Ni Catalysts for Thermochemical CO2 Methanation: A Review
Abstract
:1. Introduction
2. Mechanism of CO2 Methanation
- Step 1: H2 + 2* ⇌ 2H*; CO2 + 2H* → HCOOH*
- Step 2: HCOOH* → HCO* + OH*; OH*+ H* → H2O* → H2O
- Step 3: HCO* → *CH + *O or HCO* + H* → HCOH* → *CH + OH*
- Step 4: CH* + H* → CH2* + H* → CH3* + H* → CH4* → CH4
- Step 5: O* + H* → OH* + H* → H2O* → H2O
- Step 1: CO2 ⇌ CO2*; H2 + 2* ⇌ 2H*
- Step 2: CO2* + H* → HCOO*
- Step 3: HCOO* → CO* + *OH
- Step 4: CO* + 2H* → CH* + OH*
- Step 5: CH* + H* → CH2* + H* → CH3* + H* → CH4* → CH4
- Step 6: OH* + H* → H2O* → H2O
3. Influence of Catalyst Supports on CO2 Methanation
4. Effect of Co-Catalysts on CO2 Methanation
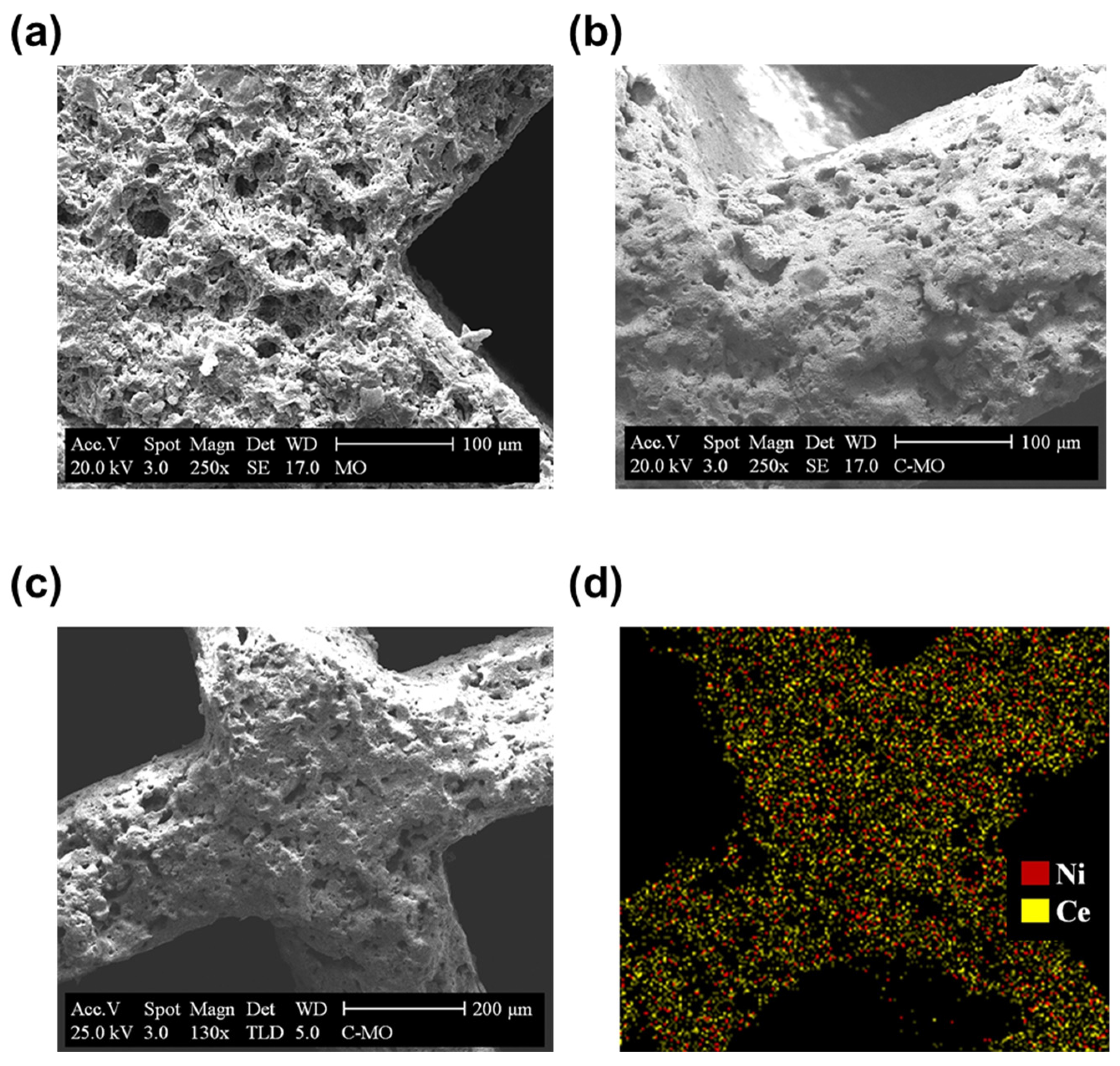
5. Performance Comparison between Powder Catalysts and Monolithic Catalysts
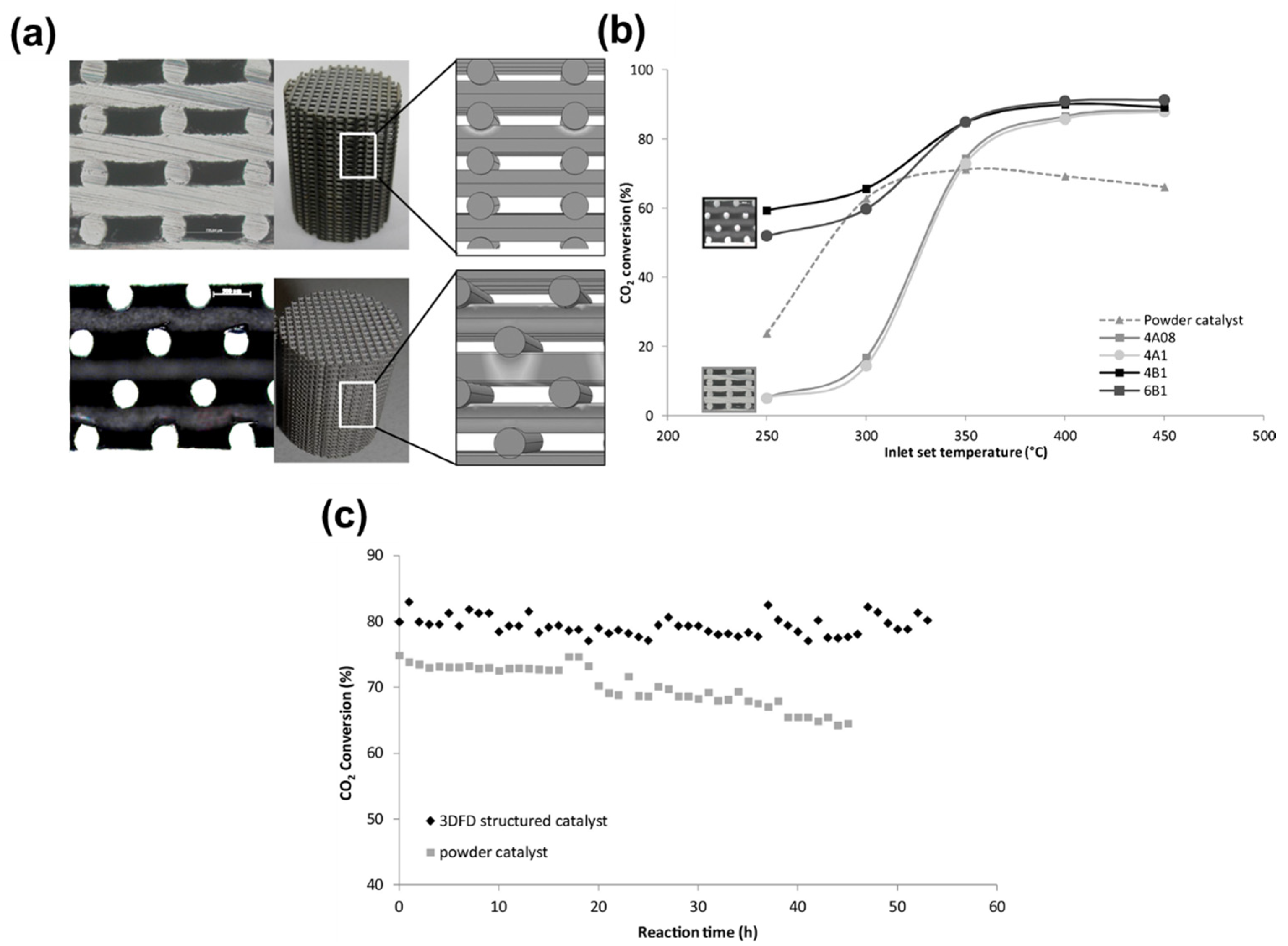
6. Performance Analysis of Nickel Catalysts Using 3D Fiber Deposition Technology
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Coumou, D.; Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2012, 2, 491–496. [Google Scholar] [CrossRef]
- Kossin, J.P.; Knapp, K.R.; Olander, T.L.; Velden, C.S. Global increase in major tropical cyclone exceedance probability over the past four decades. Proc. Natl. Acad. Sci. USA 2020, 117, 11975–11980. [Google Scholar] [CrossRef]
- van Oldenborgh, G.J.; Otto, F.E.; Haustein, K.; Cullen, H. Climate change increases the probability of heavy rains like those of storm Desmond in the UK–an event attribution study in near-real time. Hydrol. Earth Syst. Sci. Discuss. 2015, 12, 13197–13216. [Google Scholar]
- Odeyomi, O.A.; Ejimakor, G.; Isikhuemhen, O.S. Effects of Climate Change on Crop Yield: Is it a Benefit or Menace? ESI Prepr. 2024, 32, 42. [Google Scholar]
- Walsh, K.J.; McBride, J.L.; Klotzbach, P.J.; Balachandran, S.; Camargo, S.J.; Holland, G.; Knutson, T.R.; Kossin, J.P.; Lee, T.C.; Sobel, A. Tropical cyclones and climate change. Wiley Interdiscip. Rev. Clim. Chang. 2016, 7, 65–89. [Google Scholar] [CrossRef]
- Qu, Q.; Jian, S.; Chen, A.; Xiao, C. Investigating the Dynamic Change and Driving Force of Vegetation Carbon Sink in Taihang Mountain, China. Land 2024, 13, 1348. [Google Scholar] [CrossRef]
