The Impact of Thermal Treatment on the Structural, Optical and Electrochemical Characteristics of Tin Sulfide Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
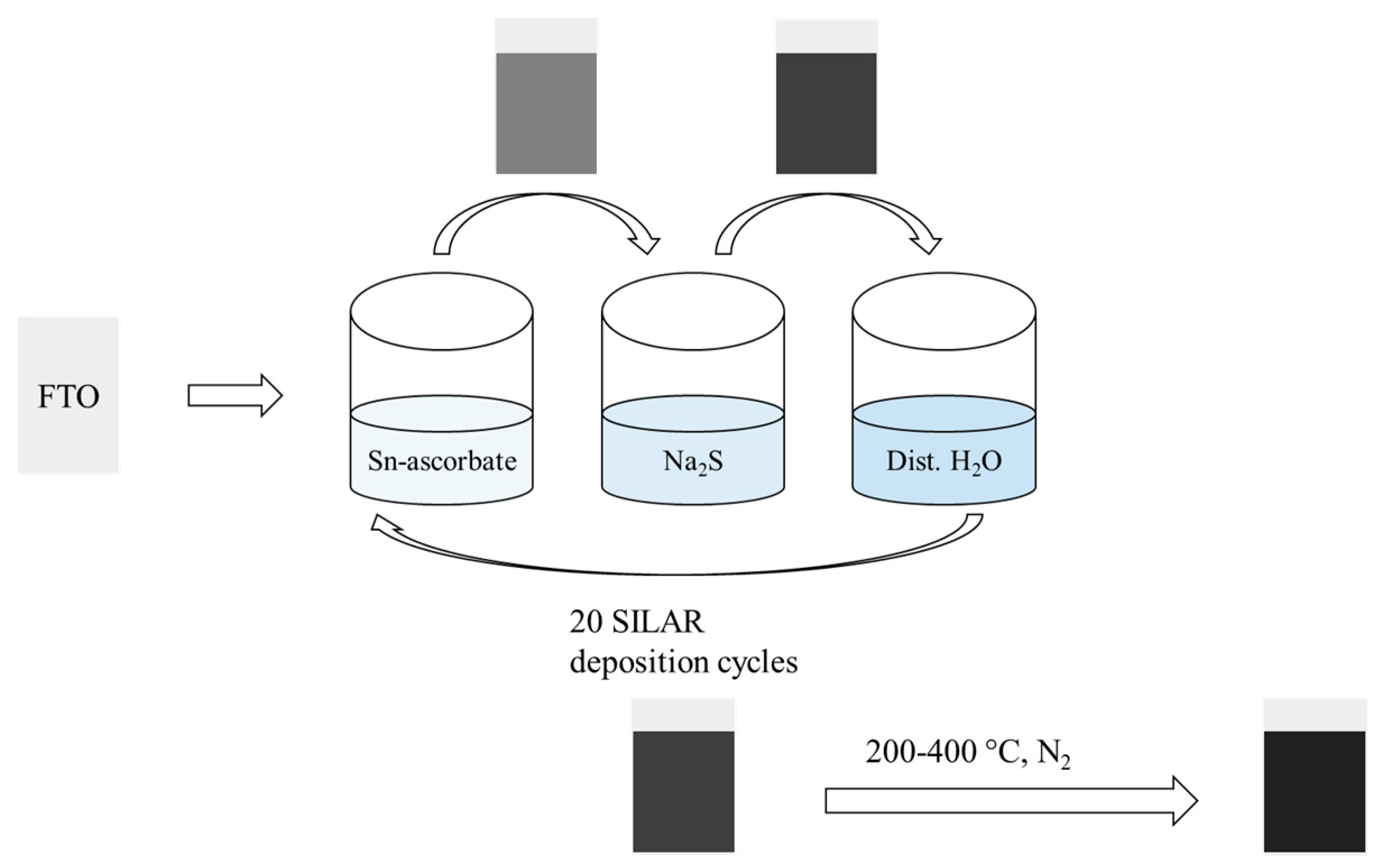
2.2. Film Deposition
2.3. Characterization of Films
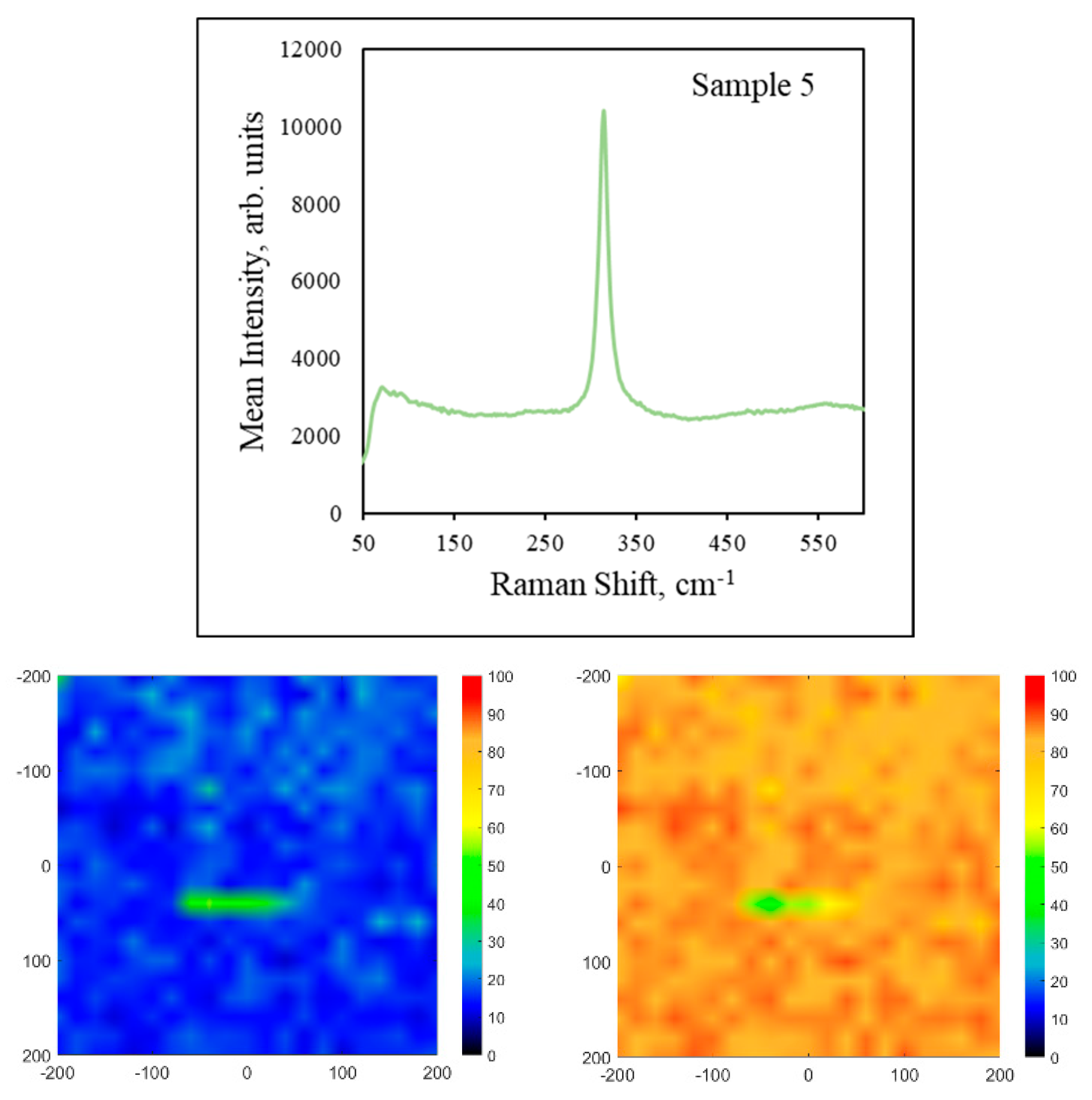
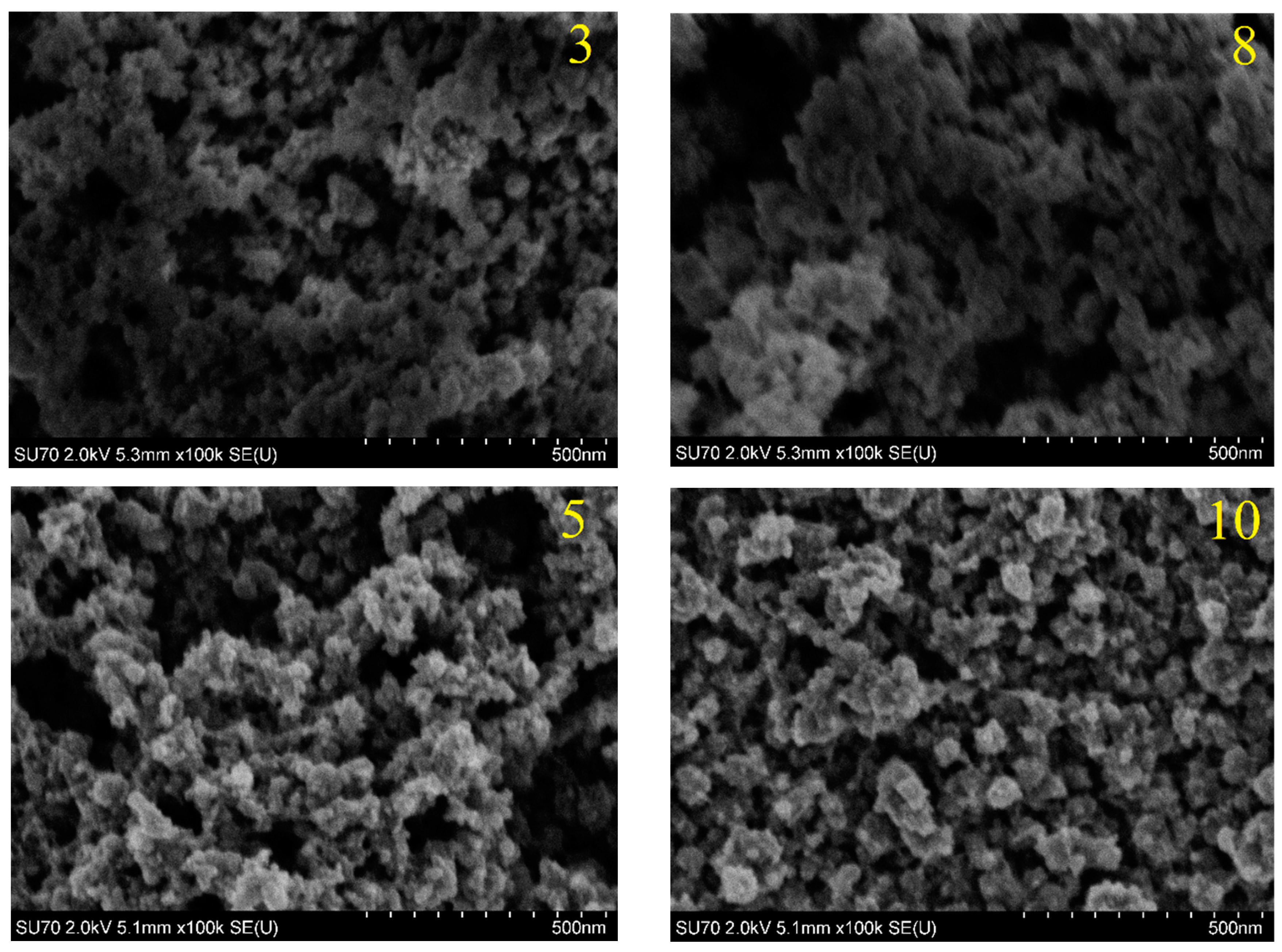
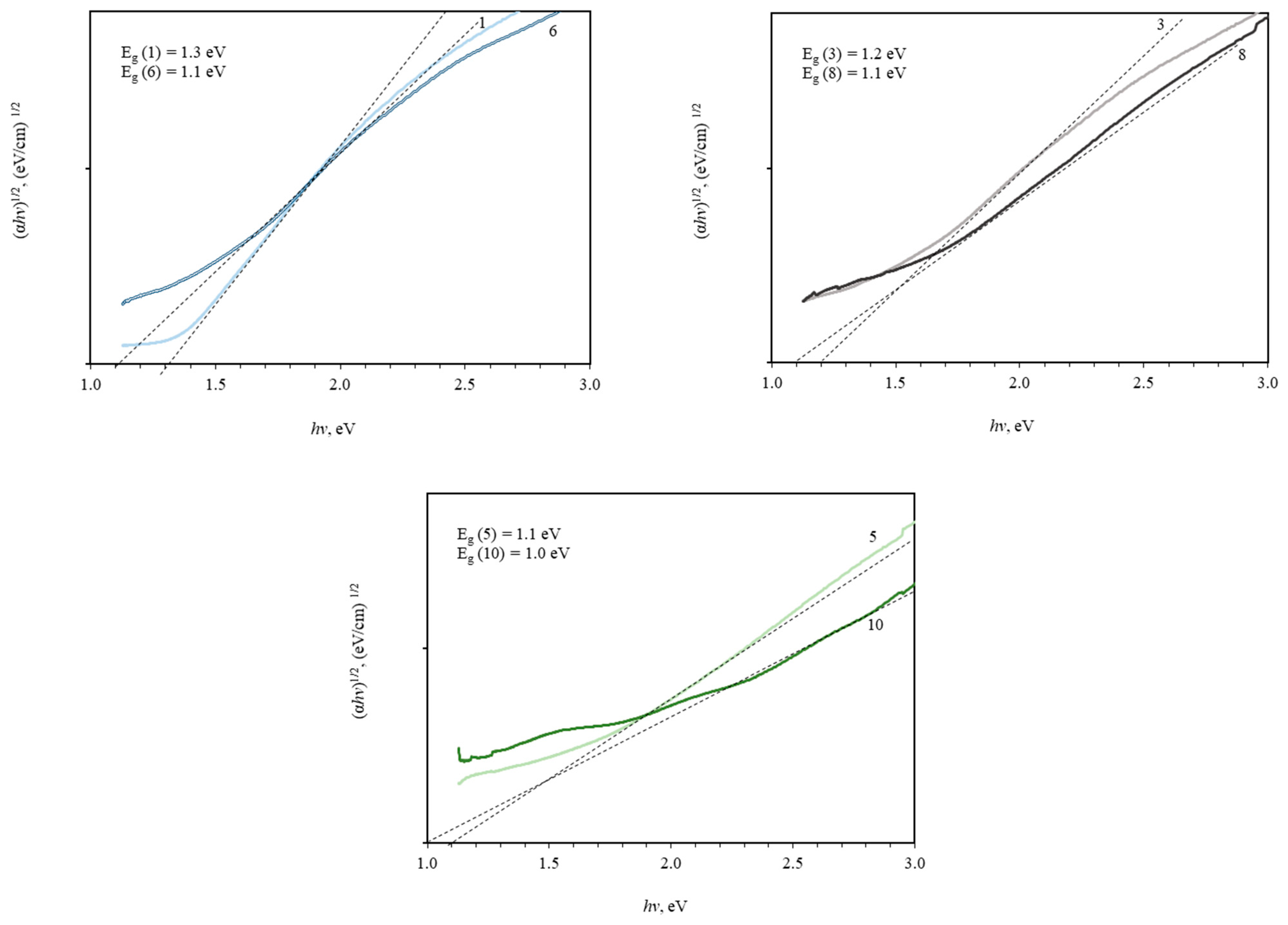
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronusiene, A.; Popov, A.; Barauskiene, I.; Ancutiene, I. Effect of Ascorbic Acid on the Properties of Tin Sulfide Films for Supercapacitor Application. Surf. Interfaces 2021, 25, 101275. [Google Scholar] [CrossRef]
- Elyas, A.A. Study of Sb Doped SnS Thin Film and Its Heterojunctions; Division of Energy and Systems Engineering: Nagano, Japan, 2022. [Google Scholar]
- Burton, L.A.; Colombara, D.; Abellon, R.D.; Grozema, F.C.; Peter, L.M.; Savenije, T.J.; Dennler, G.; Walsh, A. Synthesis, Characterization, and Electronic Structure of Single-Crystal SnS, Sn2S3, and SnS2. Chem. Mater. 2013, 25, 4908–4916. [Google Scholar] [CrossRef]
- Al-Mamun, N.S.; Wolfe, D.E.; Haque, A.; Yim, J.-G.; Kim, S.K. Room Temperature Annealing of SnS2 Films with Electron Impulse Force. Scr. Mater. 2023, 224, 1359–6462. [Google Scholar] [CrossRef]
