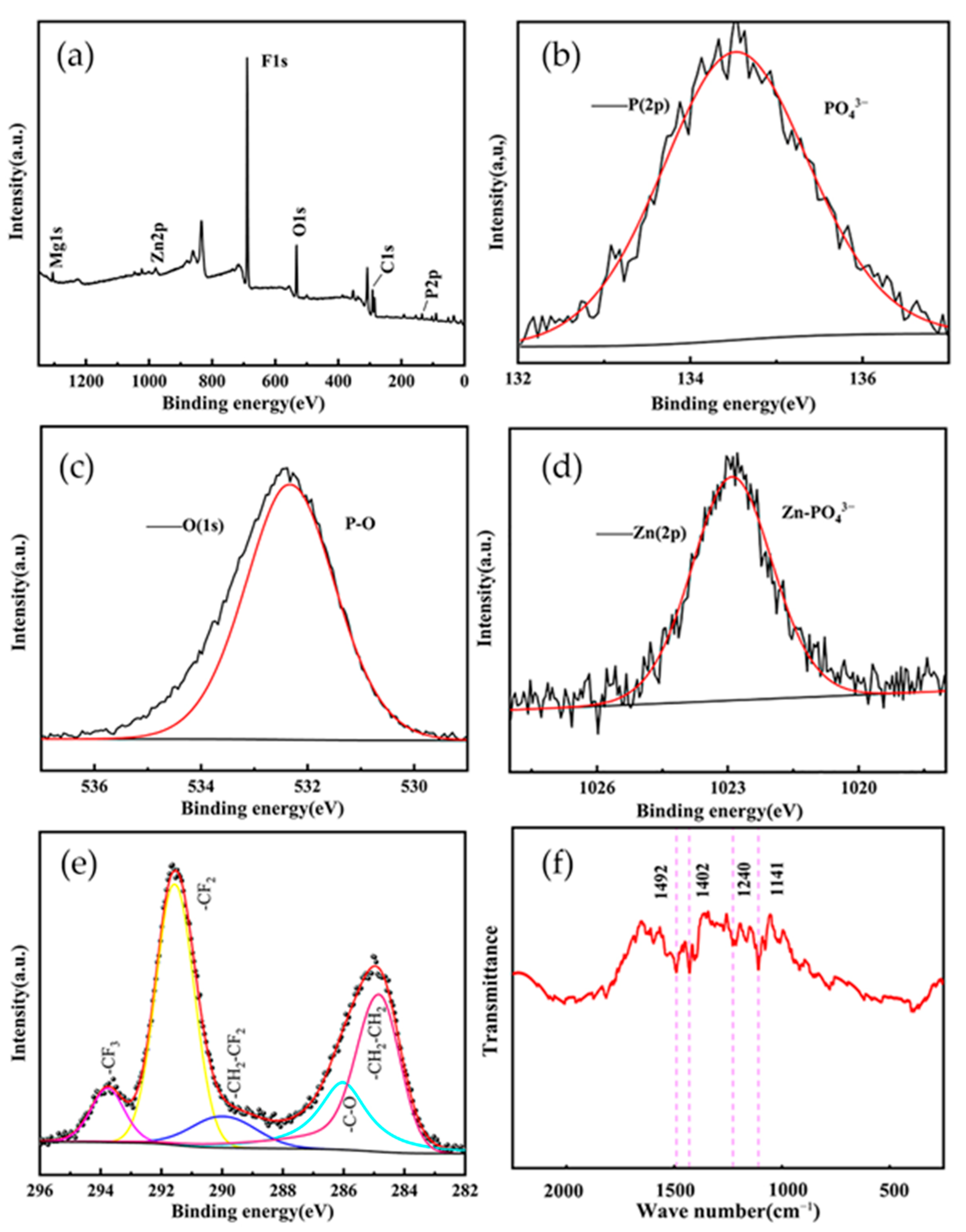
3.1. Chemical Composition
3.2. Growth Process of Zinc Phosphate Film
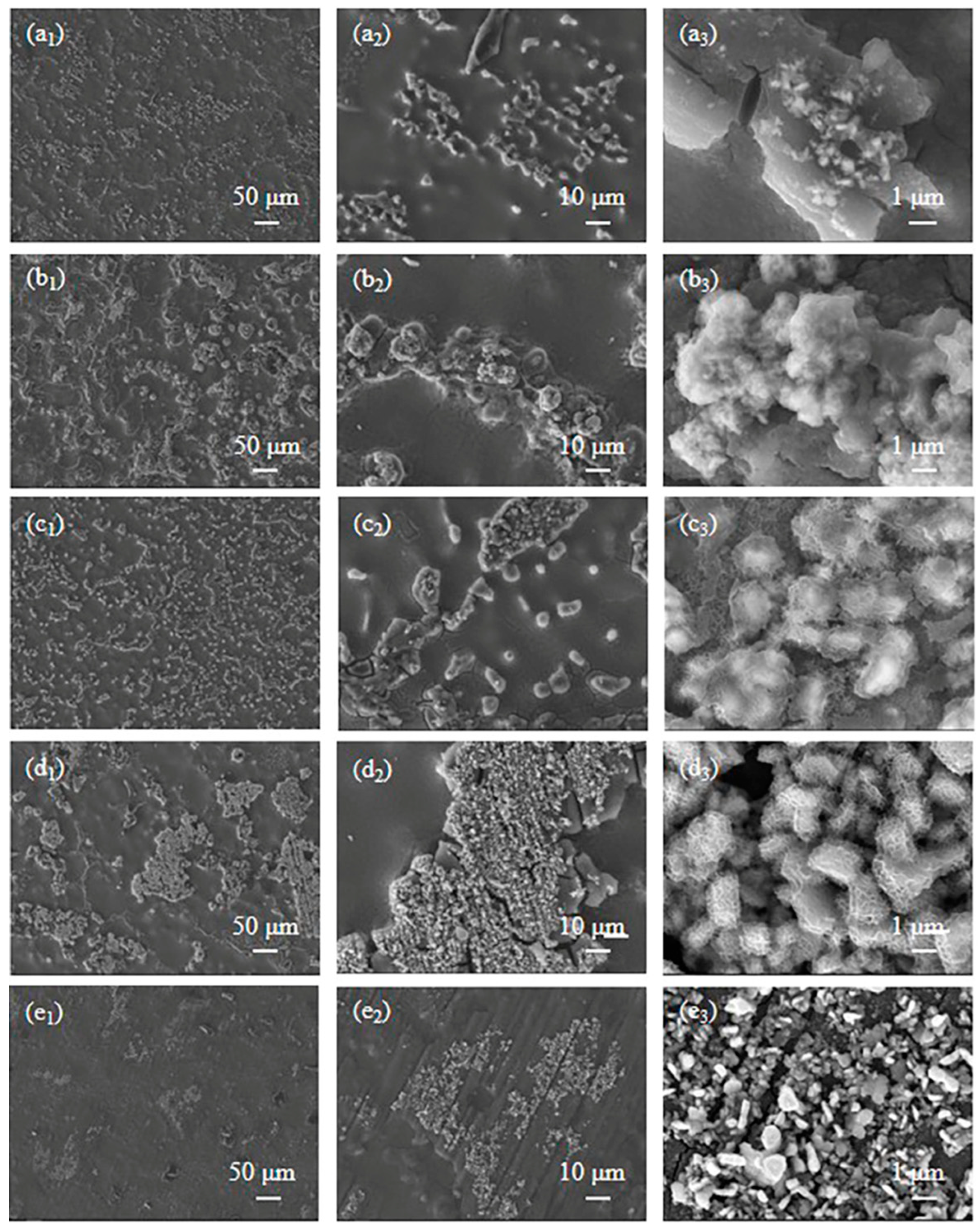
3.3. Microscopic Morphology of PCC at Different Phosphating Times
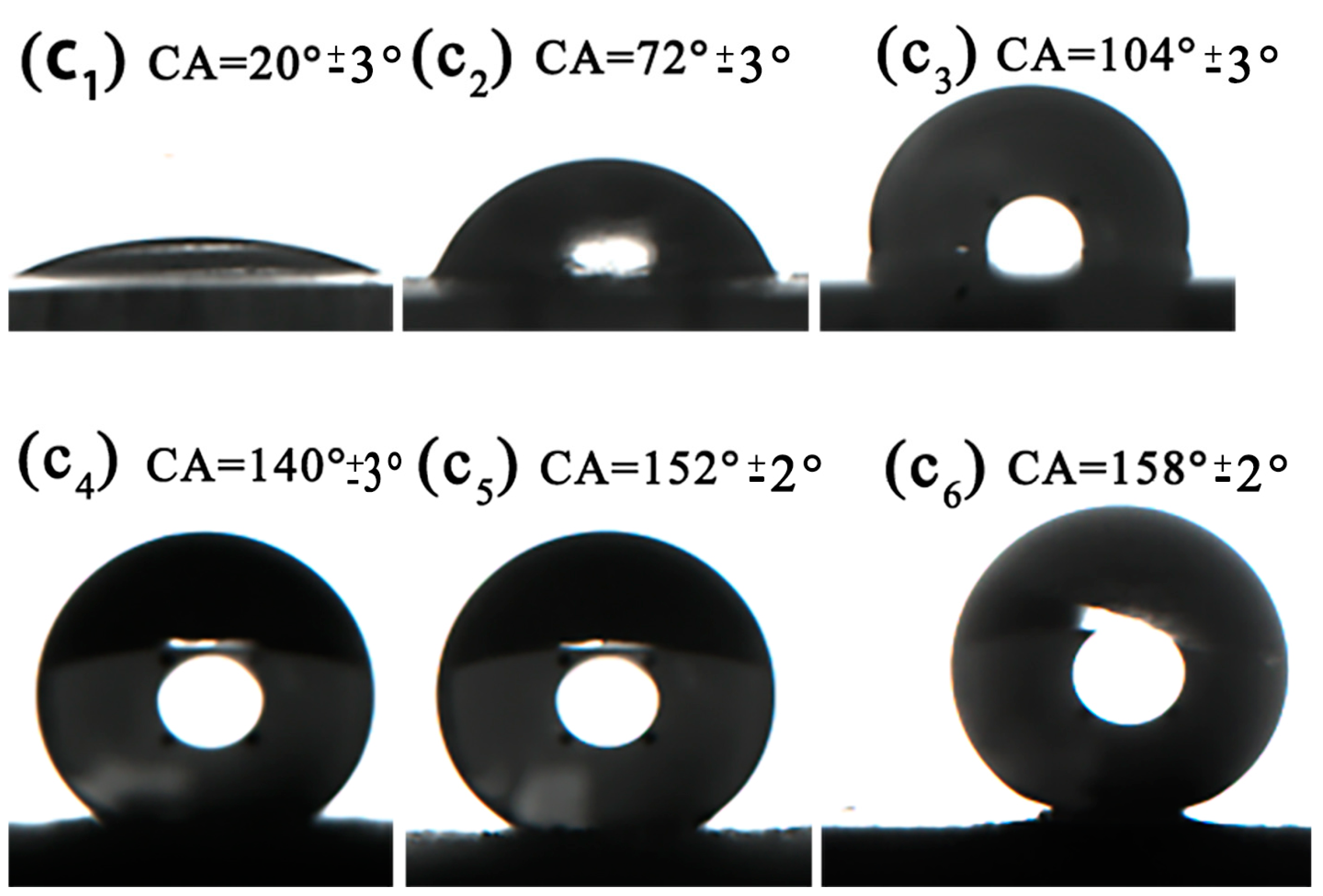
3.4. Wettability
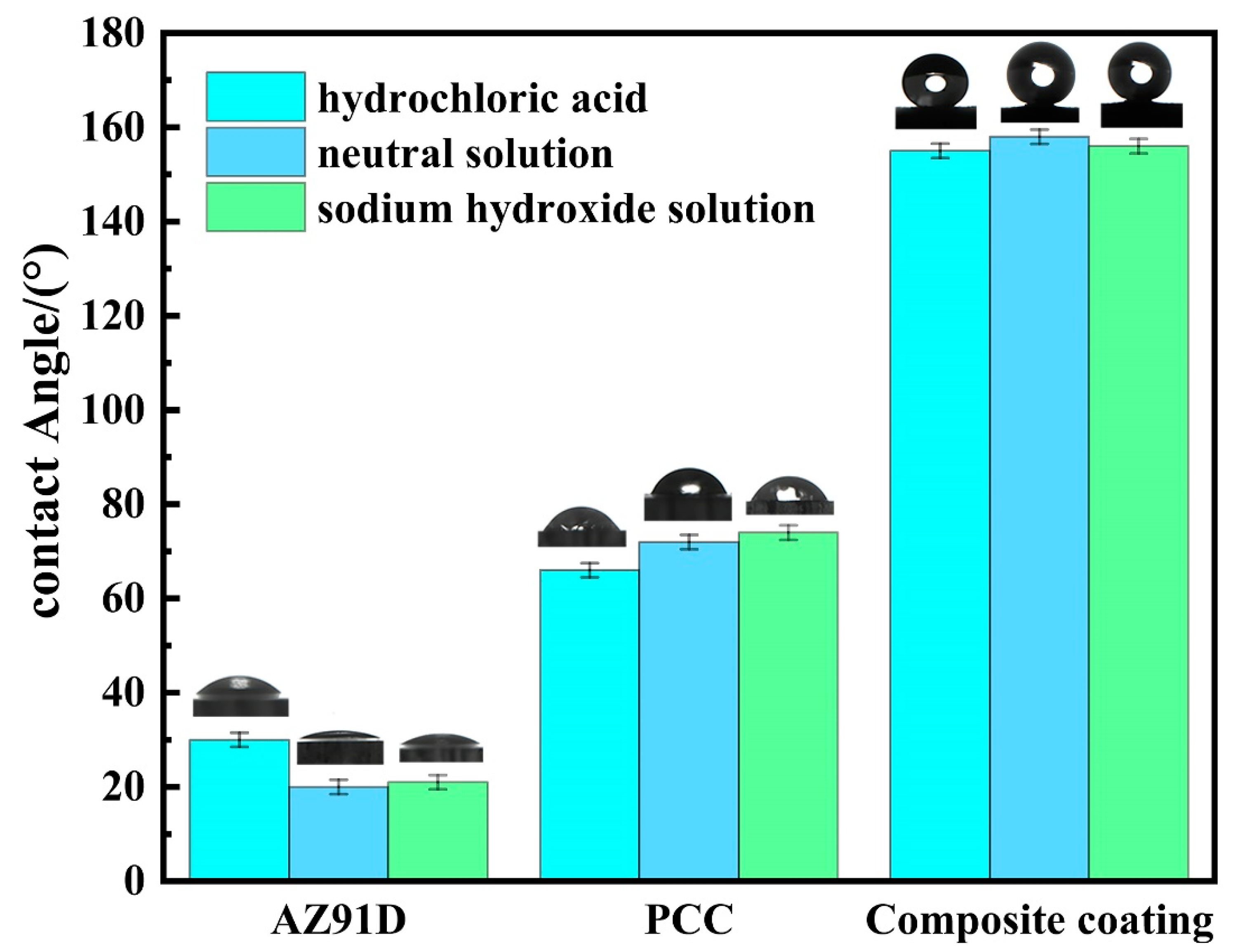
3.5. Self-Cleaning Performance and Chemical Stability Test
3.6. Anti-Corrosion Test
3.7. Anti-Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tharumarajah, A.; Koltun, P. Is there an environmental advantage of using magnesium components for light-weighting cars? J. Clean. Prod. 2007, 15, 1007–1013. [Google Scholar] [CrossRef]
- Rudajevová, A.; Lukác, P. Comparison of the thermal properties of AM20 and AS21 magnesium alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2005, 397, 16–21. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Guo, Z.; Wu, R.; Jhiao, W.; Chun, M.X.; Gorar, A.A.K. Historical Progress in Electromagnetic Interference Shielding Effectiveness of Conventional Mg Alloys Leading to Mg-Li-Based Alloys: A Review. Adv. Eng. Mater. 2023, 25, 2300732. [Google Scholar] [CrossRef]
