Enhanced Anti-Corrosion and Biological Performance of Plasma-Sprayed Nb/ZrO2/HA Coatings on ZK60 Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Characterization
2.3. Investigation of Corrosion Resistance
2.4. Cytocompatibility Evaluation
3. Results and Discussion
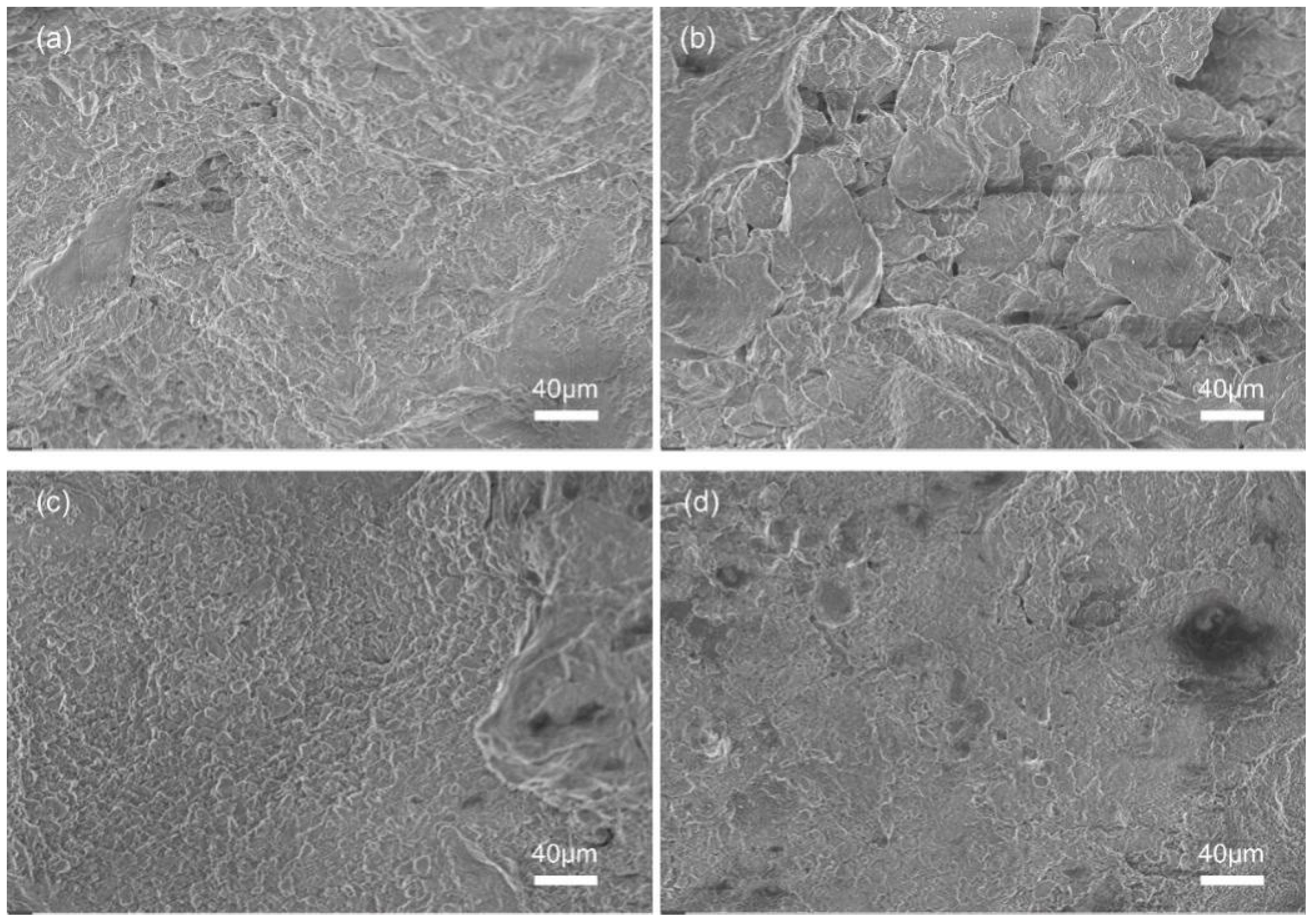
3.1. Microscopic Morphology of the Coatings
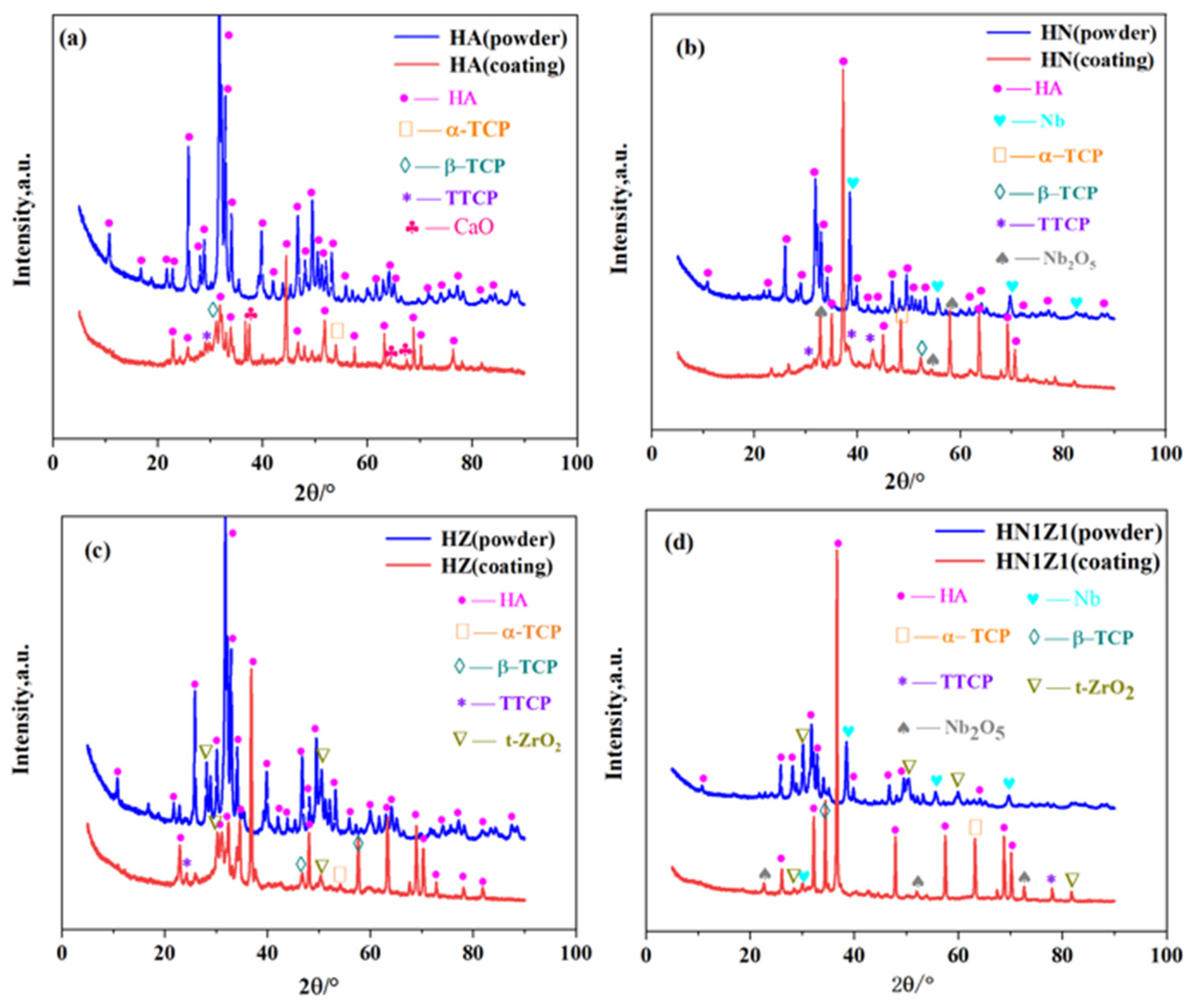
3.2. Phase Analysis of the Coatings
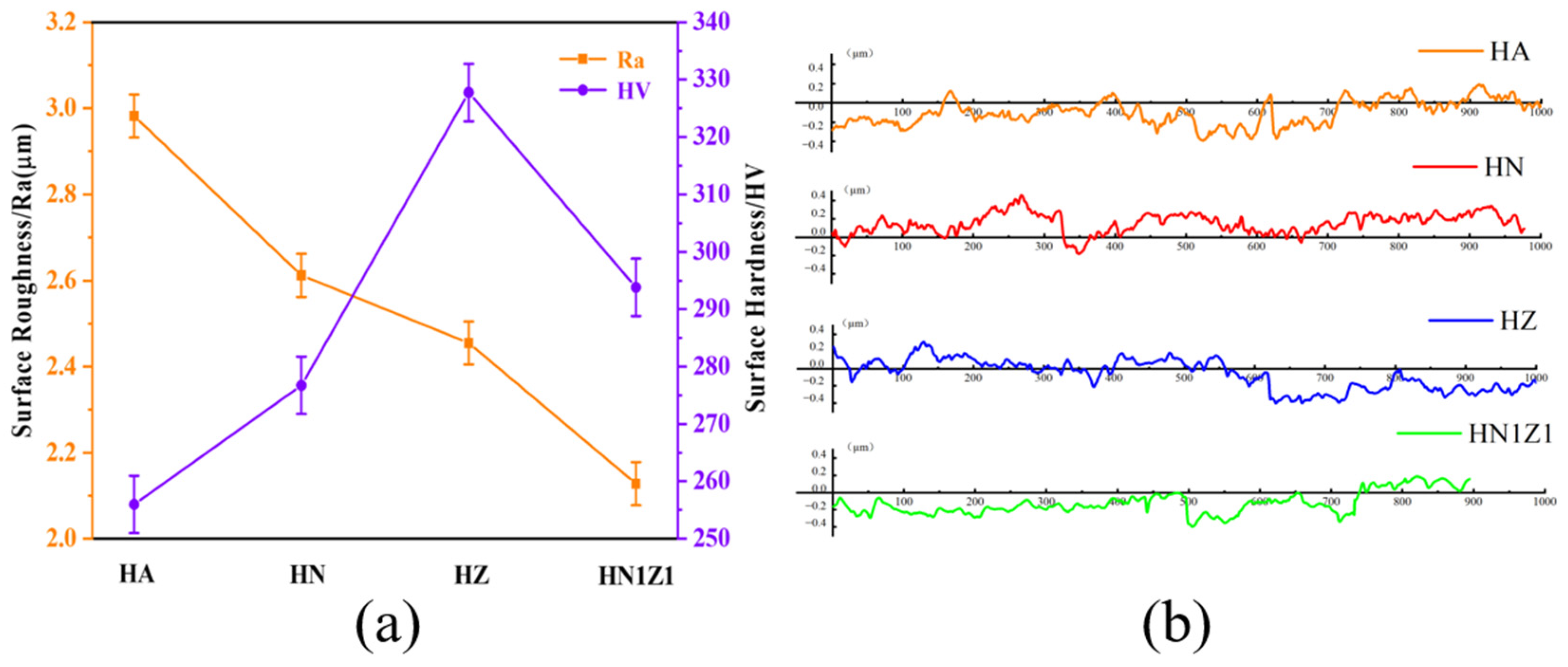
3.3. Hardness and Roughness
3.4. Wettability Analysis of Coatings
3.5. Immersion Test
3.6. Electrochemical Testing of Coatings
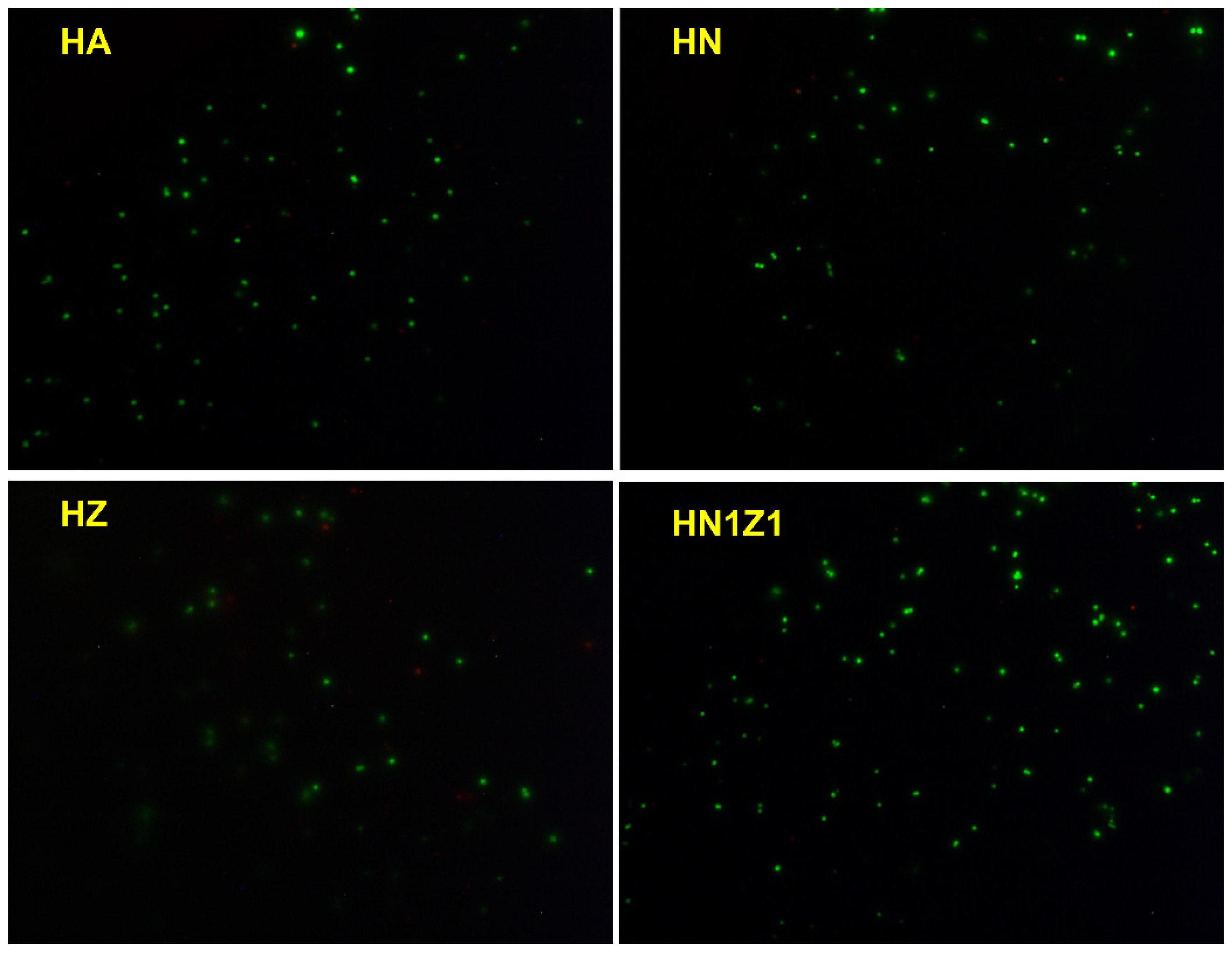
3.7. Cytocompatibility Evaluation of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Eriberto, B.; Barbara, Z. Nanostructured Biomaterials for Tissue Engineered Bone Tissue Reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Vrbka, M.; Marnat, A.B.; Stavness, I.; Roy, C.K.; Mootanah, R.; Krupka, I. The impact of surface and geometry on coefficient of friction of artificial hip joints. J. Mech. Behav. Biomed. Mater. 2017, 72, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N. Artificial organs: Recent progress in metals and ceramics. J. Artif. Organs. 2010, 13, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.Q.; Tang, Y.J.; Wang, K.S. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Tan, L.L.; Yu, X.M.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Dong, J.H.; Lin, T.; Shao, H.P.; Wang, H.; Wang, X.T.; Song, K.; Li, Q.H. Advances in degradation behavior of biomedical magnesium alloys: A review. J. Alloys Compd. 2022, 908, 16. [Google Scholar] [CrossRef]
- Ramalingam, V.V.; Ramasamy, P.; Kovukkal, M.D.; Myilsamy, G. Research and Development in Magnesium Alloys for Industrial and Biomedical Applications: A Review. Met. Mater.-Int. 2020, 26, 409–430. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Chulkova, E.V.; Semiletov, A.M.; Domantovsky, A.G.; Palacheva, V.V.; Emelyanenko, A.M.; Boinovich, L.B. The Potential of the Superhydrophobic State to Protect Magnesium Alloy against Corrosion. Coatings 2022, 12, 13. [Google Scholar] [CrossRef]
- Liu, H.G.; Cao, F.Y.; Song, G.L.; Zheng, D.J.; Shi, Z.M.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Najafi, S.; Sheikhani, A.; Sabbaghian, M.; Nagy, P.; Fekete, K.; Gubicza, J. Modification of the Tensile Performance of an Extruded ZK60 Magnesium Alloy with the Addition of Rare Earth Elements. Materials 2023, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.H.; Wang, C.L.; Sun, M.; Ding, W.J. Recent developments and applications on high-performance cast magnesium rare-earth alloys. J. Magnes. Alloy. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Xun, Q.W.; Liu, Y.H.; Pan, Z.R.; Wu, Y. Microstructure and properties of plasma cladding Ni-based alloy coated on 40Cr Surface. Surf. Topogr.-Metrol. Prop. 2023, 11, 16. [Google Scholar]
- Yao, X.Y.; Tang, J.C.; Zhou, Y.H.; Atrens, A.; Dargusch, M.S.; Wiese, B.; Ebel, T.; Yan, M. Surface modification of biomedical Mg-Ca and Mg-Zn-Ca alloys using selective laser melting: Corrosion behaviour, microhardness and biocompatibility. J. Magnes. Alloy. 2021, 9, 2155–2168. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, J.L.; Jian, S.Y.; Chen, C.A.; Aktug, S.L.; Ger, M.D. The effect of fluoride on the formation of an electroless Ni-P plating film on MAO-coated AZ31B magnesium alloy. J. Mater. Res. Technol JMRT 2022, 19, 542–556. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Y.; Dong, S.; Chen, X.B.; Dong, J. Effects of fluoride ions as electrolyte additives for a PEO/Ni-P composite coating onto Mg alloy AZ31B. Surf. Coat. Technol. 2021, 417, 11. [Google Scholar] [CrossRef]
- Pauline, S.A.; Rajendran, N. Biomimetic novel nanoporous niobium oxide coating for orthopaedic applications. Appl. Surf. Sci. 2014, 290, 448–457. [Google Scholar] [CrossRef]
- Morks, M.E. Fabrication and characterization of plasma-sprayed HA/SiO2 coatings for biomedical application. J. Mech. Behav. Biomed. Mater. 2008, 1, 105–111. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, N.N.; Bolot, R.; Planche, M.P.; Liao, H.L.; Coddet, C. Very low pressure plasma sprayed yttria-stabilized zirconia coating using a low-energy plasma gun. Appl. Phys. A Mater. Sci. Process. 2011, 105, 991–996. [Google Scholar] [CrossRef]
- Hasan, M.F.; Wang, J.; Berndt, C. Determination of the Mechanical Properties of Plasma-Sprayed Hydroxyapatite Coatings Using the Knoop Indentation Technique. J. Therm. Spray Technol. 2015, 24, 865–877. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G.; Sidhu, B.S. Analysis of Corrosion Behavior and Surface Properties of Plasma-Sprayed HA/Ta Coating on CoCr Alloy. J. Therm. Spray Technol. 2018, 27, 1401–1413. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Investigation of surface properties and corrosion behavior of plasma sprayed HA/ZnO coatings prepared on AZ31 Mg alloy. Surf. Coat. Technol. 2020, 401, 9. [Google Scholar] [CrossRef]