- Shimada, T.; Ohuchi, F.S.; Parkinson, B.A. Thermal Decomposition of SnS2 and SnSe2: Novel Molecular-Beam Epitaxy Sources for Sulfur and Selenium. J. Vac. Sci. Technol. A 1992, 10, 539–542. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.; Liu, Z.; Shang, Y.; Li, Z.; Ma, Y. Amorphous Bimetallic Sulfide Co9S8/SnS2 Used as a p-n Heterojunction to Achieve Photocatalytic Hydrogen Evolution. New J. Chem. 2023, 47, 16066–16077. [Google Scholar] [CrossRef]
- Gupta, Y.; Arun, P.; Choi, H.; Lee, J.; Shin, S.; Lee, J.; Lee, S.; Park, H.; Kwon, S.; Lee, N.; et al. Fabrication of High Crystalline SnS and SnS2 Thin Films, and Their Switching Device Characteristics. Nanotechnology 2018, 29, 215201. [Google Scholar] [CrossRef]
- Samouh, H.; Nishimoto, S.; Yoshida, H.; Sawada, S.; Kontani, O.; Suzuki, K.; Maruyama, I. Modal Analysis of Rock Forming Minerals: Contribution of XRD/Rietveld Analysis Compared to the Classic Point Counting Method. J. Adv. Concr. Technol. 2021, 19, 395–413. [Google Scholar] [CrossRef]