- Arruebarrena, G.; Hurtado, I.; Vainola, J.; Cingi, C.; Devenyi, S.; Townsend, J.; Mahmood, S.; Wendt, A.; Weiss, K.; Ben-Dov, A. Development of investment-casting process of Mg-alloys for aerospace applications. Adv. Eng. Mater. 2007, 9, 751–756. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, D.H. Dynamic patterns of technological innovation in magnesium alloys in the Korean automotive industry. Int. J. Technol. Manag. 2022, 90, 28–53. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koc, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K. Corrosion and Protection of Magnesium Alloys: Recent Advances and Future Perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Xin, Y.; Yuan, N.; Ding, J. Facile formation of super-hydrophobic nickel coating on magnesium alloy with improved corrosion resistance. Colloids Surf. A—Physicochem. Eng. Asp. 2018, 538, 500–505. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Zhong, L.; Wang, J.; Jiang, Q.; Guo, X. Super-hydrophobic surface on pure magnesium substrate by wet chemical method. Appl. Surf. Sci. 2010, 256, 3837–3840. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Facile and fast fabrication of superhydrophobic surface on magnesium alloy. Appl. Surf. Sci. 2013, 271, 182–192. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, H.; Hu, X. A rapid one-step process for the construction of corrosion-resistant bionic superhydrophobic surfaces. Prog. Org. Coat. 2016, 100, 56–62. