The Influence of Magnetic Field Orientation on the Efficacy and Structure of Ni-W-SiC Coatings Produced by Magnetic Field-Assisted Electrodeposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Coating Material
2.2. Characterization
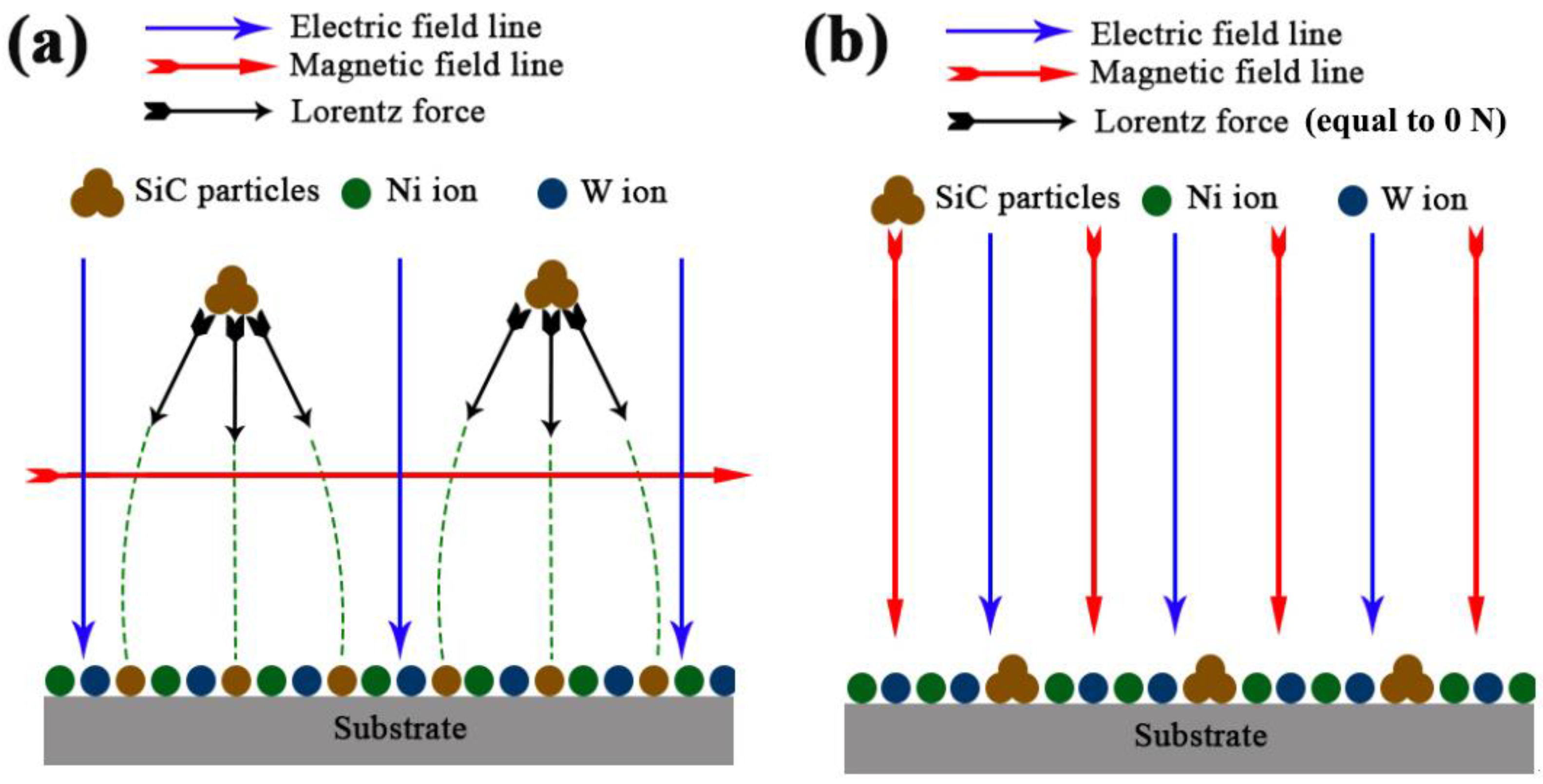
3. Results and Discussion
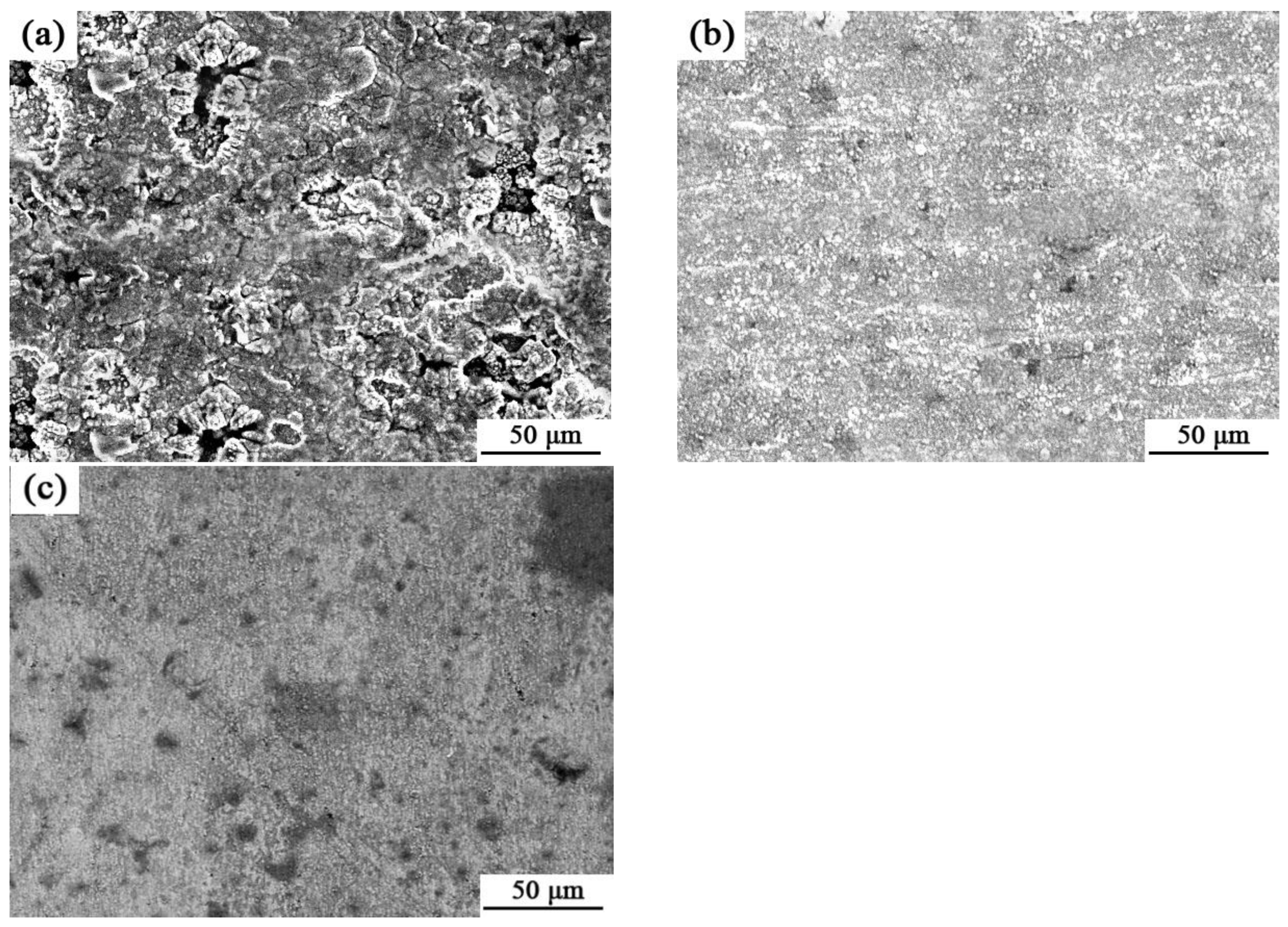
3.1. TEM and EDS Testing
3.2. AFM Measurement
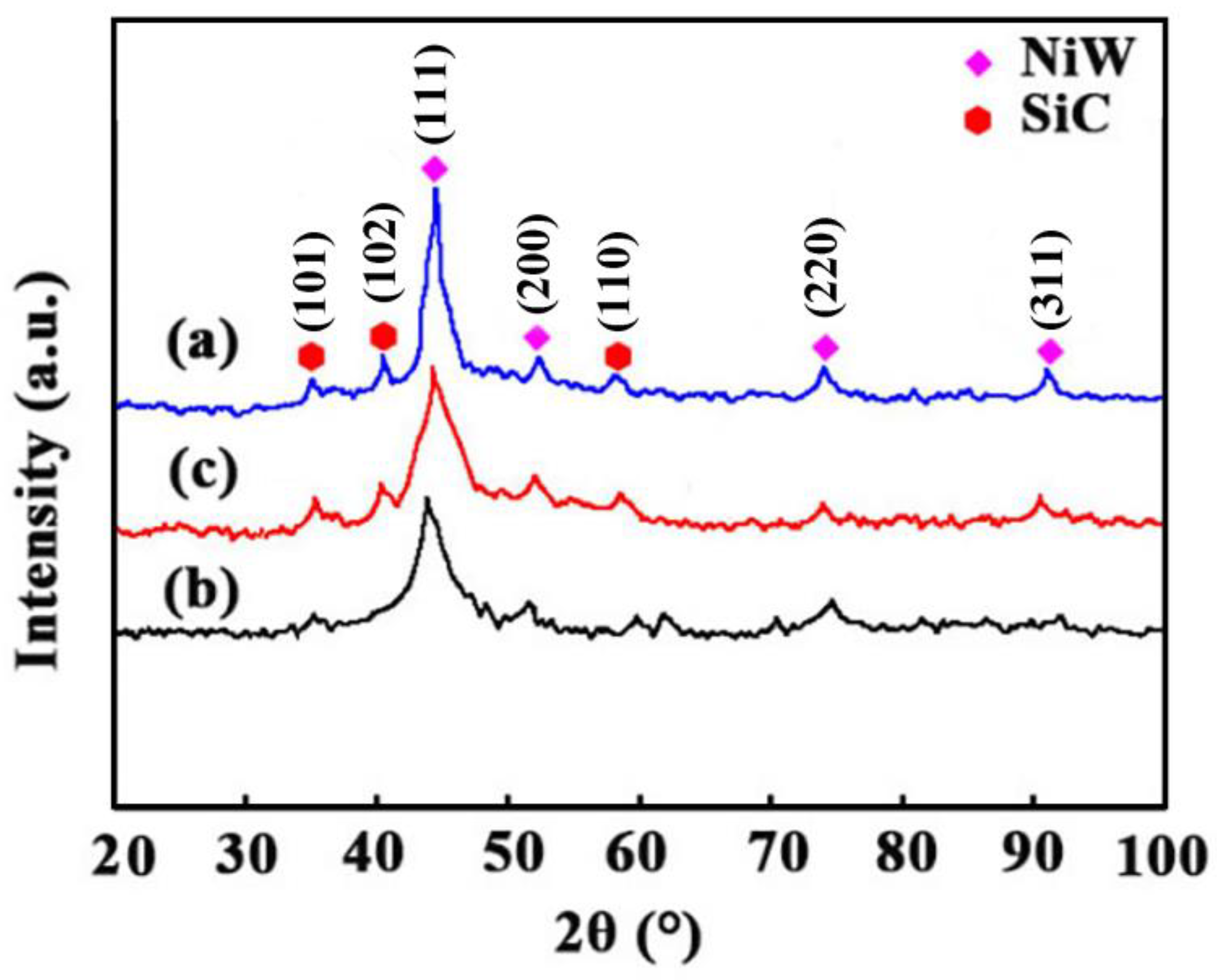
3.3. Crystallinity
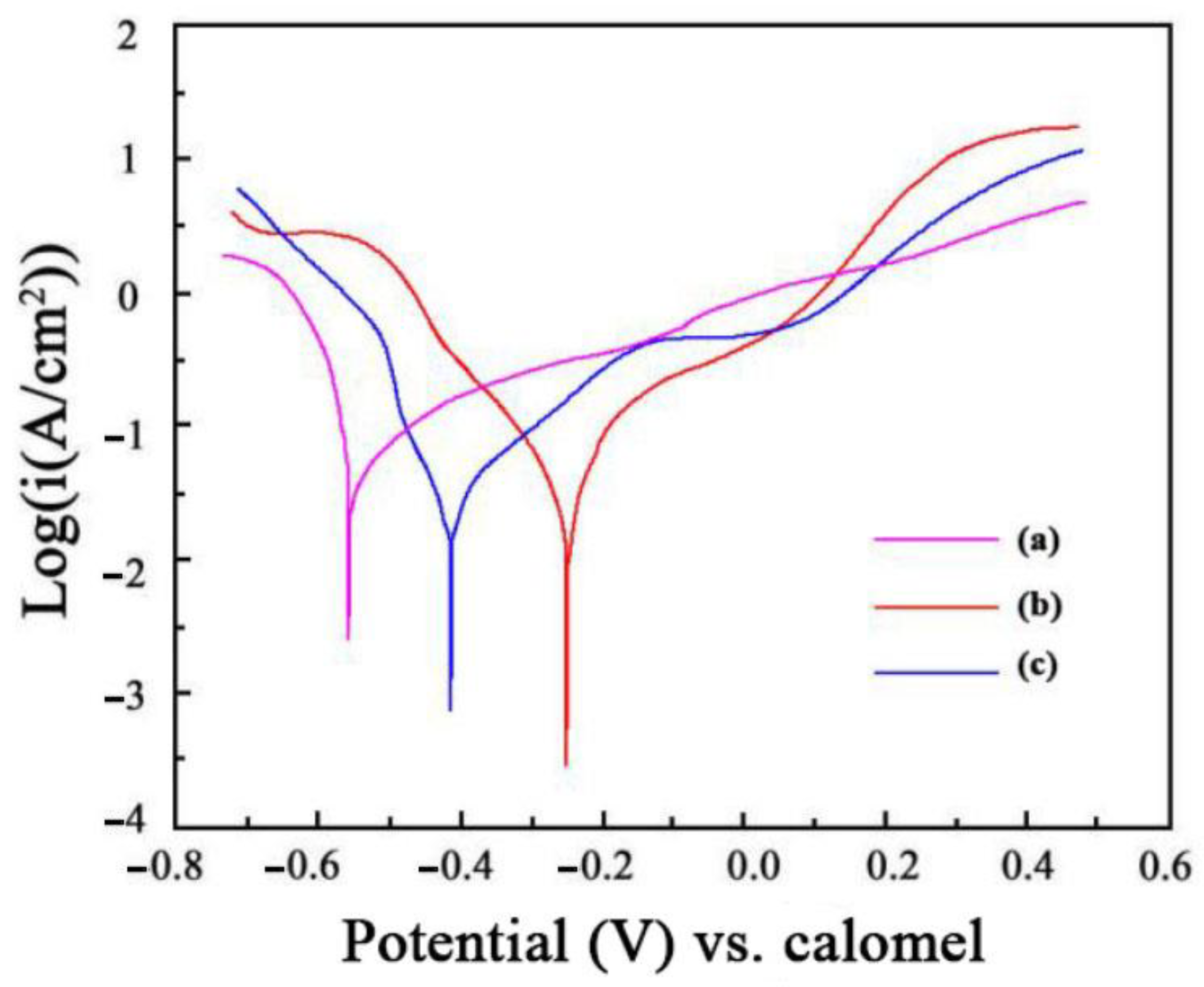
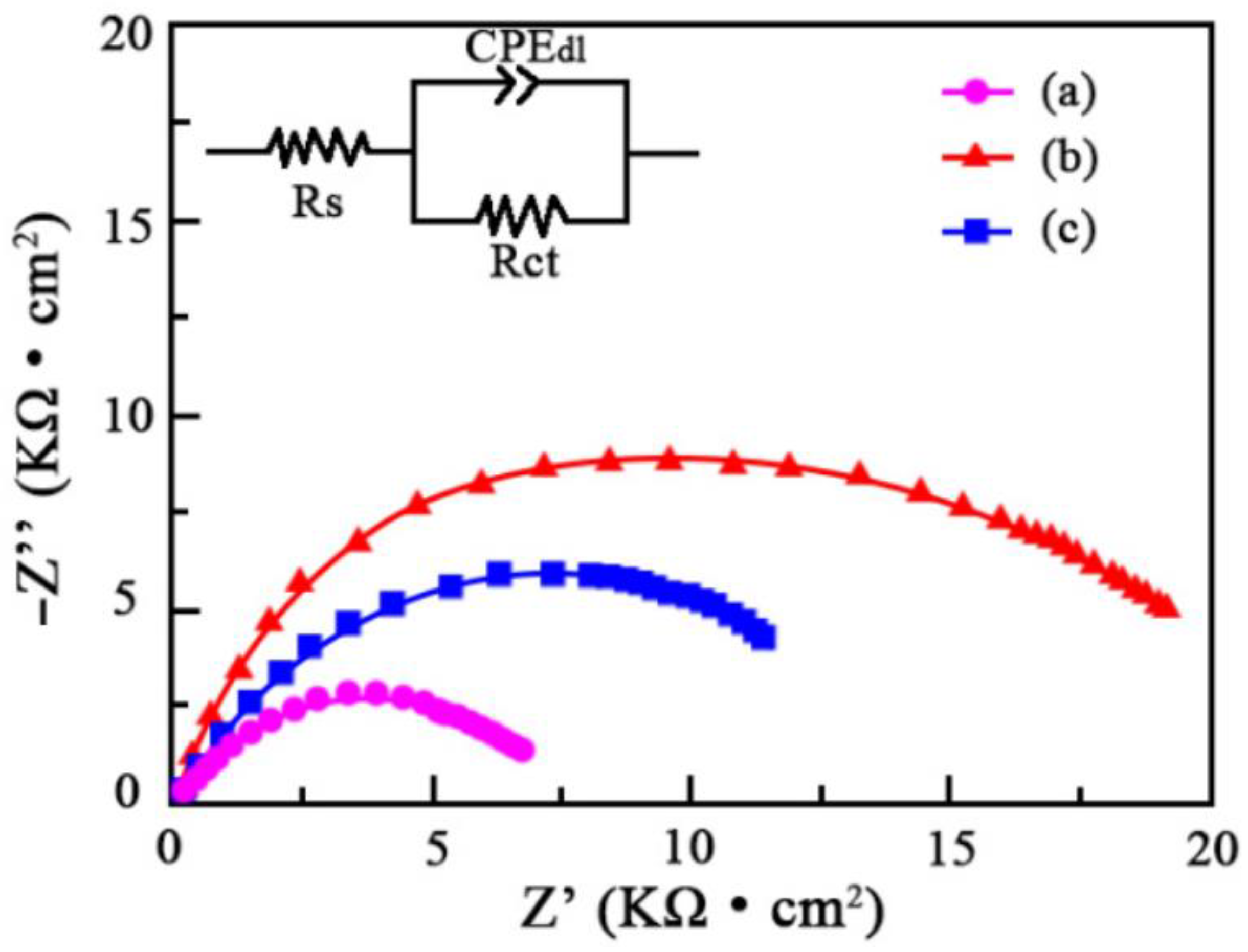
3.4. Corrosion Resistance
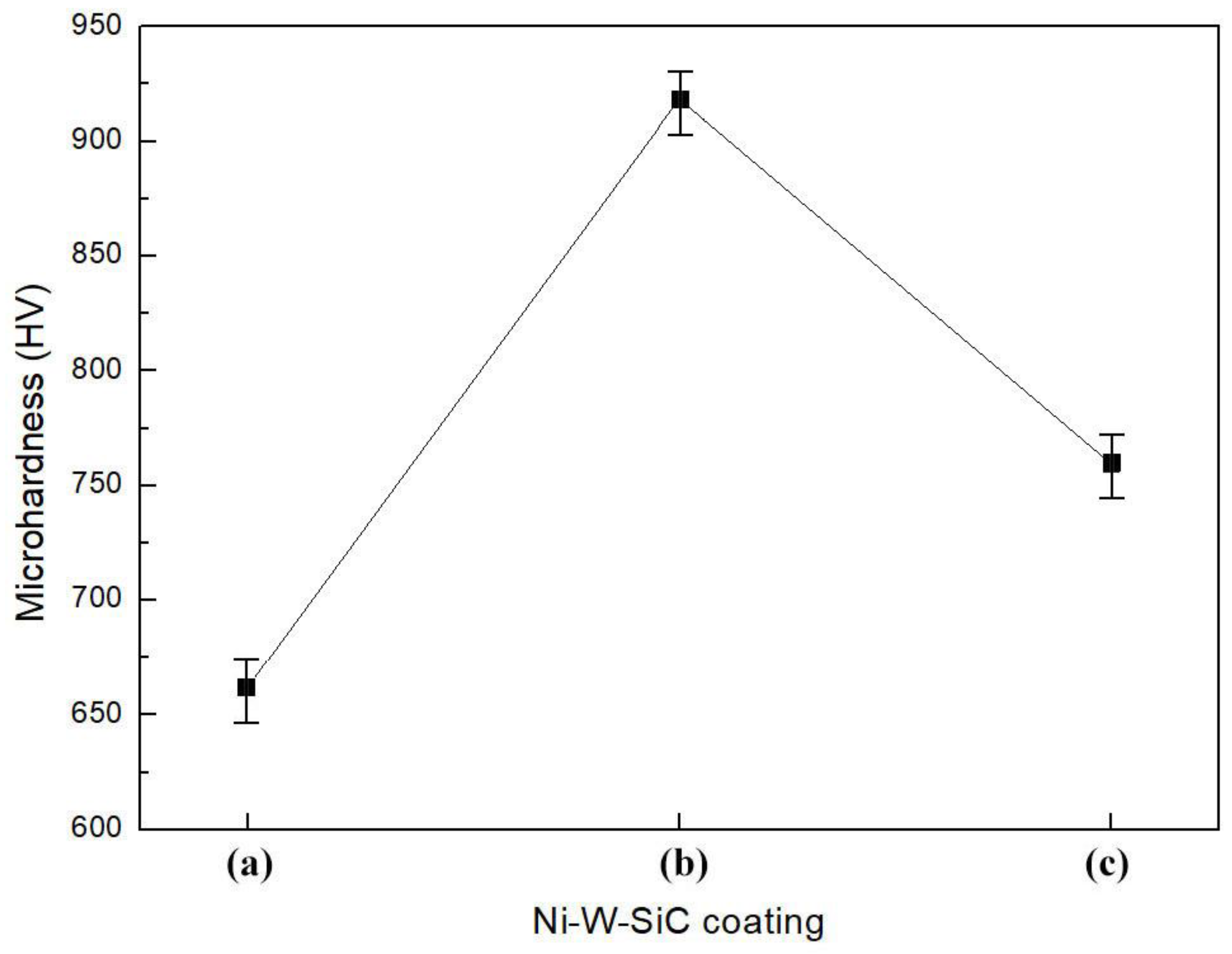
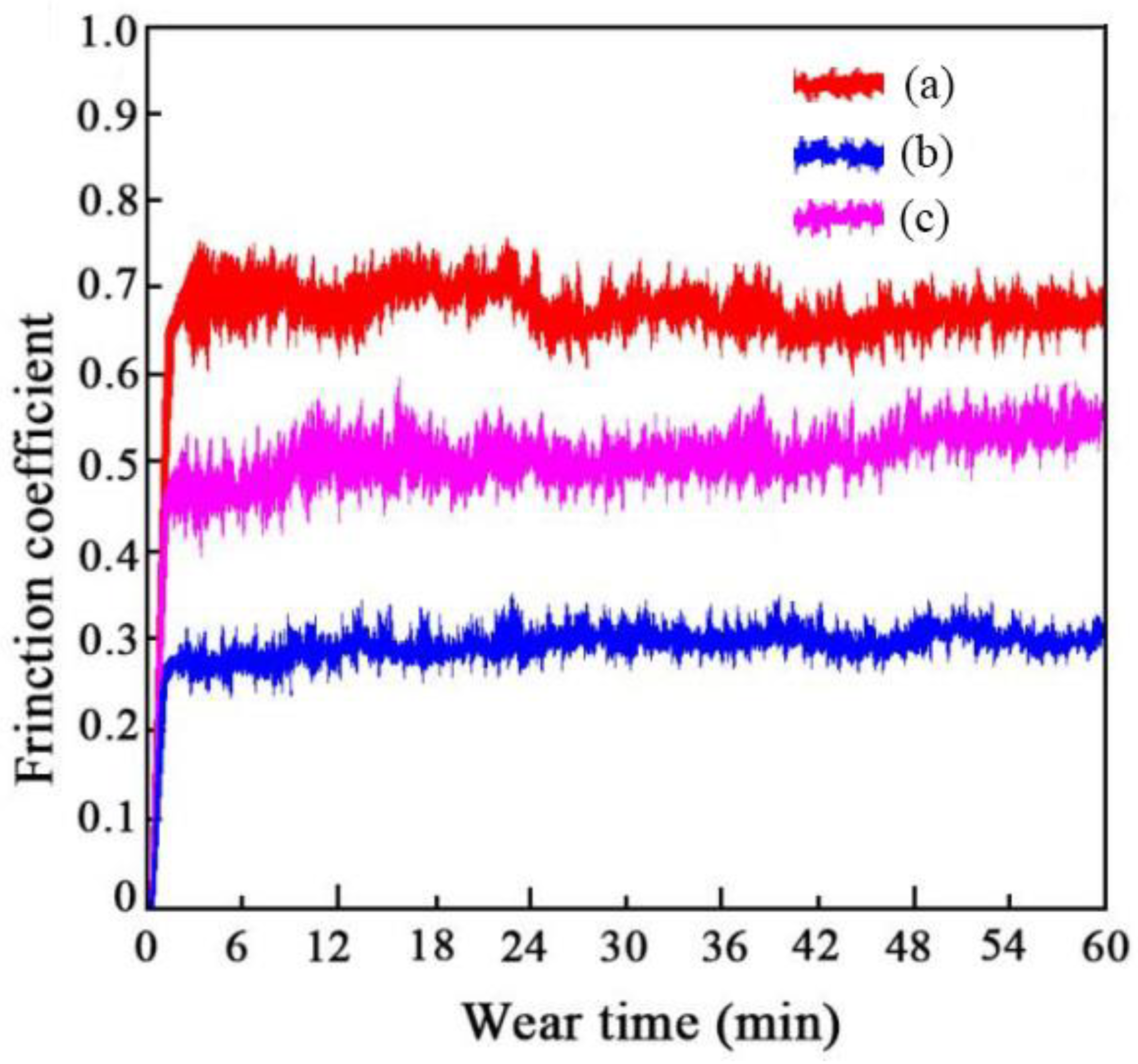
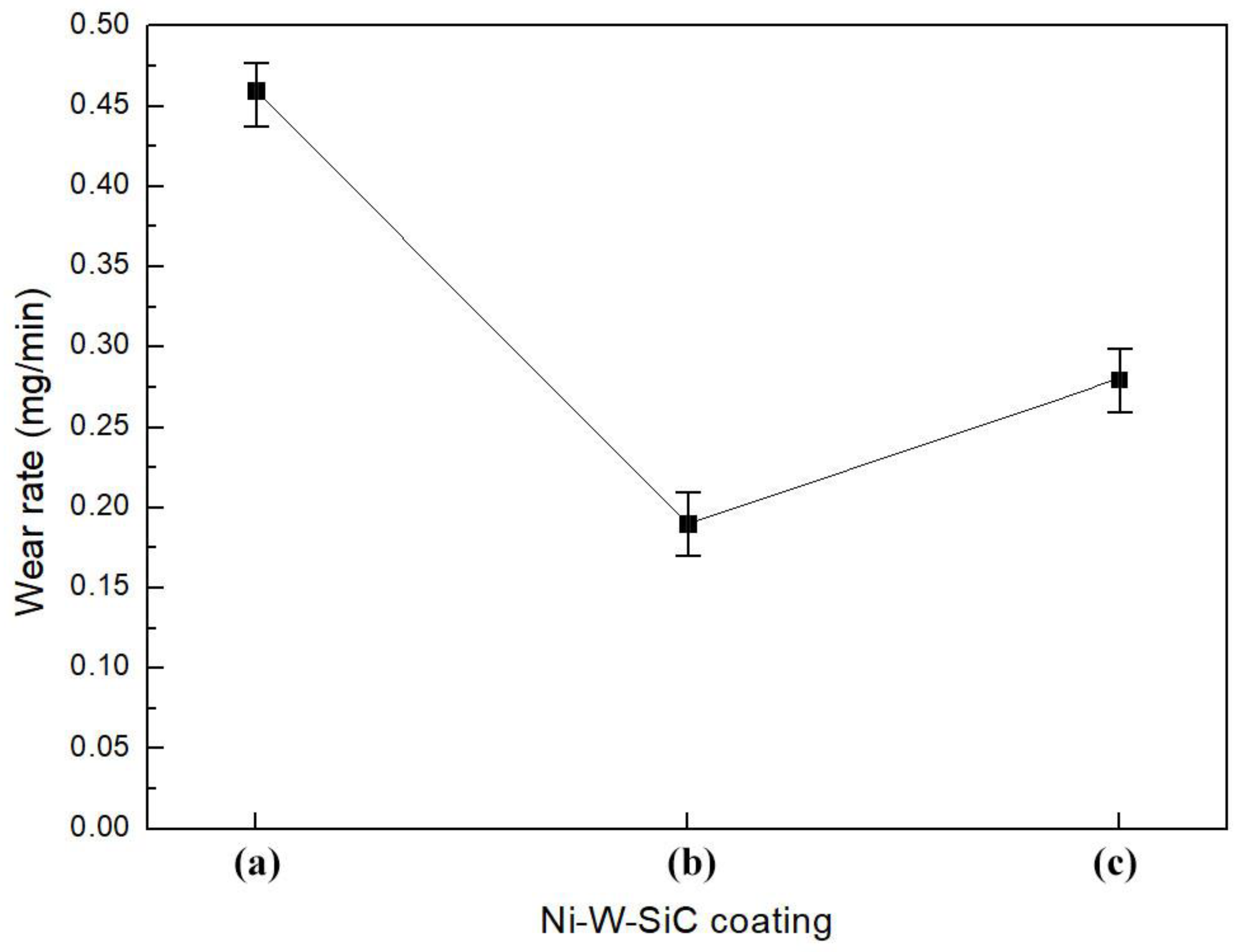
3.5. Abrasion Resistance
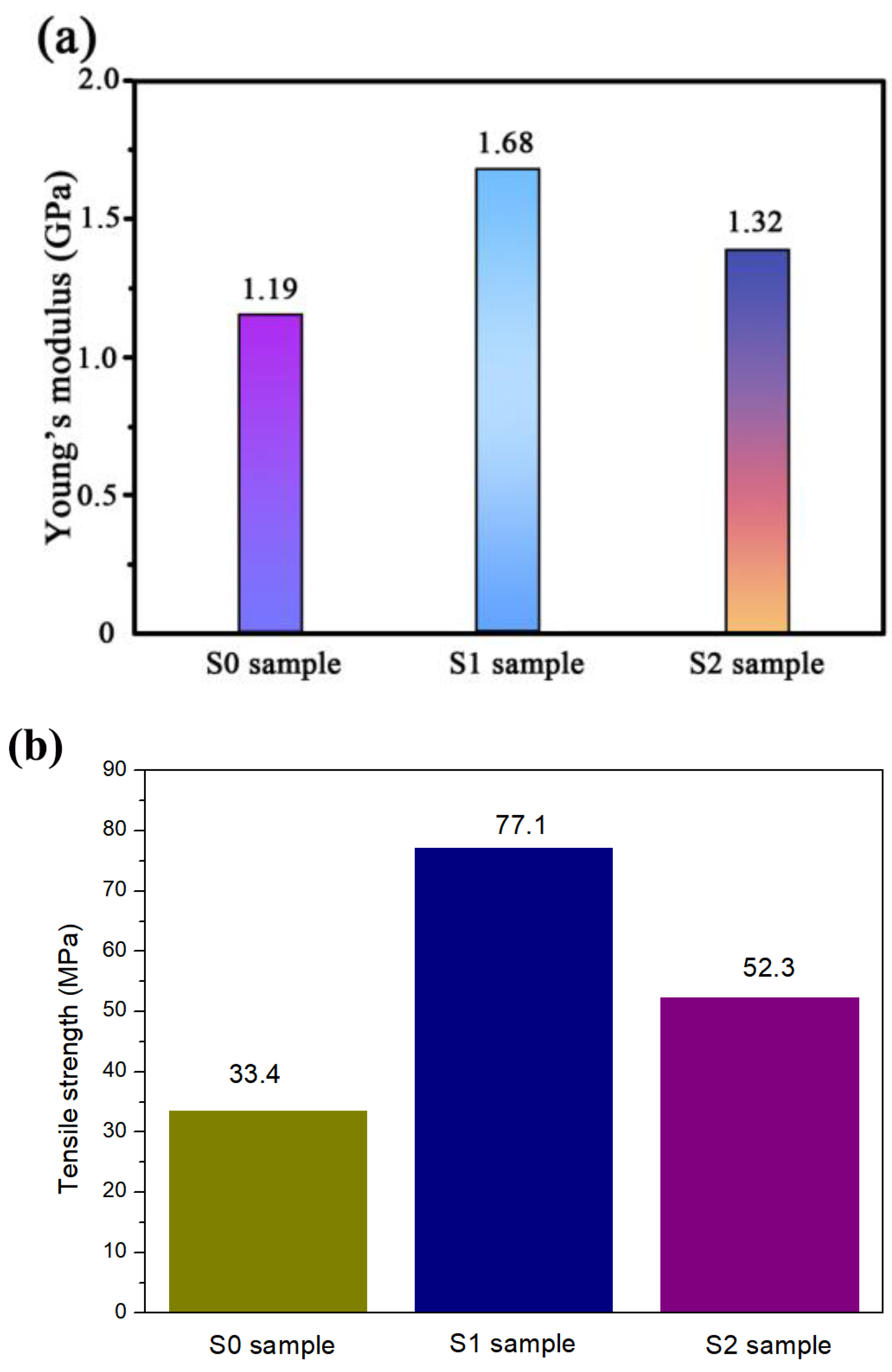
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, D.; Mukherjee, R.; Singh, M.K. A review on tribological behavior of nickel-based Inconel superalloy. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2024, 238, 706–732. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, R.L.; Lei, Y.W. Study of nickel-based composite coatings on H13 steel surface by laser. Adv. Mater. Res. 2011, 154–155, 407–411. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.G.; Kang, L.; Li, Q. Process prediction of Ni-SiC coatings based on RBF-BP model. J. Indian Chem. Soc. 2022, 99, 100513. [Google Scholar] [CrossRef]
- Kumar, K.A.; Mohan, P.; Kalaignan, G.P.; Muralidharan, V.S. Electrodeposition and characterization of Ni-ZrO2 nanocomposites by direct and pulse current methods. J. Nanosci. Nanotechnol. 2013, 12, 8364–8371. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, S.; Zhang, L. Corrosion behavior of Ni-W/SiC nanocomposite coating in NaCl solution. Surf. Rev. Lett. 2006, 13, 489–494. [Google Scholar] [CrossRef]
- Guo, Z.C.; Zhu, X.Y.; Zhai, D.C.; Yang, X.W. Electrodeposition of Ni-W amorphous alloy and Ni-W-SiC composite deposits. J. Mater. Sci. Technol. 2000, 3, 323–326. [Google Scholar]
- Ma, C.; He, H.; Xia, F.; Xiao, Z.; Liu, Y. Performance of Ni-SiC composites deposited using magnetic-field-assisted electrodeposition under different magnetic-field directions. Ceram. Int. 2023, 49, 35907–35916. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Zhang, H.; Li, Z.; Wei, L.; Xia, F. Impact of magnetic field direction on performance and structure of Ni-Co-SiC coatings fabricated via magnetic-field-induced electrodeposition. Coatings 2024, 14, 672. [Google Scholar] [CrossRef]
- Xia, F.F.; Tian, J.Y.; Ma, C.Y.; Xu, X.Y.; Huang, M. Microstructural, nanomechanical and wear properties of magnetic pulse electrodeposited Ni-TiN composite coatings. Sci. Eng. Compos. Mater. 2016, 23, 535–541. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.X.; Zhang, H.B.; Wang, J.D.; Ma, C.Y.; Xu, F.X. Study on magnetic-field-assisted electrodeposited Ni-SiC nanocomposites. J. Mater. Eng. Perform. 2021, 31, 602–612. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Hegde, A.C. Magnetically induced codeposition of Ni-Cd alloy coatings for better corrosion protection. Ind. Eng. Chem. Res. 2014, 53, 5490–5497. [Google Scholar] [CrossRef]
- Yih, K.A. Free convection effect on MHD coupled heat and mass transfer of a moving permeable vertical surface. Int. Commun. Heat Mass. 1999, 26, 95–104. [Google Scholar] [CrossRef]