- Ye, J.; Fanyang, Y.; Wang, J.; Meng, S.; Tang, D. A Literature Review of Green Building Policies: Perspectives from Bibliometric Analysis. Buildings 2024, 14, 2607. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, W.M. Optimizing LCD structures to mitigate carbon emissions based on root-mean-square values. Appl. Opt. 2024, 63, 6603–6615. [Google Scholar] [CrossRef]
- Hasan, G.G.; Laouini, S.E.; Osman, A.I.; Bouafia, A.; Althamthami, M.; Meneceur, S.; Al-Hazeef, M.S.; Al-Fatesh, A.S.; Rooney, D.W. Green Synthesis of Mn3O4@ CoO Nanocomposites Using Rosmarinus officinalis L. Extract for Enhanced Photocatalytic Hydrogen Production and CO2 Conversion. J. Environ. Chem. Eng. 2024, 12, 113911. [Google Scholar] [CrossRef]
- Ming, L.; Dae-Yeong, K.; Shutaro, N.; Tomohiro, N. CO2 methanation through gliding arc discharge over Ni/Al2O3. Int. J. Plasma Environ. Sci. Technol. 2024, 18, e02005. [Google Scholar]
- Farshchi, M.E.; Asgharizadeh, K.; Jalili, H.; Nejatbakhsh, S.; Azimi, B.; Aghdasinia, H.; Mohammadpourfard, M. Bimetallic MOF@CdS Nanorod Composite for Highly Efficient Piezo-photocatalytic CO2 Methanation under Visible Light. J. Environ. Chem. Eng. 2024, 12, 113909. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Liu, W.; Cheng, Y.; Yang, X.; Wang, W. Rational H2 Partial Pressure over Nickel/Ceria Crystal Enables Efficient and Durable Wide-Temperature-Zone Air-Level CO2 Methanation. Chem. Eur. J. 2024, e202402516. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, T.; Qiu, J.; Bai, J.; Li, Z.; Wang, H.; Cai, X.; Yang, Y.; Xu, Y. Cobalt-Doped Ni-Based Catalysts for Low-Temperature CO2 Methanation. Renew. Energy 2024, 236, 121512. [Google Scholar] [CrossRef]
- Chopendra, G.W.; Nakamura, M.; Shimada, T.; Machida, H.; Norinaga, K. 6-2-3 Development of CO2 Methanation Catalysts Supported on CeO2 and SiC At Elevated Temperatures. In Proceedings of the Annual Conference of the Japan Institute of Energy, Tokyo, Japan, 7–9 August 2024. [Google Scholar]
- Busca, G.; Spennati, E.; Riani, P.; Garbarino, G. Looking for an optimal composition of nickel-based catalysts for CO2 methanation. Energies 2023, 16, 5304. [Google Scholar] [CrossRef]