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Wang, D.; Wen, Y.; Peng, N.; Shang, W. ZIF-8-based micro-arc oxidation composite coatings enhanced the corrosion resistance and superhydrophobicity of a Mg alloy. J. Magnes. Alloys 2023, 11, 1367–1380. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Fu, X.; Wang, L.; Fan, Y.; Xu, J.; Yin, Y.; Tian, L.; Zhao, J. Fluorinated epoxy based superhydrophobic coating with robust self-healing and anticorrosive performances. Prog. Org. Coat. 2022, 171, 107145. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, K.A.; Emelyanenko, A.M. The mechanisms and advances in magnesium-based materials protection against corrosion by the superhydrophobic coatings. Surf. Coat. Technol. 2024, 481, 130607. [Google Scholar] [CrossRef]
- Chen, D.; He, H.; Kang, Z.; Li, W. Research advances on superhydrophobic coating for metal surfaces via one-step electrodeposition. Anti-Corros. Methods Mater. 2024, 71, 179–190. [Google Scholar] [CrossRef]
- Umehara, H.; Takaya, M.; Terauchi, S. Permanganate conversion coatings for magnesium alloys. Mater. Sci. Forum 2003, 419–422, 883–888. [Google Scholar] [CrossRef]
- Kang, Y.; Yan, S.; Li, Z.; Wang, Z.; Yang, A.; Ma, W.; Chen, W.; Qu, Y. Influence of Anodic Oxidation on the Organizational Structure and Corrosion Resistance of Oxide Film on AZ31B Magnesium Alloy. Coatings 2024, 14, 271. [Google Scholar] [CrossRef]