- Li, F.C.; Kunugi, T.; Serizawa, A. MHD effect on flow structures and heat transfer characteristics of liquid metal-gas annular flow in a vertical pipe. Int. Commun. Heat Mass. 2005, 48, 2571–2581. [Google Scholar] [CrossRef]
- Ke, W.; Jiang, W.; Han, Y.; Chen, Y.; Lao, Y. Effect of parallel magnetic field on growth and properties of jet electrodeposited Ni-Co-SiC composite coatings. J. Alloys Compd. 2023, 946, 169407. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cui, H.X.; Zhou, Q.; Zhang, X.L.; Zhang, Y.R. Microhardness and corrosion resistance of electrodeposited Ni-SiC-BN composite coatings. Int. J. Electrochem. Sci. 2022, 17, 220677. [Google Scholar] [CrossRef]
- Zhao, Y.; Opata, Y.A.; Wu, W.; Grivel, J.C. Surface defects on the Gd2Zr2O7 oxide films grown on textured NiW technical substrates by chemical solution method. Mater. Charact. 2017, 124, 58–64. [Google Scholar] [CrossRef]
- Yang, F.Z.; Guo, Y.F.; Huang, L.; Xu, S.K.; Zhou, S.M. Electrodeposition, structure and corrosion resistance of nanocrystalline NiW alloy. Chin. J. Chem. 2004, 3, 228–231. [Google Scholar] [CrossRef]
- Iwasaki, H.; Higashi, K.; Nich, T.G. Tensile deformation and microstructure of a nanocrystalline Ni-W alloy produced by electrodeposition. Scr. Mater. 2004, 50, 395–399. [Google Scholar] [CrossRef]
- Liu, W.Q.; Lei, W.N.; Shen, Y.; Wang, C.Y.; Qian, H.F.; Li, Q.L. Performance characterization and preparation of Ni-SiC nanocomposites based on SCF-CO2. Integr. Ferroelectr. 2017, 179, 45–55. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Ma, C.Y.; Li, Q. Research on the corrosion behavior of Ni-SiC nanocoating prepared using a jet electrodeposition technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Li, J.; Liu, Q.; Zhang, J.; Wei, T. Optimization design of environmental-friendly Cu-Fe laser cladding coating for self-grown microchannel in a marine corrosive environment. J. Alloys Compd. 2023, 940, 168820. [Google Scholar] [CrossRef]
