Synthesis and Characterization of TiO2 Thin Films Modified with Anderson-Type Polyoxometalates (Ni, Co, and Fe)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Adsorption and Photocatalytic Study
3. Results and Discussion
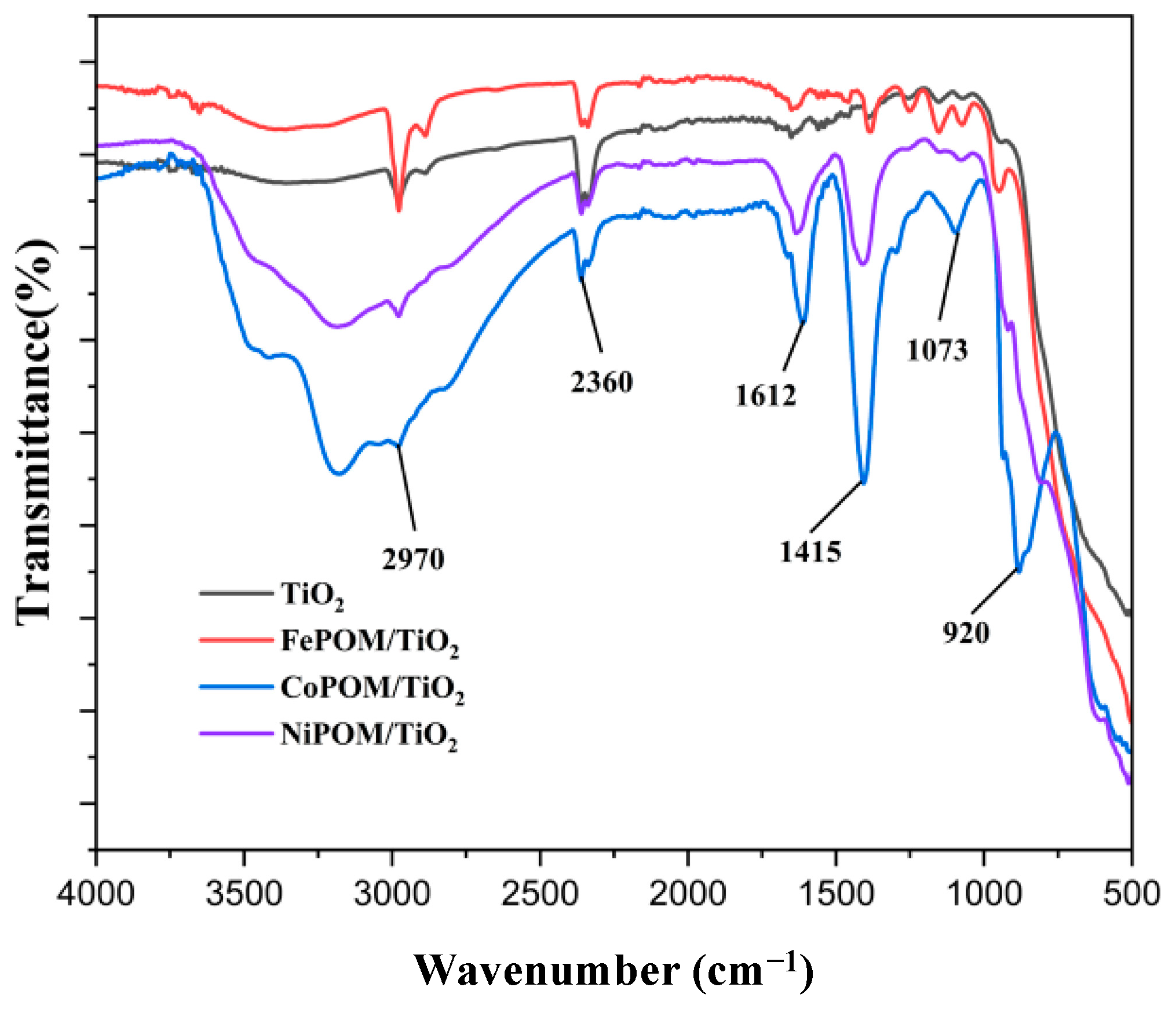
3.1. FTIR Characterization
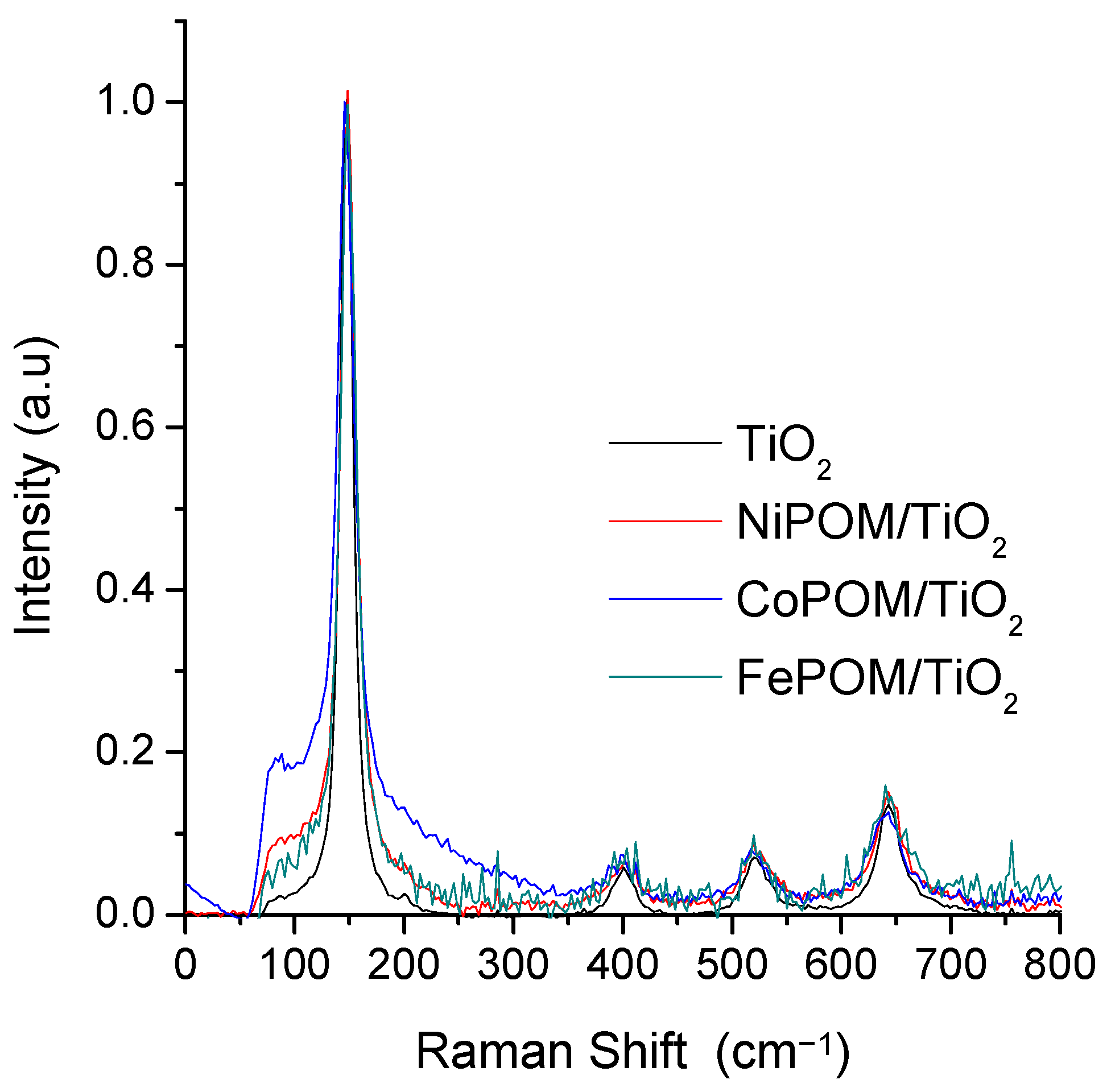
3.2. Raman Characterization
3.3. Optical Characterization
3.4. Morphological Characterization
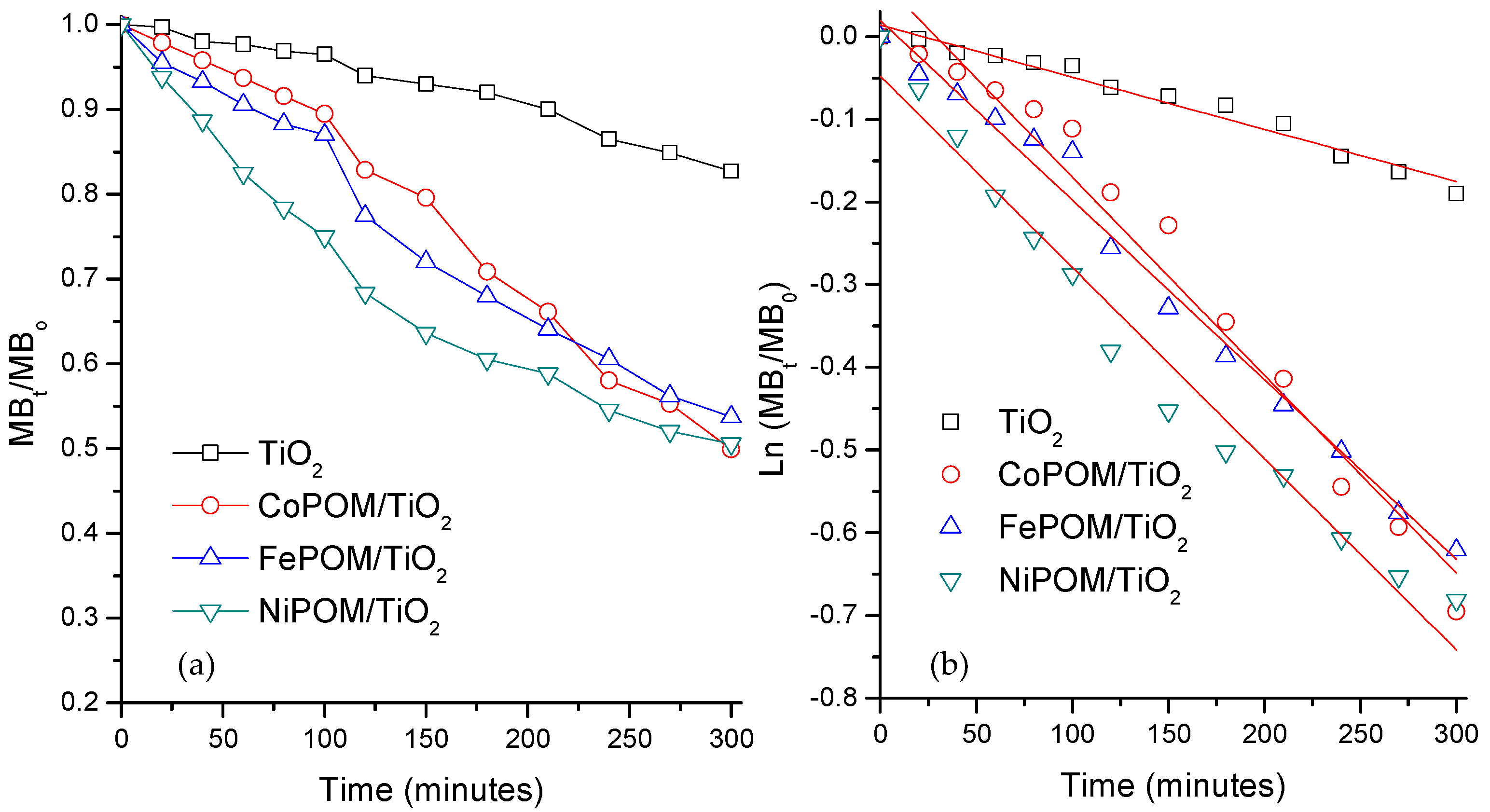
3.5. Removal and Degradation Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the economic impact of water scarcity in a changing world. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Bassyouni, M.; Alalm, M.G.; Saleh, M.Y. Recent developments in recalcitrant organic pollutants degradation using immobilized photocatalysts. Appl. Phys. A 2021, 127, 612. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. NPJ Clean Water 2022, 5, 1–25. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Su, R.; Zhu, Y.; Gao, B.; Li, Q. Progress on mechanism and efficacy of heterogeneous photocatalysis coupled oxidant activation as an advanced oxidation process for water decontamination. Water Res. 2024, 251, 121119. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Taneja, Y.; Dube, D.; Singh, R. Recent Advances in Elemental Doping and Simulation Techniques: Improving Structural, Photophysical and Electronic Properties of Titanium Oxide. J. Mater. Chem. C 2024, 12, 14774–14808. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Vallejo, W.; Rueda, A.; Díaz-Uribe, C.; Grande, C.; Quintana, P. Photocatalytic activity of graphene oxide-TiO2 thin films sensitized by natural dyes extracted from Bactris guineensis. R. Soc. Open Sci. 2019, 6, 181824. [Google Scholar] [CrossRef] [PubMed]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing visible light: Enhancing TiO2 photocatalysis with photosensitizers for sustainable and efficient environmental solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Balapure, A.; Dutta, J.R.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Rajput, R.B.; Jamble, S.N.; Kale, R.B. A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manag. 2022, 307, 114533. [Google Scholar] [CrossRef]
- Jayswal, S.; Moirangthem, R.S. Construction of a solar spectrum active SnS/ZnO p–n heterojunction as a highly efficient photocatalyst: The effect of the sensitization process on its performance. New J. Chem. 2018, 42, 13689–13701. [Google Scholar] [CrossRef]
- Lan, J.; Wang, Y.; Huang, B.; Xiao, Z.; Wu, P. Application of polyoxometalates in photocatalytic degradation of organic pollutants. Nanoscale Adv. 2021, 3, 4646–4658. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Florez, J.; Vallejo, W.; Duran, F.; Puello, E.; Roa, V.; Schott, E.; Zarate, X. Removal and photocatalytic degradation of methylene blue on ZrO2 thin films modified with Anderson-Polioxometalates (Cr3+, Co3+, Cu2+): An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2024, 454, 115689. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Shi, X.; Wu, L. Polyoxometalate-based frameworks for photocatalysis and photothermal catalysis. Nanoscale 2023, 15, 9242–9255. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, D.; Peng, W.; Ji, Y.; Tong, H. Research progress of polyoxometalates photocatalyst for degradation of organic wastewater. Appl. Chem. Eng. 2022, 5, 92–106. [Google Scholar] [CrossRef]
- Streb, C.; Kastner, K.; Tucher, J. Polyoxometalates in photocatalysis. Phys. Sci. Rev. 2019, 4, 20170177. [Google Scholar] [CrossRef]
- Liu, Q.; Su, T.; Zhang, H.; Liao, W.; Ren, W.; Zhu, Z.; Yang, K.; Len, C.; Yu, J.; Zhao, D.; et al. Development of TiO2 catalyst based on Anderson-polyoxometalates for efficient visible-light-driven photocatalytic oxidative desulfurization. Fuel 2023, 333, 126286. [Google Scholar] [CrossRef]