- Natarajan, S.; Sivan, V.; Tennyson, P.G.; Kiran, V.R. Protective coatings on magnesium and its alloys: A critical review. Corros. Prev. Control 2004, 51, 142–163. [Google Scholar]
- Li, G.Y.; Lian, J.S.; Niu, L.Y.; Jiang, Z.H.; Jiang, Q. Growth of zinc phosphate coatings on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 201, 1814–1820. [Google Scholar] [CrossRef]
- Amini, R.; Sarabi, A.A. The corrosion properties of phosphate coating on AZ31 magnesium alloy: The effect of sodium dodecyl sulfate (SDS) as an eco-friendly accelerating agent. Appl. Surf. Sci. 2011, 257, 7134–7139. [Google Scholar] [CrossRef]
- Nguyen, T.-L.; Cheng, T.-C.; Yang, J.-Y.; Pan, C.-J.; Lin, T.-H. A zinc–manganese composite phosphate conversion coating for corrosion protection of AZ91D alloy: Growth and characteristics. J. Mater. Res. Technol. 2022, 19, 2965–2980. [Google Scholar] [CrossRef]
- Shi, T.; Kong, J.; Wang, X.; Li, X. Preparation of multifunctional Al-Mg alloy surface with hierarchical micro/nanostructures by selective chemical etching processes. Appl. Surf. Sci. 2016, 389, 335–343. [Google Scholar] [CrossRef]
- Li, X.; Yin, S.; Huang, S.; Luo, H.; Tang, Q. Fabrication of durable superhydrophobic Mg alloy surface with water-repellent, temperature-resistant, and self-cleaning properties. Vacuum 2020, 173, 109172. [Google Scholar] [CrossRef]