- Xin, L.; Li, P.; Li, H.; Sun, W.; Zhang, C.; Zhang, K.; Yin, X.; Yu, S. Fabrication of decoupling coatings with robustness and superhydrophobicity for anti-icing and anti-corrosion applications. J. Mater. Sci. 2023, 58, 6038–6054. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, D.Q.; Xia, H.Z.; Xia, F.F.; Ma, Z.P.; Williams, T. Microstructure and properties of Ni-SiC nanocomposites fabricated by ultrasonic-assisted electrodeposition. Int. J. Electrochem. Sci. 2020, 15, 4015–4031. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Lv, X.; Jie, X. Wear and corrosion resistance of cold-sprayed Cu-based composite coatings on magnesium substrate. J. Therm. Spray Technol. 2019, 28, 1212–1224. [Google Scholar] [CrossRef]
- Lou, B.Y.; Xu, B.; Zhou, Y.B.; Bai, W.J.; Du, H.L. Microstructure and wear response of Ni/SiC laser cladding composite coatings on a carbon steel. Key Eng. Mater. 2008, 373–374, 375–378. [Google Scholar] [CrossRef]
- Hu, F.; Chan, K.C.; Song, S.Z.; Yang, X.J. Enhancement of corrosion resistance of electrocodeposited Ni-SiC composites by magnetic field. J. Solid State Electrochem. 2007, 11, 745–750. [Google Scholar] [CrossRef]