- Zainul, R.; Ali, A.B.; Jasim, D.J.; Al-Bayati, A.D.J.; Kaur, I.; Kumar, A.; Mahariq, I.; Hasan, M.A.; Islam, S.; Kareem, M. Biphenyl monolayer construction with single transition metal doping as electrocatalysts for conversion CO2 to fuel. Int. J. Hydrogen Energy 2024, in press. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.P.; Eng, F.; Fuess, L.T.; Pozzi, E.; Nolasco, M.A.; De Wever, H.; Pant, D.; Zaiat, M. Hydrogen Production from Fermented Sugarcane Vinasse and Its Utilization by Biosynthesis Processes in a Single-Chambered Microbial Electrolysis Cell. 2024. Available online: https://ssrn.com/abstract=4914019 (accessed on 18 September 2024).
- Lin, L.; Wang, K.; Yang, K.; Chen, X.; Fu, X.; Dai, W. The visible-light-assisted thermocatalytic methanation of CO2 over Ru/TiO2-xNx. Appl. Catal. B Environ. 2017, 204, 440–455. [Google Scholar] [CrossRef]
- Do, J.Y.; Park, N.-K.; Seo, M.W.; Lee, D.; Ryu, H.-J.; Kang, M. Effective thermocatalytic carbon dioxide methanation on Ca-inserted NiTiO3 perovskite. Fuel 2020, 271, 117624. [Google Scholar] [CrossRef]
- Rusdan, N.A.; Timmiati, S.N.; Isahak, W.N.R.W.; Yaakob, Z.; Lim, K.L.; Khaidar, D. Recent application of core-shell nanostructured catalysts for CO2 thermocatalytic conversion processes. Nanomaterials 2022, 12, 3877. [Google Scholar] [CrossRef]
- Gao, J.; Shiong, S.C.S.; Liu, Y. Reduction of CO2 to chemicals and Fuels: Thermocatalysis versus electrocatalysis. Chem. Eng. J. 2023, 472, 145033. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Simakov, D.S.A. Thermocatalytic Conversion of CO2. In Renewable Synthetic Fuels and Chemicals from Carbon Dioxide: Fundamentals, Catalysis, Design Considerations and Technological Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–25. [Google Scholar]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Li, L.; Zeng, W.; Song, M.; Wu, X.; Li, G.; Hu, C. Research progress and reaction mechanism of CO2 methanation over Ni-based catalysts at low temperature: A review. Catalysts 2022, 12, 244. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Liu, Q.; Sun, J.; Ji, S.; Wang, Z.J. Enhanced low-temperature activity for CO2 methanation over NiMgAl/SiC composite catalysts. J. Chem. Technol. Biotechnol. 