- Bronusiene, A.; Barauskiene, I.; Popov, A.; Ancutiene, I. Green Synthesis of Tin Sulfide Films for Potential Applications in Supercapacitors and the Effect of Annealing. Sustain. Chem. Pharm. 2023, 34, 101150. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Li, T.; Chen, Y.; Ding, H.; Lin, Y.; Pan, N.; Wang, X. Revealing Anisotropy and Thickness Dependence of Raman Spectra for SnS Flakes. RSC Adv. 2017, 7, 48759–48765. [Google Scholar] [CrossRef]
- Xu, R.; Gong, H.; Yao, W.; Peng, X.; Li, D.; Yuan, H.; Wang, X. SnS Nanoflake-Based Field Effect Transistor with an Anisotropic Gate Effect and a Polarization-Dependent Raman Response. J. Phys. Chem. C 2022, 126, 12660–12668. [Google Scholar] [CrossRef]
- Gong, X.; Yan, T.; Li, J.; Liu, J.; Zou, H.; Zhang, B.; Wu, H.; Zhou, Z.; Zhou, X. Revealing the Anisotropic Phonon Behaviours of Layered SnS by Angle/Temperature-Dependent Raman Spectroscopy. RSC Adv. 2022, 12, 32262–32269. [Google Scholar] [CrossRef] [PubMed]
- Kamali, K. UV Excited Enhanced Raman Scattering on Carbon-Doped SnS2 Nanoflowers. Mater. Res. Bull. 2022, 150, 111757. [Google Scholar] [CrossRef]
- Azhniuk, Y.; Hasynets, S.; Lopushansky, V.; Kryshenik, V.; Solomon, A.M.; Gomonnai, A.V. Raman Study of Photoinduced Crystallization of SnS2 in As2S3:Sn Glasses. Vib. Spectrosc. 2023, 128, 103583. [Google Scholar] [CrossRef]