- Li, B.X.; Niu, J.L.; Liu, H.Y.; Li, G.Y. Fabrication and corrosion property of novel 3-aminopropyltriethoxy-modified calcium phosphate/poly(lactic acid) composite coating on AZ60 Mg alloy. Appl. Phys. A Mater. Sci. Process. 2018, 124, 13. [Google Scholar] [CrossRef]
- Yugeswaran, S.; Yoganand, C.P.; Kobayashi, A.; Paraskevopoulos, K.M.; Subramanian, B. Mechanical properties, electrochemical corrosion and in-vitro bioactivity of yttria stabilized zirconia reinforced hydroxyapatite coatings prepared by gas tunnel type plasma spraying. J. Mech. Behav. Biomed. Mater. 2012, 9, 22–33. [Google Scholar] [CrossRef]
- Hussain, S.; Shah, Z.A.; Sabiruddin, K.; Keshri, A.K. Characterization and tribological behaviour of Indian clam seashell-derived hydroxyapatite coating applied on titanium alloy by plasma spray technique. J. Mech. Behav. Biomed. Mater. 2023, 137, 15. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G.; Sidhu, B.S.; Bhatia, N. In-vitro assessment of HA-Nb coating on Mg alloy ZK60 for biomedical applications. Mater. Chem. Phys. 2019, 231, 138–149. [Google Scholar] [CrossRef]
- Singh, B.; Singh, G.; Sidhu, B.S. Investigation of the in vitro corrosion behavior and biocompatibility of niobium (Nb)-reinforced hydroxyapatite (HA) coating on CoCr alloy for medical implants. J. Mater. Res. 2019, 34, 1678–1691. [Google Scholar] [CrossRef]