2019, 94, 3780–3786. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Cui, Y.; He, S.; Yang, J.; Gao, R.; Hu, K.; Chen, X.; Xu, L.; Deng, C.; Lin, C.; Peng, S. Research Progress of Non-Noble Metal Catalysts for Carbon Dioxide Methanation. Molecules 2024, 29, 374. [Google Scholar] [CrossRef] [PubMed]
- Gac, W.; Zawadzki, W.; Rotko, M.; Greluk, M.; Słowik, G.; Kolb, G. Effects of support composition on the performance of nickel catalysts in CO2 methanation reaction. Catal. Today 2020, 357, 468–482. [Google Scholar] [CrossRef]
- Delmelle, R.; Duarte, R.B.; Franken, T.; Burnat, D.; Holzer, L.; Borgschulte, A.; Heel, A. Development of improved nickel catalysts for sorption enhanced CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 20185–20191. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293, 89–96. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, L.; Zheng, Y.; Zhang, S.; Liu, Q.; Gao, G.; Dong, D.; Wang, Y.; Xu, L.; Hu, X. Methanation of CO2 over nickel catalysts: Impacts of acidic/basic sites on formation of the reaction intermediates. Fuel 2020, 262, 116521. [Google Scholar] [CrossRef]
- Lif, J.; Odenbrand, I.; Skoglundh, M. Sintering of alumina-supported nickel particles under amination conditions: Support effects. Appl. Catal. A Gen. 2007, 317, 62–69. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, J.J.; Hwang, S.-M.; Seo, M.W.; Lee, D.; Jeong, S.K.; Ryu, H.-J.; Choi, S.-A.; Hwang, B.; Nam, H. Optimizing heat transfer rate for efficient CO2-to-chemical conversion in CO2 methanation and CO2 hydrogenation reactions. J. CO2 Util. 2024, 81, 102730. [Google Scholar] [CrossRef]
- Ren, J.; Guo, H.; Yang, J.; Qin, Z.; Lin, J.; Li, Z. Insights into the mechanisms of CO2 methanation on Ni (111) surfaces by density functional theory. Appl. Surf. Sci. 2015, 351, 504–516. [Google Scholar] [CrossRef]
- Schmider, D.; Maier, L.; Deutschmann, O. Reaction kinetics of CO and CO2 methanation over nickel. Ind. Eng. Chem. Res. 2021, 60, 5792–5805. [Google Scholar] [CrossRef]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Eckle, S.; Anfang, H.G.; Behm, R.J. Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J. Phys. Chem. C 2011, 115, 1361–1367. [Google Scholar] [CrossRef]