- Nisha; Kumar, P.; Sarkar, P.; Katiyar, R.S. Influence of S/Sn Ratio on Microstructural, Morphological and Optical Properties of Tin Monosulfide Thin Films. Opt. Mater. 2022, 133, 112792. [Google Scholar] [CrossRef]
- Son, S.I.; Shin, D.; Son, Y.G.; Son, C.S.; Kim, D.R.; Park, J.H.; Kim, S.; Hwang, D.; Song, P. Effect of Working Pressure on the Properties of RF Sputtered SnS Thin Films and Photovoltaic Performance of SnS-Based Solar Cells. J. Alloys Compd. 2020, 831, 154626. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, D.; Wu, Y.; Cheng, X.; Kang, W.; Xu, J. Phase Transformation and Sulfur Vacancy Modulation of 2D Layered Tin Sulfide Nanoplates as Highly Durable Anodes for Pseudocapacitive Lithium Storage. Chem. Eng. J. 2020, 392, 123722. [Google Scholar] [CrossRef]
- Tulenin, S.S.; Timina, A.A.; Maskaeva, L.N.; Markov, V.F. Chemical Bath Deposition of Thin Nanocrystalline Tin(II) Sulfide Films with Thioacetamide. Russ. J. Appl. Chem. 2017, 90, 91–96. [Google Scholar] [CrossRef]
- Data Compiled and Evaluated by Alexander V. Naumkin; Anna Kraut-Vass; Stephen W. Gaarenstroom; Cedric J. Powell NIST X-Ray Photoelectron Spectroscopy (XPS) Database, Version 4.1. Available online: https://srdata.nist.gov/xps/ (accessed on 13 January 2023).