- Chen, M.; Lu, C.; Yu, J. Improvement in performance of MgO–CaO refractories by addition of nano-sized ZrO2. J. Eur. Ceram. Soc. 2007, 27, 4633–4638. [Google Scholar] [CrossRef]
- Hung, K.Y.; Lai, H.C.; Yang, Y.C.; Feng, H.-P. Characterization of hydroxyapatite (HA) sputtering targets by APS methods. Coatings 2017, 7, 197. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings. Corrosion Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Lv, M.; Lv, W.J.; Chen, H.Z.; Zheng, F.; Liu, J.; Kong, F.D.; Liu, S.L.; Wang, L.T. Biotribological properties of nano zirconium dioxide and hydroxyapatite-reinforced polyetheretherketone (HA/ZrO2/PEEK) biocomposites. Iran. Polym. J. 2021, 30, 1127–1136. [Google Scholar] [CrossRef]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Prymak, O.; Epple, M.; Litvinova, L.S.; Shupletsova, V.V.; Malashchenko, V.V.; Yurova, K.A.; Dzyuman, A.N.; et al. Zn-or Cu-containing CaP-based coatings formed by micro-arc oxidation on titanium and Ti-40Nb alloy: Part II—Wettability and biological performance. Materials 2020, 13, 4366. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zheng, X.X.; Wang, Y.X.; Tao, T.Y.; Wang, Z.L.; Yuan, L.; Han, B. The Biomimetics of Mg2+-Concentration-Resolved Microenvironment for Bone and Cartilage Repairing Materials Design. Biomimetics 2022, 7, 21. [Google Scholar] [CrossRef] [PubMed]