- Peng, X.S.; Xu, X.Y.; Wang, J. Characterization and performance prediction of jet pulse electrodeposited Ni-SiC nanocomposites by means of artificial neural networks. Ceram. Int. 2018, 44, 8599–8604. [Google Scholar] [CrossRef]
- Li, Z.J.; Shi, G.M.; Zhao, Q. Improved microwave absorption properties of core–shell type Ni@SiC nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 5887–5897. [Google Scholar] [CrossRef]

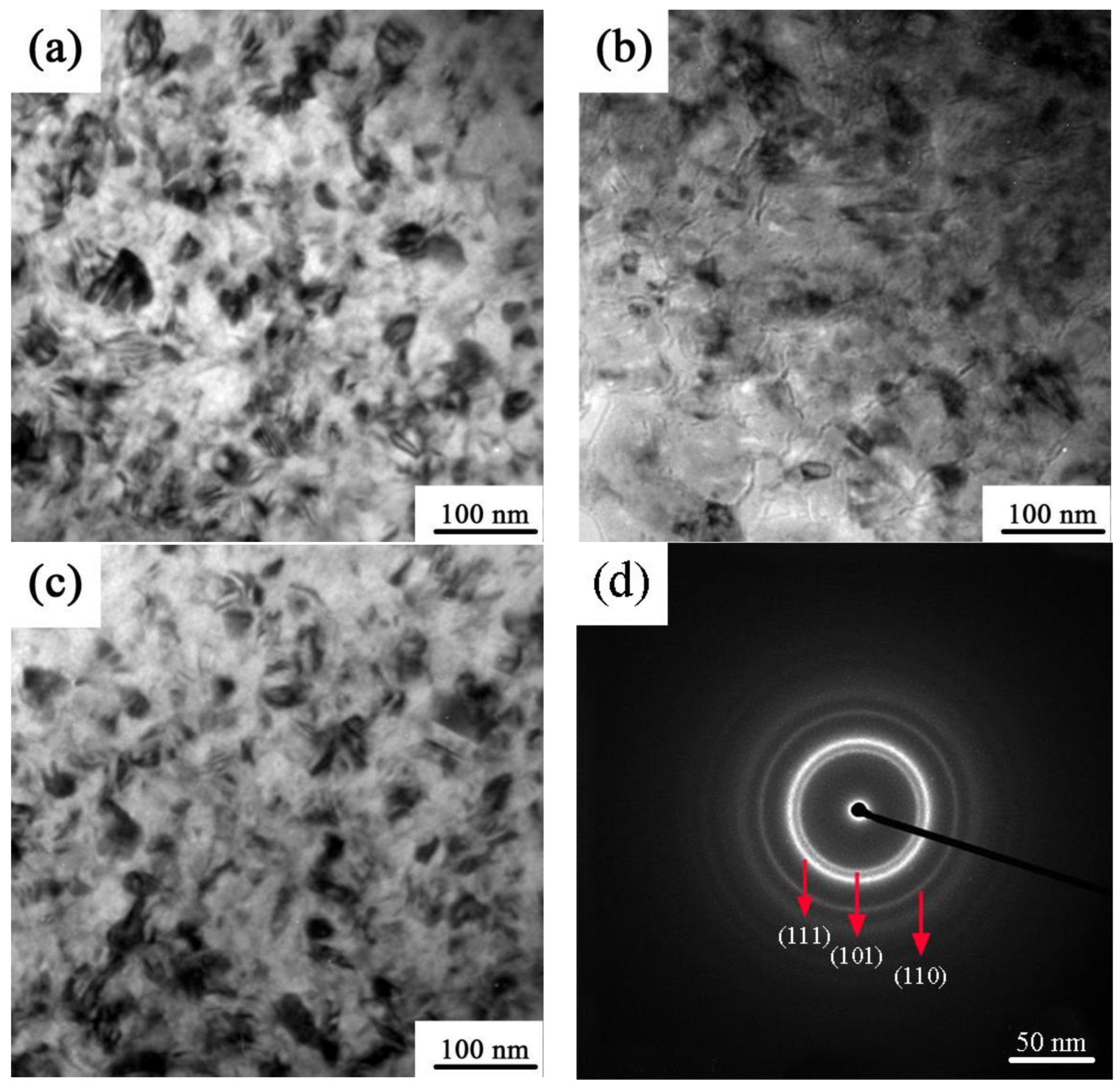
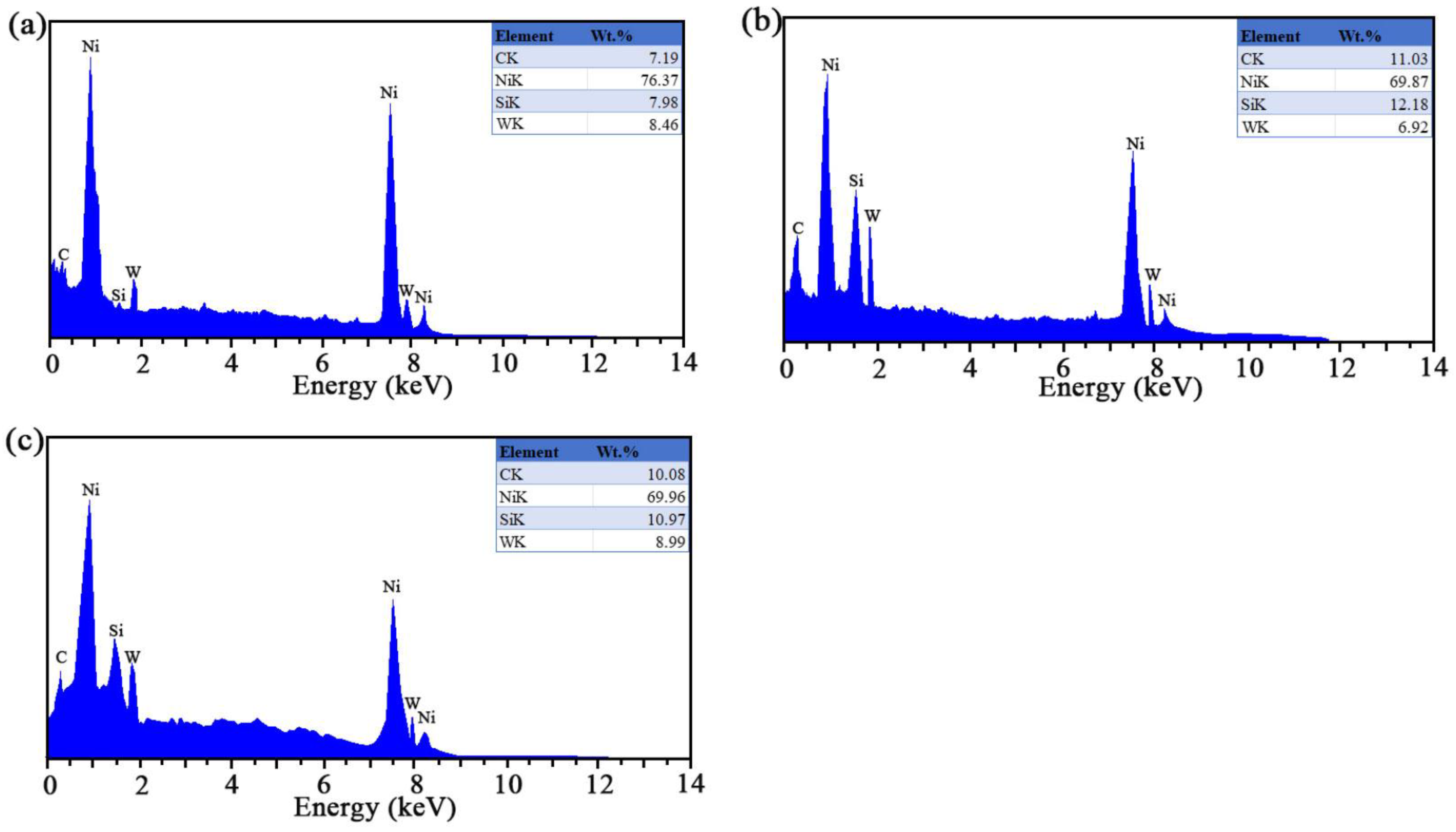
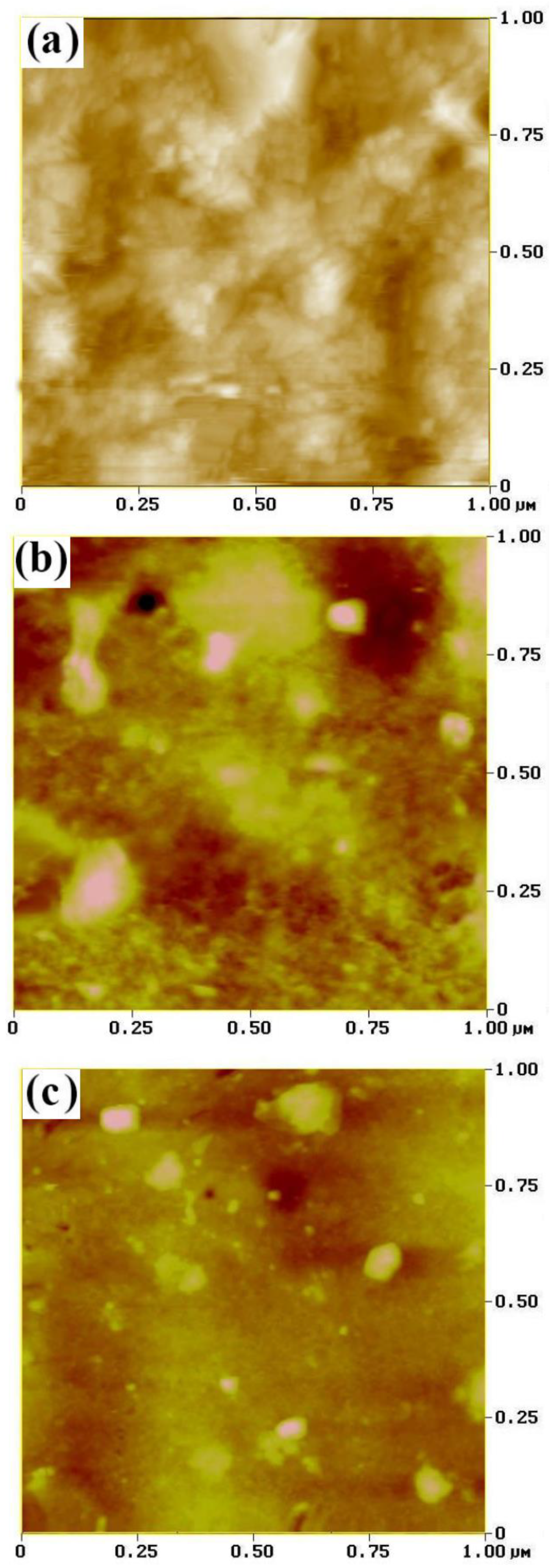
| Sample | Ni-W (nm) | SiC (nm) |
|---|---|---|
| S0 | 133 ± 1 | 76 ± 1 |
| S1 | 84 ± 1 | 53 ± 1 |
| S2 | 103 ± 1 | 71 ± 1 |
| Peak Position (2θ) | Plane Direction | Peak Height (a.u.) | Peak Width (FWHM, 2θ) | Crystallite Size |
|---|---|---|---|---|
| 35.62° | SiC(101) | 131 | 0.11 | 76.31 |
| 41.44° | SiC(102) | 254 | 0.16 | 52.08 |
| 44.62° | Ni-W(111) | 1237 | 0.07 | 131.69 |
| 52.44° | Ni-W(200) | 109 | 0.10 | 85.10 |
| 57.82° | SiC(110) | 188 | 0.13 | 70.95 |
| 76.78° | Ni-W(220) | 159 | 0.12 | 72.60 |