- Cao, M.; Yao, K.; Wu, C.; Huang, J.; Yang, W.; Zhang, L.; Lei, F.; Sun, Y.; Wang, L.; Shen, Y. Facile Synthesis of SnS and SnS 2 Nanosheets for FTO/SnS/SnS 2 /Pt Photocathode. ACS Appl. Energy. Mater. 2018, 1, 6497–6504. [Google Scholar] [CrossRef]
- Guillen, G.G.; Isabel Mendivil Palma, M.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Tin Sulfide Nanoparticles by Pulsed Laser Ablation in Liquid. J. Mater. Sci. Mater. Electron. 2016, 27, 6859–6871. [Google Scholar] [CrossRef]
- Hu, X.; Song, G.; Li, W.; Peng, Y.; Jiang, L.; Xue, Y.; Liu, Q.; Chen, Z.; Hu, J. Phase-Controlled Synthesis and Photocatalytic Properties of SnS, SnS 2 and SnS/SnS2 Heterostructure Nanocrystals. Mater. Res. Bull. 2013, 48, 2325–2332. [Google Scholar] [CrossRef]
- Higareda-Sánchez, A.; Mis-Fernández, R.; Rimmaudo, I.; Camacho-Espinosa, E.; Peña, J.L. Evaluation of PH and Deposition Mechanisms Effect on Tin Sulfide Thin Films Deposited by Chemical Bath Deposition. Superlattices Microstruct. 2021, 151, 106831. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.; Lee, N.; Kim, B.; Lee, J.; Lee, G.; Jeon, H. Deposition of the Tin Sulfide Thin Films Using ALD and a Vacuum Annealing Process for Tuning the Phase Transition. J. Alloys Compd. 2022, 896, 162806. [Google Scholar] [CrossRef]
- Kim, D.G.; Lee, J.M.; Choi, J.H.; Ahn, J.H. Phase Control of Two-Dimensional Tin Sulfide Compounds Deposited via Atomic Layer Deposition. Appl. Surf. Sci. 2023, 612, 155887. [Google Scholar] [CrossRef]
- Deshpande, N.G.; Sagade, A.A.; Gudage, Y.G.; Lokhande, C.D.; Sharma, R. Growth and Characterization of Tin Disulfide (SnS2) Thin Film Deposited by Successive Ionic Layer Adsorption and Reaction (SILAR) Technique. J. Alloys Compd. 2007, 436, 421–426. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef]
- Bang, J.H.; Lee, N.; Mirzaei, A.; Choi, M.S.; Na, H.G.; Jin, C.; Oum, W.; Shin, S.; Choi, H.S.; Park, H.; et al. Effect of Microwave Irradiation on the Electrical and Optical Properties of SnO 2 Thin Films. Ceram. Int. 2019, 45, 7723–7729. [Google Scholar] [CrossRef]
- Shi, C.; Chen, Z.; Shi, G.; Sun, R.; Zhan, X.; Shen, X. Influence of Annealing on Characteristics of Tin Disulfide Thin Films by Vacuum Thermal Evaporation. Thin Solid Film. 2012, 520, 4898–4901. [Google Scholar] [CrossRef]