- Ilsemann, J.; Murshed, M.M.; Gesing, T.M.; Kopyscinski, J.; Bäumer, M. On the support dependency of the CO2 methanation–decoupling size and support effects. Catal. Sci. Technol. 2021, 11, 4098–4114. [Google Scholar] [CrossRef]
- Zhen, W.; Gao, F.; Tian, B.; Ding, P.; Deng, Y.; Li, Z.; Gao, Z.; Lu, G. Enhancing activity for carbon dioxide methanation by encapsulating (111) facet Ni particle in metal–organic frameworks at low temperature. J. Catal. 2017, 348, 200–211. [Google Scholar] [CrossRef]
- Alarcon, A.; Guilera, J.; Soto, R.; Andreu, T. Higher tolerance to sulfur poisoning in CO2 methanation by the presence of CeO2. Appl. Catal. B Environ. 2020, 263, 118346. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of highly active nickel catalysts supported on mesoporous nanocrystalline γ-Al2O3 for CO2 methanation. J. Ind. Eng. Chem. 2014, 20, 1346–1352. [Google Scholar] [CrossRef]
- Mutz, B.; Carvalho, H.W.; Mangold, S.; Kleist, W.; Grunwaldt, J.-D. Methanation of CO2: Structural response of a Ni-based catalyst under fluctuating reaction conditions unraveled by operando spectroscopy. J. Catal. 2015, 327, 48–53. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Zhang, Z.; Ma, Z.; Wang, L.; Liu, Y. Highly dispersed and stable Ni nanoparticles confined by MgO on ZrO2 for CO2 methanation. Appl. Surf. Sci. 2019, 481, 1538–1548. [Google Scholar] [CrossRef]
- Ashok, J.; Ang, M.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Li, Z. Highly efficient Ni/ZrO2 catalysts prepared via combustion method for CO2 methanation. J. CO2 Util. 2016, 16, 236–244. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C.; Wang, Q.; Xu, Y.; Ma, G.; Wang, J.; Wang, H.; Dong, C.; Zhang, C.; Ding, M. Enhanced low-temperature performance of CO2 methanation over mesoporous Ni/Al2O3-ZrO2 catalysts. Appl. Catal. B Environ. 2019, 243, 262–272. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, X.; Rui, N.; Hu, X.; Liu, C.-J. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl. Catal. B Environ. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Saad, M. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The effect of impregnation strategy on structural characters and CO2 methanation properties over MgO modified Ni/SiO2 catalysts. Catal. Commun. 2014, 54, 55–60. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, Y.; Wang, S. CO2 methanation on the catalyst of Ni/MCM-41 promoted with CeO2. Sci. Total Environ. 2018, 625, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Vrijburg, W.L.; Moioli, E.; Chen, W.; Zhang, M.; Terlingen, B.J.; Zijlstra, B.; Filot, I.A.; Zuttel, A.; Pidko, E.A.; Hensen, E.J. Efficient base-metal NiMn/TiO2 catalyst for CO2 methanation. ACS Catal. 2019, 9, 7823–7839. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Nayak, B.B.; Purohit, G.; Mondal, A.; Purohit, R.; Sinha, P. Efficient way of precipitation to synthesize Ni2+-ion stabilized tetragonal zirconia nanopowders. Mater. Lett. 2011, 65, 959–961. [Google Scholar] [CrossRef]
- Bacariza, M.; Graça, I.; Bebiano, S.; Lopes, J.; Henriques, C. Micro-and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef]
- Mohammadijoo, M.; Khorshidi, Z.N.; Sadrnezhaad, S.; Mazinani, V. Synthesis and characterization of nickel oxide nanoparticle with wide band gap energy prepared via thermochemical processing. Nanosci. Nanotechnol. Int. J. 2014, 4, 6–9. [Google Scholar]
- Westermann, A.; Azambre, B.; Bacariza, M.; Graça, I.; Ribeiro, M.; Lopes, J.; Henriques, C. The promoting effect of Ce in the CO2 methanation performances on Ni-USY zeolite: A FTIR in situ/operando study. Catal. Today 2017, 283, 74–81. [Google Scholar] [CrossRef]
- Jiao, F.; Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. 2009, 121, 1873–1876. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.A.; Triwahyono, S.; Mukti, R.; Taufiq-Yap, Y.; Sazegar, M. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Sidik, S. Methanation of carbon dioxide on metal-promoted mesostructured silica nanoparticles. Appl. Catal. A Gen. 2014, 486, 115–122. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential role of the support for nickel-based CO2 methanation catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.