- Hikita, M.; Tanaka, K.; Nakamura, T.; Kajiyama, T.; Takahara, A. Super-liquid-repellent surfaces prepared by colloidal silica nanoparticles covered with fluoroalkyl groups. Langmuir 2005, 21, 7299–7302. [Google Scholar] [CrossRef]
- Gao, J.; Liu, H.; Tong, C.; Pang, L.; Feng, Y.; Zuo, M.; Wei, Z.; Li, J. Hemoglobin-Mn3(PO4)2 hybrid nanoflower with opulent electroactive centers for high-performance hydrogen peroxide electrochemical biosensor. Sens. Actuators B—Chem. 2020, 307, 127628. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, J.; Yan, F. The preparation and characterization of a nano-CeO2/phosphate composite coating on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 328, 335–343. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, R.; Wang, J.; Li, Q.; Xing, X.; Liu, X.; Hu, W. Fabrication and corrosion resistance of phosphate/ZnO multilayer protective coating on magnesium alloy. Surf. Coat. Technol. 2018, 352, 74–83. [Google Scholar] [CrossRef]
- Chen, X.; Kong, L.; Dong, D.; Yang, G.; Yu, L.; Chen, J.; Zhang, P. Synthesis and characterization of superhydrophobic functionalized Cu(OH)2 nanotube arrays on copper foil. Appl. Surf. Sci. 2009, 255, 4015–4019. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Zhu, J.; Duan, Y. Facilely etching of superhydrophobic surface with regular multiple hierarchical micro-nano structures for crowning wettability. Appl. Surf. Sci. 2024, 648, 159009. [Google Scholar] [CrossRef]
- LNiu, Y.; Jiang, Z.H.; Li, G.Y.; Gu, C.D.; Lian, J.S. A study and application of zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 3021–3026. [Google Scholar]
- Wesolowski, D.J.; Bénézeth, P.; Palmer, D.A. ZnO Solubility and Zn2 Complexation by Chloride and Sulfate in Acidic Solutions to 290 °C with In-Situ pH Measurement. Geochim. Et Cosmochim. Acta 1998, 62, 971–984. [Google Scholar] [CrossRef]
- Lian, J.S.; Li, G.Y.; Niu, L.Y.; Gu, C.D.; Jiang, Z.H.; Jiang, Q. Electroless Ni–P deposition plus zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 5956–5962. [Google Scholar] [CrossRef]
- Zhou, W.; Shan, D.; Han, E.-H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. Formation mechanism of phosphate conversion film on Mg–8.8Li alloy. Corros. Sci. 2009, 51, 62–69. [Google Scholar] [CrossRef]
- Fouladi, M.; Amadeh, A. Effect of phosphating time and temperature on microstructure and corrosion behavior of magnesium phosphate coating. Electrochim. Acta 2013, 106, 1–12. [Google Scholar] [CrossRef]