- Sreekala, A.P.; Krishnan, B.; Pelaes, R.F.C.; Avellaneda, D.A.; Palma, M.I.M.; Shaji, S. Tin Sulfide Thin Films by Spin Coating of Laser Ablated Nanocolloids for UV–Vis–NIR Photodetection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128382. [Google Scholar] [CrossRef]
- Sainbileg, B.; Hayashi, M. Possible Indirect to Direct Bandgap Transition in SnS 2 via Nickel Doping. Chem. Phys. 2019, 522, 59–64. [Google Scholar] [CrossRef]
- Arulanantham, A.M.S.; Valanarasu, S.; Jeyadheepan, K.; Kathalingam, A. Effect of Thermal Annealing on Nebulizer Spray Deposited Tin Sulfide Thin Films and Their Application in a Transparent Oxide/CdS/SnS Heterostructure. Thin Solid Film. 2018, 666, 85–93. [Google Scholar] [CrossRef]
- Luan, Y.; Zobeiri, H.; Wang, X.; Sutter, E.; Sutter, P.; Fei, Z. Imaging Anisotropic Waveguide Exciton Polaritons in Tin Sulfide. Nano Lett. 2022, 22, 1497–1503. [Google Scholar] [CrossRef]
- Mohan, R.N.; Gomez Daza, O.; García-Angelmo, A.R.; Espinosa Santana, A.L.; Beristain Bautista, A.; Nair, M.T.S.; Nair, P.K. Thin Films of P-SnS and n-Sn2S3 for Solar Cells Produced by Thermal Processing of Chemically Deposited SnS. J. Alloys Compd. 2022, 892, 162036. [Google Scholar] [CrossRef]
- Luo, J.; Song, X.; Lu, Y.; Hu, Y.; Lv, X.; Li, L.; Li, X.; Deng, J.; Yan, Y.; Jiang, Y.; et al. Phase-Controlled Synthesis of SnS2 and SnS Flakes and Photodetection Properties. J. Phys. Condens. Matter 2022, 34, 285701. [Google Scholar] [CrossRef]
- Afzali, N.; Torka Beydokhti, M.; Khodadadi, A.A.; Mortazavi, Y. Tuning the Band-Gap and Enhancing the Trichloroethylene Photocatalytic Degradation Activities of Flower-like Ni-Doped SnS2/SnO2heterostructures by Partial Oxidation. J. Environ. Chem. Eng. 2022, 10, 107793. [Google Scholar] [CrossRef]
- Buniyamin, I.; Akhir, R.M.; Asli, N.A.; Khusaimi, Z.; Rusop, M. Green Synthesis of Tin Oxide Nanoparticles by Using Leaves Extract of Chromolaena Odorata: The Effect of Different Thermal Calcination Temperature to the Energy Band Gap. Mater. Today Proc. 2021, 48, 1805–1809. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review—Recent Advances in Tin Oxide Nanomaterials as Electrochemical/Chemiresistive Sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Kim, C.Y.; Riu, D.H. Raman Scattering, Electrical and Optical Properties of Fluorine-Doped Tin Oxide Thin Films with (200) and (301) Preferred Orientation. Mater. Chem. Phys. 2014, 148, 810–817. [Google Scholar] [CrossRef]