-E.; Yang, B.; Miao, Z.; Hu, X. Recent progresses in constructing the highly efficient Ni-based catalysts with advanced low-temperature activity toward CO2 methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-based catalysts for CO2 methanation: A review. Nanomaterials 2020, 11, 28. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Italiano, C.; Ferrante, G.D.; Pino, L.; Vita, A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Sharan, A. Ni-noble metal bimetallic catalysts for improved low temperature CO2 methanation. Appl. Surf. Sci. 2024, 646, 158945. [Google Scholar] [CrossRef]
- Guo, M.; Lu, G. The regulating effects of cobalt addition on the catalytic properties of silica-supported Ni–Co bimetallic catalysts for CO2 methanation. React. Kinet. Mech. Catal. 2014, 113, 101–113. [Google Scholar] [CrossRef]
- Dias, Y.R.; Perez-Lopez, O.W. Carbon dioxide methanation over Ni-Cu/SiO2 catalysts. Energy Convers. Manag. 2020, 203, 112214. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Frey, M.; Edouard, D.; Roger, A.-C. Optimization of structured cellular foam-based catalysts for low-temperature carbon dioxide methanation in a platelet milli-reactor. Comptes Rendus Chim. 2015, 18, 283–292. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Martino, M.; Meloni, E. Innovative catalyst design for methane steam reforming intensification. Fuel 2017, 198, 175–182. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Specchia, S.; Cipitì, F.; Specchia, V. Methane oxy-steam reforming reaction: Performances of Ru/γ-Al2O3 catalysts loaded on structured cordierite monoliths. Int. J. Hydrogen Energy 2014, 39, 18592–18603. [Google Scholar] [CrossRef]
- Vita, A.; Cristiano, G.; Italiano, C.; Pino, L.; Specchia, S. Syngas production by methane oxy-steam reforming on Me/CeO2 (Me = Rh, Pt, Ni) catalyst lined on cordierite monoliths. Appl. Catal. B Environ. 2015, 162, 551–563. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Lagana, M.; Pino, L. Influence of Ce-precursor and fuel on structure and catalytic activity of combustion synthesized Ni/CeO2 catalysts for biogas oxidative steam reforming. Mater. Chem. Phys. 2015, 163, 337–347. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al2O3 coated structured catalysts. Catal. Today 2016, 273, 234–243. [Google Scholar] [CrossRef]