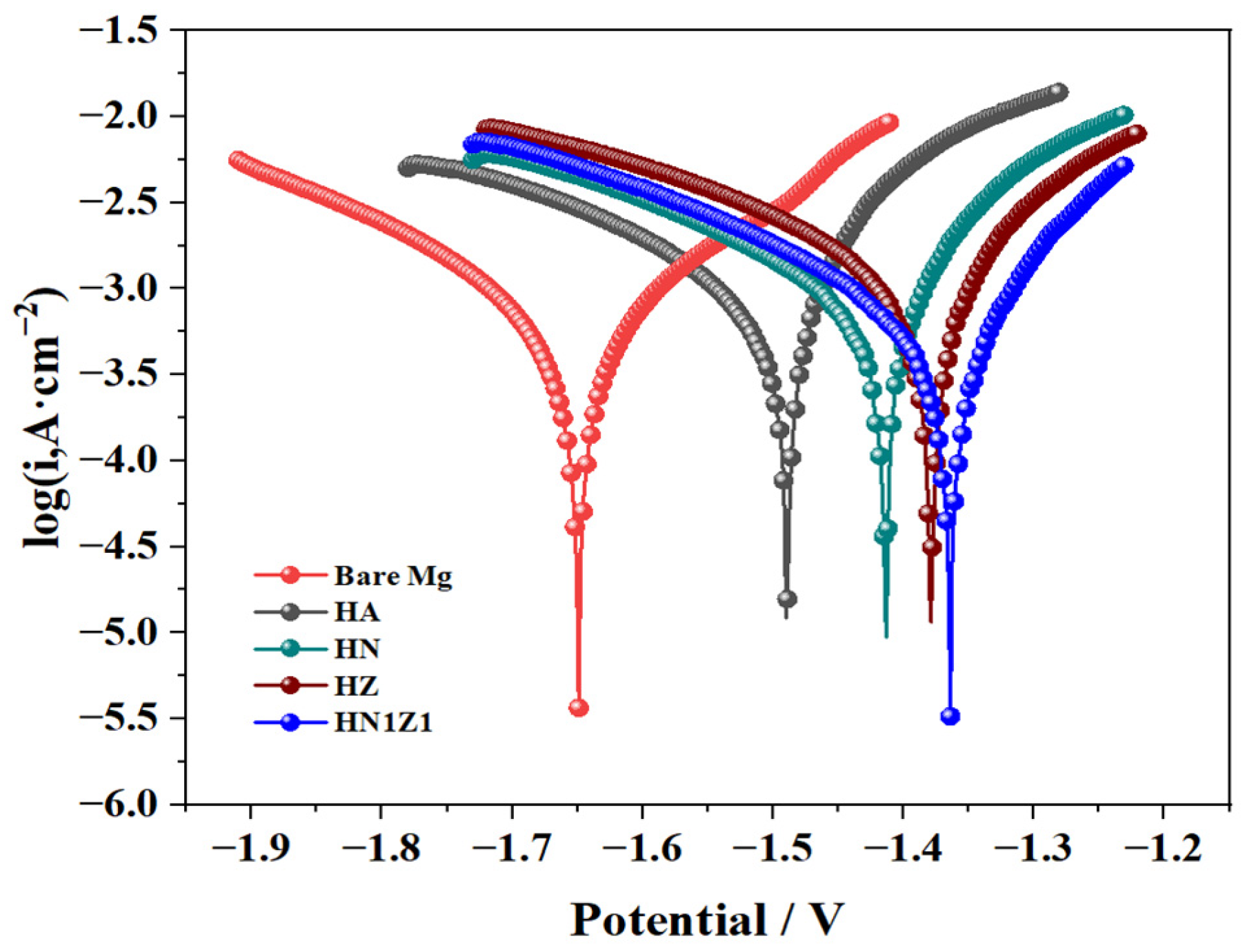

| Sample | (V) | (A·cm−2) |
|---|---|---|
| Mg | −1.649 | 1.030 × 10−2 |
| HA | −1.490 | 1.169 × 10−3 |
| HN | −1.413 | 1.148 × 10−3 |
| HZ | −1.379 | 1.134 × 10−3 |
| HN1Z1 | −1.364 | 5.109 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Fang, S.; Xu, S.; Yu, L.; Zhou, J.; Qian, S.; Huang, F.; Ma, C. Enhanced Anti-Corrosion and Biological Performance of Plasma-Sprayed Nb/ZrO2/HA Coatings on ZK60 Mg Alloy. Coatings 2024, 14, 1282. https://doi.org/10.3390/coatings14101282
Wan X, Fang S, Xu S, Yu L, Zhou J, Qian S, Huang F, Ma C. Enhanced Anti-Corrosion and Biological Performance of Plasma-Sprayed Nb/ZrO2/HA Coatings on ZK60 Mg Alloy. Coatings. 2024; 14(10):1282. https://doi.org/10.3390/coatings14101282
Chicago/Turabian StyleWan, Xiaofeng, Siyi Fang, Shouwei Xu, Lu Yu, Jingling Zhou, Shuangqing Qian, Fenglai Huang, and Chunhui Ma. 2024. "Enhanced Anti-Corrosion and Biological Performance of Plasma-Sprayed Nb/ZrO2/HA Coatings on ZK60 Mg Alloy" Coatings 14, no. 10: 1282. https://doi.org/10.3390/coatings14101282