- Singh, D.J. Optical and Electronic Properties of Semiconducting Sn2S3. Appl. Phys. Lett 2016, 109, 32102. [Google Scholar] [CrossRef]
- Arepalli, V.K.; Kim, J. Effect of Substrate Temperature on the Structural and Optical Properties of Radio Frequency Sputtered Tin Sulfide Thin Films for Solar Cell Application. Thin Solid Film. 2018, 666, 34–39. [Google Scholar] [CrossRef]
- Mohan, R.N.; Nair, M.T.S.; Nair, P.K. Thin Film Sn2S3 via Chemical Deposition and Controlled Heating –Its Prospects as a Solar Cell Absorber. Appl. Surf. Sci. 2020, 504, 144162. [Google Scholar] [CrossRef]
- Li, G.; Su, R.; Rao, J.; Wu, J.; Rudolf, P.; Blake, G.R.; de Groot, R.A.; Besenbacher, F.; Palstra, T.T.M. Band Gap Narrowing of SnS 2 Superstructures with Improved Hydrogen Production †. J. Mater. Chem. A 2016, 4, 209–216. [Google Scholar] [CrossRef]
- Yu, J.; Xu, C.-Y.; Li, Y.; Zhou, F.; Chen, X.-S.; Hu, P.-A.; Zhen, L. Ternary SnS 2-x Se x Alloys Nanosheets and Nanosheet Assemblies with Tunable Chemical Compositions and Band Gaps for Photodetector Applications OPEN. Sci. Rep. 2015, 5, 17109. [Google Scholar] [CrossRef] [PubMed]
- Taleblou, M.; Borhani, E.; Yarmand, B.; Kolahi, A.R. Structural and Optoelectrical Properties of Single Phase SnS2thin Films at Various Substrate Temperatures by Spray Pyrolysis. Iran. J. Mater. Sci. Eng. 2018, 15, 43–52. [Google Scholar] [CrossRef]
- Conway, B.E.; Pell, W.G. Double-Layer and Pseudocapacitance Types of Electrochemical Capacitors and Their Applications to the Development of Hybrid Devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]
- Kuddus, A.; Hossain, J.; Mostaque, S.K. Simulating the Performance of a High-Efficiency SnS-Based Dual-Heterojunction Thin Film Solar Cell. Opt. Mater. Express 2021, 11, 3812–3826. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef]





| Annealing Temperature, °C | 200 | 250 | 300 | 350 | 400 | |
|---|---|---|---|---|---|---|
| Quantity of L-ascorbic Acid, g | ||||||
| 0.8 | 1 | 2 | 3 | 4 | 5 | |
| 1.0 | 6 | 7 | 8 | 9 | 10 | |
| Observed d-Values, Å | SnS (83-47) d-Values, Å | Sn2S3 (75-2183) d-Values, Å | SnO2 (46-1088) d-Values, Å | SnS2 (83-1705) d-Values, Å |
|---|---|---|---|---|
| 5.9416 | 5.8800 | |||