- Junaedi, C.; Hawley, K.; Walsh, D.; Roychoudhury, S.; Abney, M.; Perry, J. Compact and lightweight sabatier reactor for carbon dioxide reduction. In Proceedings of the 41st International Conference on Environmental Systems, Portland, OR, USA, 17–21 July 2011. [Google Scholar]
- Lefevere, J.; Gysen, M.; Mullens, S.; Meynen, V.; Van Noyen, J. The benefit of design of support architectures for zeolite coated structured catalysts for methanol-to-olefin conversion. Catal. Today 2013, 216, 18–23. [Google Scholar] [CrossRef]
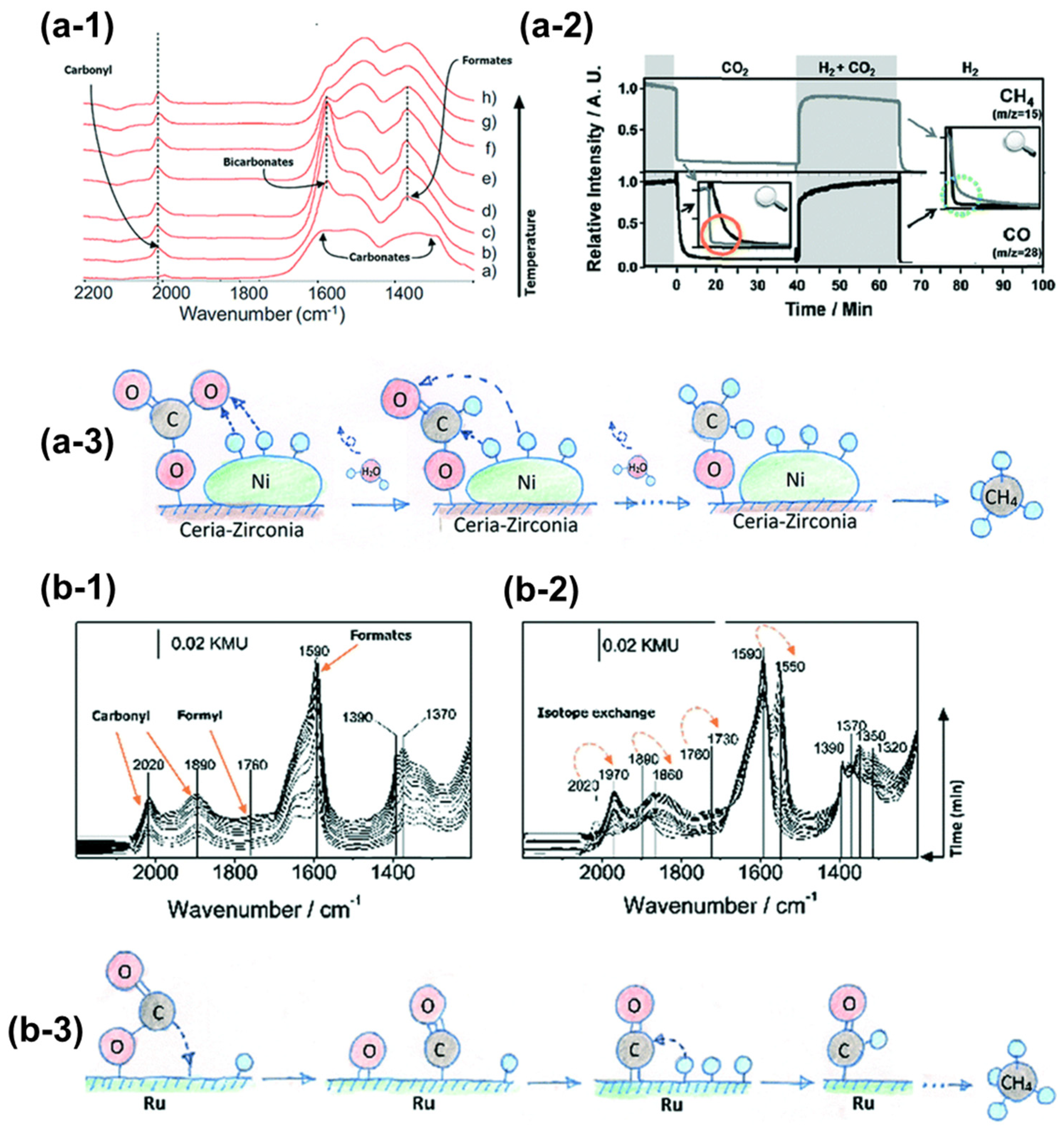
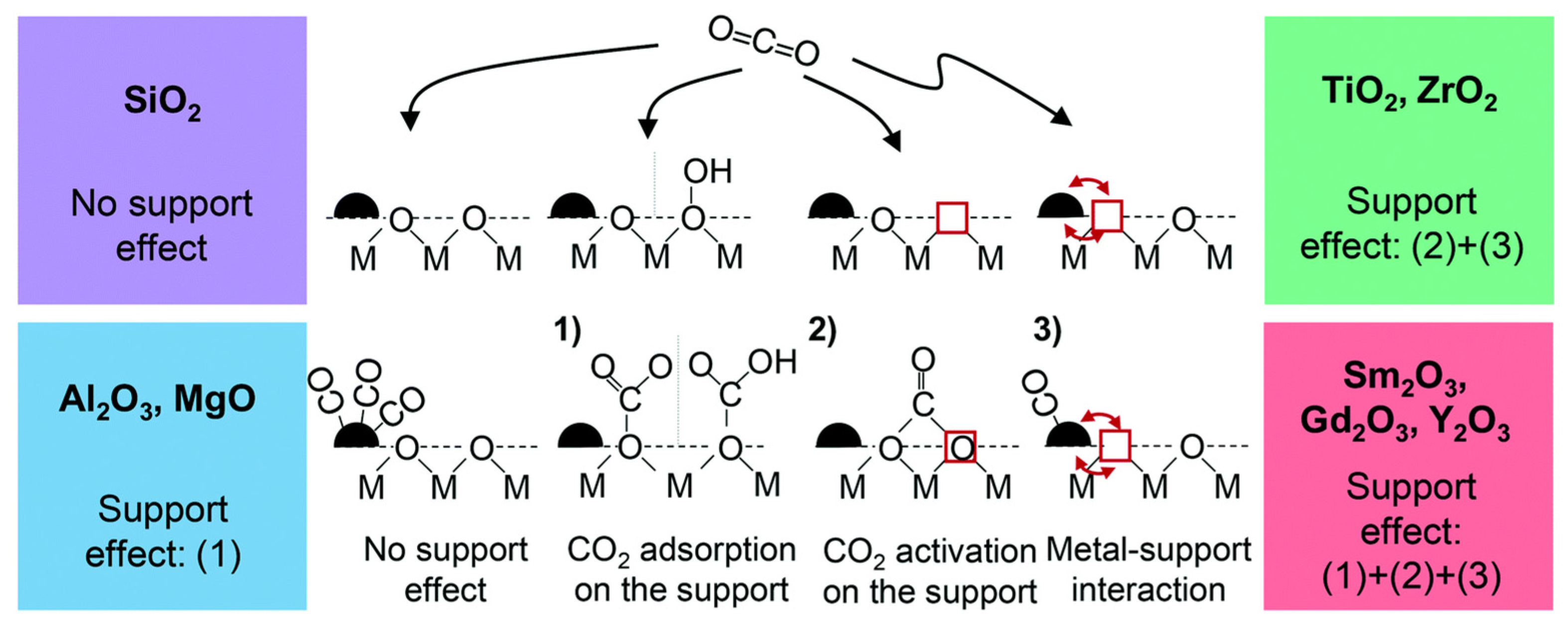
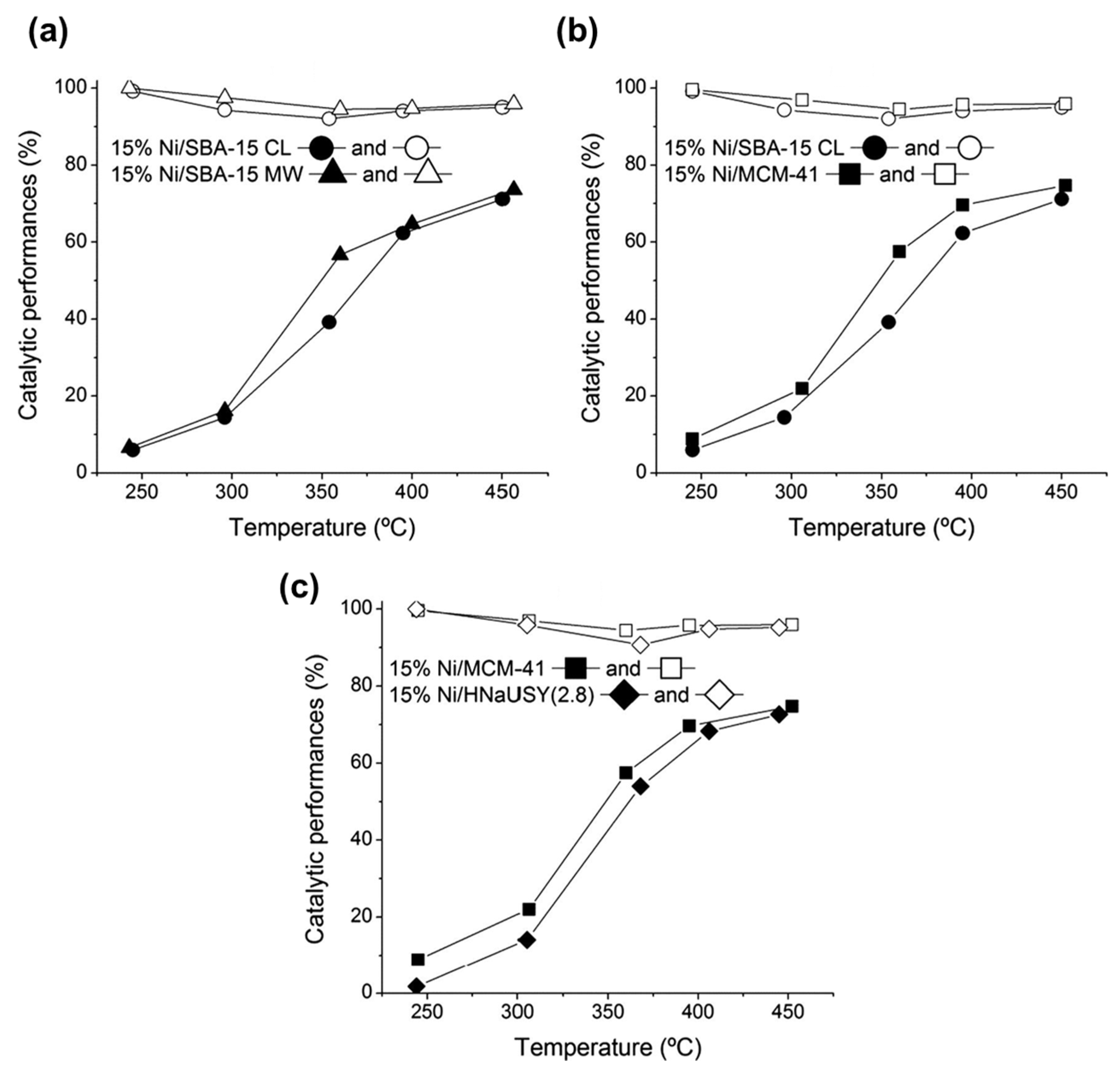
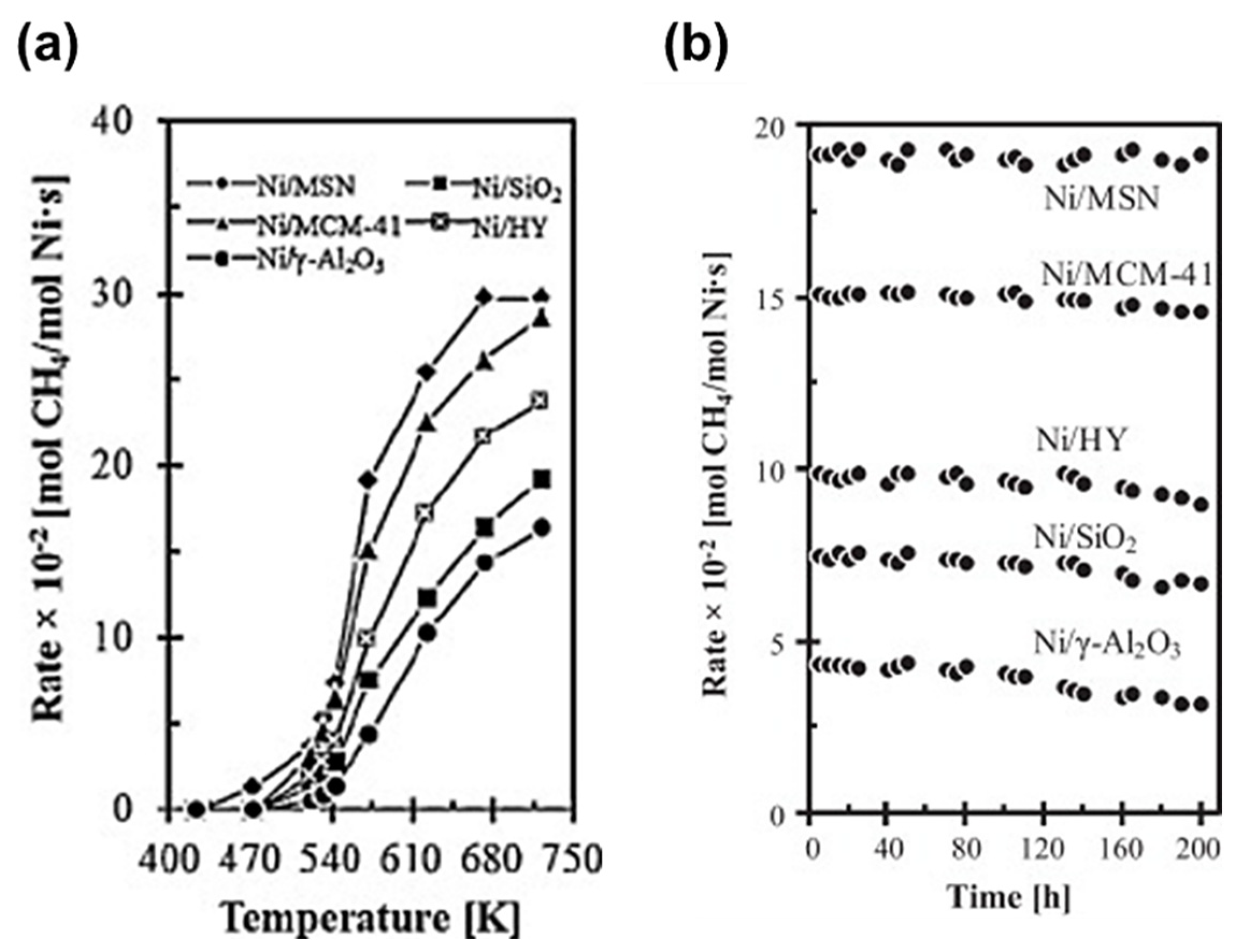


| Type | Catalysts | CO2 Conv. (%) | CH4 Sel. (%) | Temp. (°C) | Pressure (bar) | Ref. |
|---|---|---|---|---|---|---|
| Alumina, zirconia, and ceria | 25% Ni-CeO2/γ-Al2O3 | 21 | 100 | 250 | 6 | [44] |
| 20% Ni/Al2O3 | 82.4 | 100 | 350 | 1 | [45] | |
| 23% Ni/CaO-Al2O3 | 81 | 99 | 400 | 1 | [46] | |
| 6% Ni-MgO/ZrO2 | 90 | 100 | 250 | 1 | [47] | |
| 10% Ni/CeO2-ZrO2 | 55 | 99.8 | 275 | 1 | [48] | |
| 15% Ni/ZrO2 | 60 | 97.5 | 300 | 1 | [49] | |
| 10% Ni/ZrO2-Al2O3 | 77 | 100 | 300 | 1 | [50] | |
| 20% Ni/ZrO2 | 79.1 | 96.7 | 350 | 1 | [51] | |
| 10% Ni/CeO2 | 83.3 | 100 | 350 | 1 | [52] | |
| Silica | 10% Ni/MSN | 85 | 100 | 350 | 1 | [53] |
| 10% Ni-MgO/SiO2 | 73.2 | 98.7 | 400 | 1 | [54] | |
| 20% Ni-CeO2/MCM-41 | 85.6 | 99.8 | 380 | 1 | [55] | |
| 10% Ni-CeO2/SBA-15 | 68.8 | 99.0 | 400 | 1 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J. Ni Catalysts for Thermochemical CO2 Methanation: A Review. Coatings 2024, 14, 1322. https://doi.org/10.3390/coatings14101322
Kim J. Ni Catalysts for Thermochemical CO2 Methanation: A Review. Coatings. 2024; 14(10):1322. https://doi.org/10.3390/coatings14101322
Chicago/Turabian StyleKim, Jungpil. 2024. "Ni Catalysts for Thermochemical CO2 Methanation: A Review" Coatings 14, no. 10: 1322. https://doi.org/10.3390/coatings14101322
APA StyleKim, J. (2024). Ni Catalysts for Thermochemical CO2 Methanation: A Review. Coatings, 14(10), 1322. https://doi.org/10.3390/coatings14101322