| 92.46° | Ni-W(311) | 183 | 0.09 | 102.87 |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | CPEdl (F·cm−2) |
|---|---|---|---|
| S0 | 152 | 6391 | 8.72 × 10−6 |
| S1 | 187 | 10,582 | 5.38 × 10−6 |
| S2 | 206 | 8143 | 6.94 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Z.; Wei, L.; Xia, F. The Influence of Magnetic Field Orientation on the Efficacy and Structure of Ni-W-SiC Coatings Produced by Magnetic Field-Assisted Electrodeposition. Coatings 2024, 14, 1272. https://doi.org/10.3390/coatings14101272
Zhang H, Li Z, Wei L, Xia F. The Influence of Magnetic Field Orientation on the Efficacy and Structure of Ni-W-SiC Coatings Produced by Magnetic Field-Assisted Electrodeposition. Coatings. 2024; 14(10):1272. https://doi.org/10.3390/coatings14101272
Chicago/Turabian StyleZhang, Hongbin, Zhiping Li, Lixin Wei, and Fafeng Xia. 2024. "The Influence of Magnetic Field Orientation on the Efficacy and Structure of Ni-W-SiC Coatings Produced by Magnetic Field-Assisted Electrodeposition" Coatings 14, no. 10: 1272. https://doi.org/10.3390/coatings14101272