| 3.3757 | 3.3774 | 3.3510 | ||
| 2.6680 | 2.6521 | 2.6518 | ||
| 2.3727 | 2.3751 | 2.3707 | 2.3799 | |
| 1.7762 | 1.7792 | 1.7752 | 1.7649 | |
| 1.6841 | 1.6810 | |||
| 1.5064 | 1.5001 | 1.5012 | ||
| 1.4270 | 1.4342 | 1.4251 |
| Sample Number | Sample Thickness | |
|---|---|---|
| The Area of Sample Thickness, nm | The Average Size of Sample Thickness, nm | |
| 1 | 35–590 | 240 |
| 2 | 30–670 | 235 |
| 3 | 20–460 | 230 |
| 4 | 30–470 | 160 |
| 5 | 90 | 90 |
| 6 | 50–1900 | 430 |
| 7 | 40–910 | 340 |
| 8 | 30–590 | 250 |
| 9 | 20–450 | 210 |
| 10 | 100 | 100 |
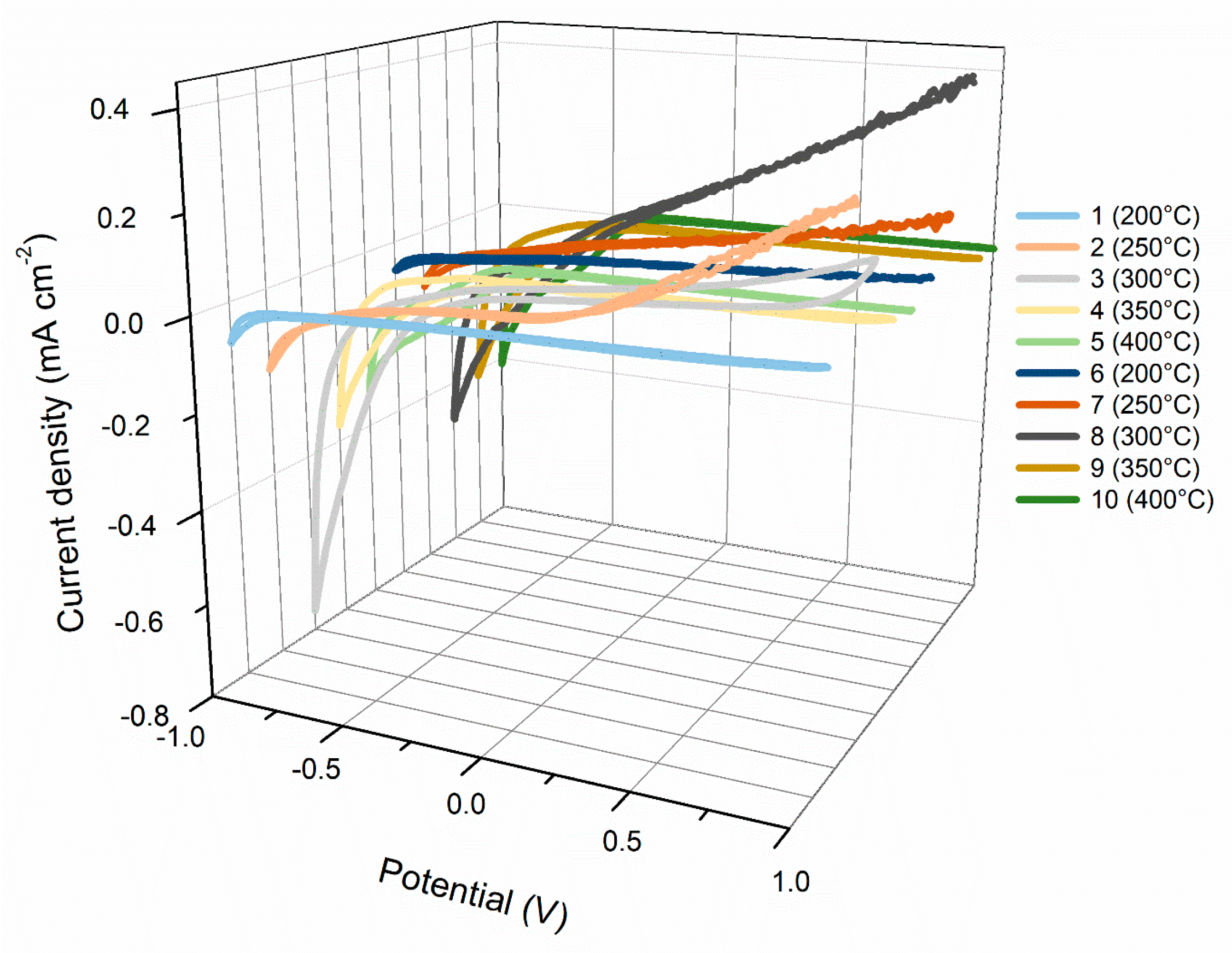
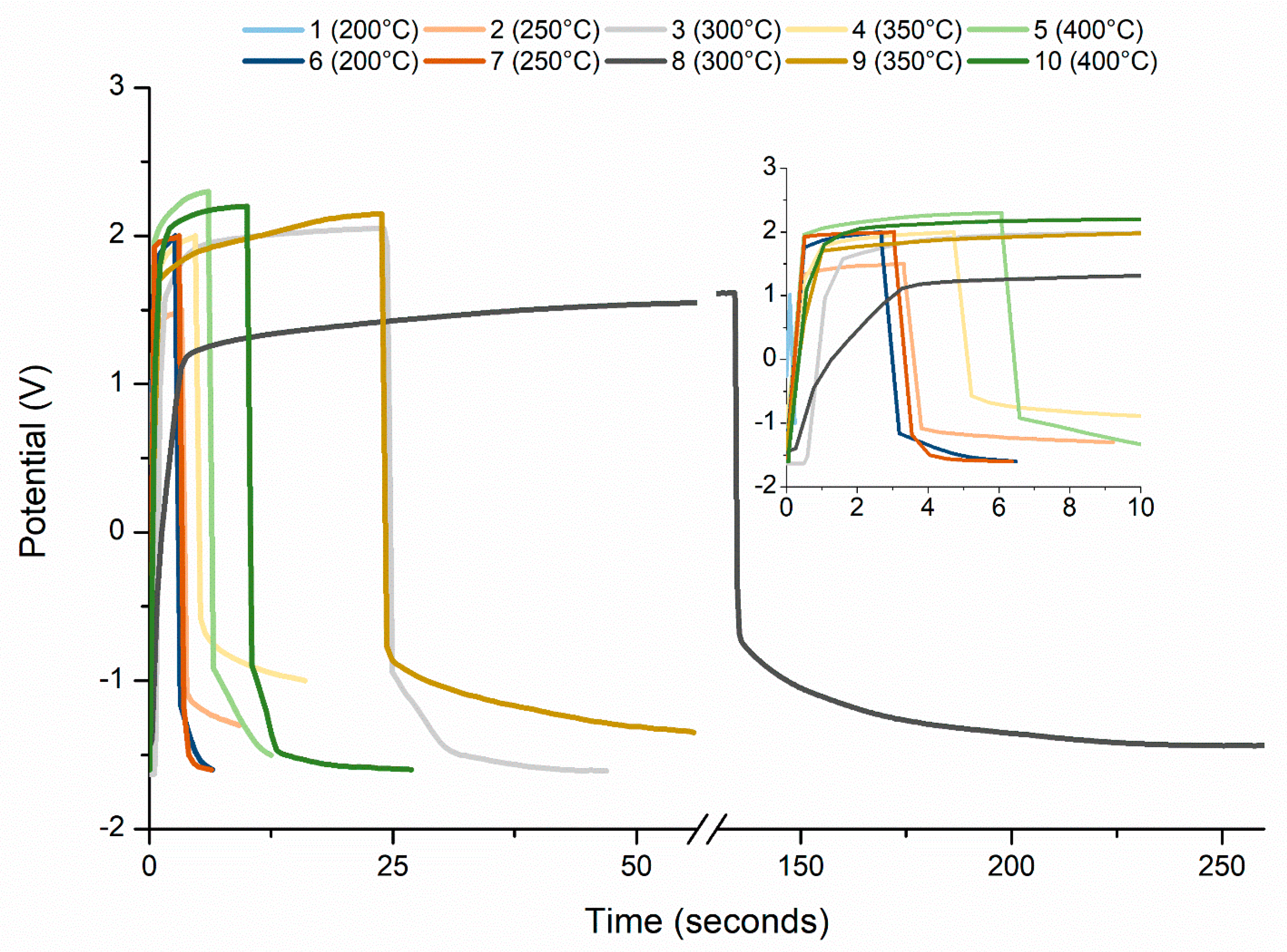
| Sample Number | Temperature (°C) | SC (F g−1) | SE (Wh kg−1) | SP (W kg−1) |
|---|---|---|---|---|
| 1 | 200 | 0.07 | 0.04 | 1000 |
| 2 | 250 | 1.05 | 1.14 | 697 |
| 3 | 300 | 6.90 | 13.11 | 1847 |
| 4 | 350 | 3.50 | 4.37 | 1498 |
| 5 | 400 | 1.71 | 3.43 | 1898 |
| 6 | 200 | 1.02 | 1.84 | 1800 |
| 7 | 250 | 1.11 | 2.00 | 1798 |
| 8 | 300 | 39.00 | 50.06 | 1521 |
| 9 | 350 | 12.67 | 22.17 | 1773 |
| 10 | 400 | 4.44 | 8.90 | 1899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronusiene, A.; Barauskiene, I.; Popov, A.; Zsiros, B.; Farkas, A.; Plšek, J.; Ancutiene, I. The Impact of Thermal Treatment on the Structural, Optical and Electrochemical Characteristics of Tin Sulfide Films. Coatings 2024, 14, 1284. https://doi.org/10.3390/coatings14101284
Bronusiene A, Barauskiene I, Popov A, Zsiros B, Farkas A, Plšek J, Ancutiene I. The Impact of Thermal Treatment on the Structural, Optical and Electrochemical Characteristics of Tin Sulfide Films. Coatings. 2024; 14(10):1284. https://doi.org/10.3390/coatings14101284
Chicago/Turabian StyleBronusiene, Asta, Ieva Barauskiene, Anton Popov, Boldizsar Zsiros, Attila Farkas, Jan Plšek, and Ingrida Ancutiene. 2024. "The Impact of Thermal Treatment on the Structural, Optical and Electrochemical Characteristics of Tin Sulfide Films" Coatings 14, no. 10: 1284. https://doi.org/10.3390/coatings14101284
APA StyleBronusiene, A., Barauskiene, I., Popov, A., Zsiros, B., Farkas, A., Plšek, J., & Ancutiene, I. (2024). The Impact of Thermal Treatment on the Structural, Optical and Electrochemical Characteristics of Tin Sulfide Films. Coatings, 14(10), 1284. https://doi.org/10.3390/coatings14101284