- Mahto, V.K.; Singh, A.K.; Malik, A. Surface modification techniques of magnesium-based alloys for implant applications. J. Coat. Technol. Res. 2023, 20, 433–455. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, Q.; Zhou, H.; Wang, G.; Peng, X.; Jin, W.; Yu, Z.; Chu, P.K.; Li, W. Titania-zinc phosphate/nanocrystalline zinc composite coatings for corrosion protection of biomedical WE43 magnesium alloy. Surf. Coat. Technol. 2021, 410, 126940. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Comparative study on corrosion protection properties of electroless Ni-P-ZrO2 and Ni-P coatings on AZ91D magnesium alloy. Mater. Corros.-Werkst. Und Korros. 2007, 58, 506–510. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Sun, Y.; Xiong, Z.; Qi, C.; Zhang, Y.; Liu, Y. Robust Superhydrophobic Surface Based on Multiple Hybrid Coatings for Application in Corrosion Protection. Acs Appl. Mater. Interfaces 2019, 11, 6512–6526. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, W.; Wu, L.; Chen, J.; Pan, F. Effect of Microstructure on Layered Double Hydroxides Film Growth on Mg-2Zn-xMn Alloy. Coatings 2021, 11, 59. [Google Scholar] [CrossRef]



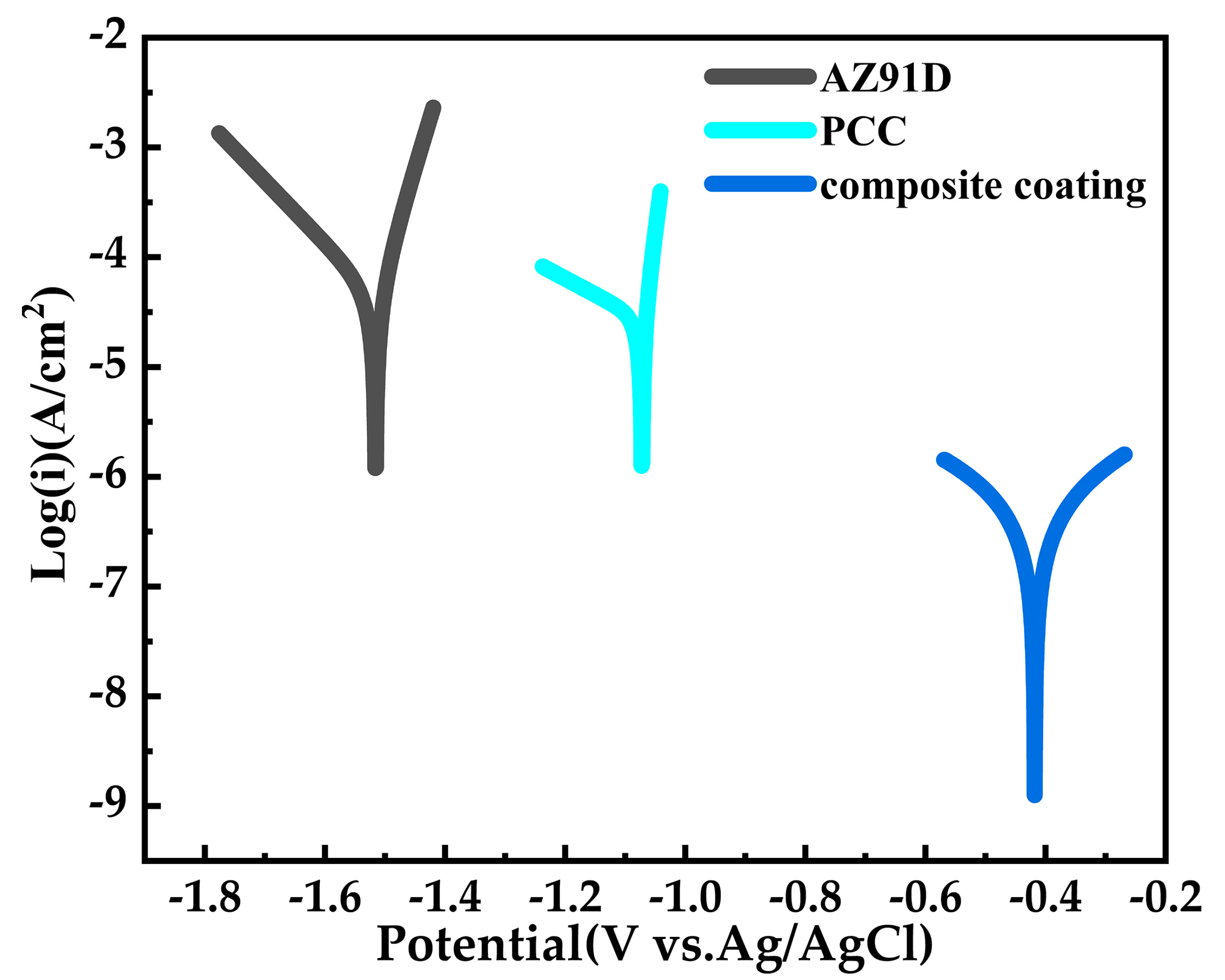
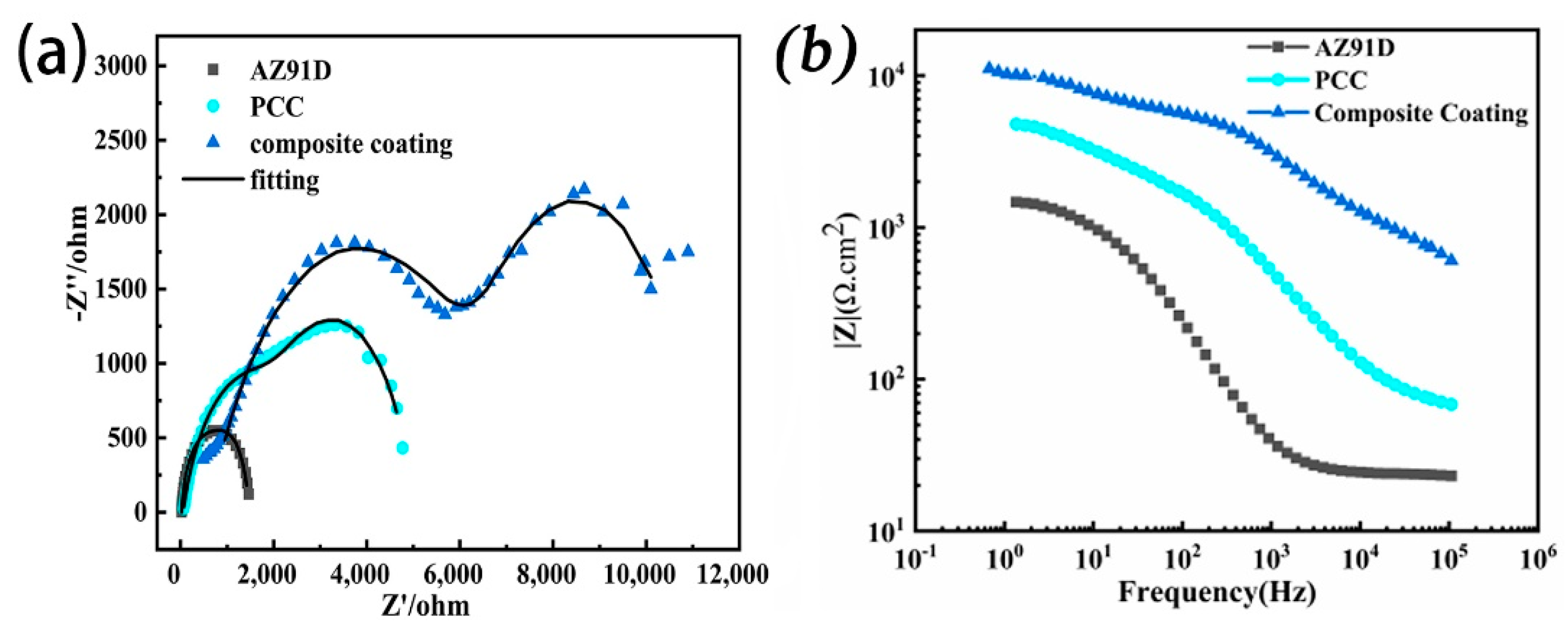


| Sample | Icorr (A/cm2) | Ecorr (V) | η (%) | Corrosion Rate (mm/a) |
|---|---|---|---|---|
| AZ91D | 6.5121 × 10–5 | −1.5154 | − − | 1.3813 |
| PCC | 2.7325 × 10–5 | −1.0722 | 58.0396 | 0.5791 |
| Composite coating | 9.1224 × 10–7 | −0.4181 | 98.5991 | 0.01933 |
| Sample | Rs·(Ω·cm2) | Rc·(Ω·cm2) | CPEd·(μF/cm−2·S(α − 1)) | Rct (Ω·cm2) |
|---|---|---|---|---|
| AZ91D | 24.29 | − − | 0.93 | 285 |
| PCC | 56.91 | 2811 | 0.91 | 2115 |
| Composite coating | 62.36 | 6337 | 0.88 | 4380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, S.; Liu, X.; Cheng, L.; Zhu, J. Superhydrophobic Corrosion-Resistant Coating of AZ91D Magnesium Alloy: Preparation and Performance. Coatings 2024, 14, 1237. https://doi.org/10.3390/coatings14101237
Qi S, Liu X, Cheng L, Zhu J. Superhydrophobic Corrosion-Resistant Coating of AZ91D Magnesium Alloy: Preparation and Performance. Coatings. 2024; 14(10):1237. https://doi.org/10.3390/coatings14101237
Chicago/Turabian StyleQi, Shucheng, Xiang Liu, Lei Cheng, and Jiyuan Zhu. 2024. "Superhydrophobic Corrosion-Resistant Coating of AZ91D Magnesium Alloy: Preparation and Performance" Coatings 14, no. 10: 1237. https://doi.org/10.3390/coatings14101237
APA StyleQi, S., Liu, X., Cheng, L., & Zhu, J. (2024). Superhydrophobic Corrosion-Resistant Coating of AZ91D Magnesium Alloy: Preparation and Performance. Coatings, 14(10), 1237. https://doi.org/10.3390/coatings14101237