- Tang, Q.; An, X.; Lan, H.; Liu, H.; Qu, J. Polyoxometalates/TiO2 photocatalysts with engineered facets for enhanced degradation of bisphenol A through persulfate activation. Appl. Catal. B Environ. 2020, 268, 118394. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Duran, F.; Vallejo, W.; Puello, E.; Zarate, X.; Schott, E. Photocatalytic study of TiO2 thin films modified with Anderson-type polyoxometalates (Cr, Co and Ni): Experimental and DFT study. Polyhedron 2023, 231, 116253. [Google Scholar] [CrossRef]
- Vargas, X.; Tauchert, E.; Marin, J.M.; Restrepo, G.; Dillert, R.; Bahnemann, D. Fe-doped titanium dioxide synthesized: Photocatalytic activity and mineralization study for azo dye. J. Photochem. Photobiol. A Chem. 2012, 243, 17–22. [Google Scholar] [CrossRef]
- Lee, U.; Joo, H.-C.; Kwon, J.-S. Tetraammonium hexahydrogen hexamolybdonickelate(II) tetrahydrate, (NH4)4[H6NiMo6O24]4H2O. Acta Crystallogr. Sect. E Struct. Rep. Online 2002, 58, i6–i8. [Google Scholar] [CrossRef]
- Kontos, A.I.; Kontos, A.G.; Tsoukleris, D.S.; Bernard, M.-C.; Spyrellis, N.; Falaras, P. Nanostructured TiO2 films for DSSCS prepared by combining doctor-blade and sol–gel techniques. J. Mater. Process. Technol. 2008, 196, 243–248. [Google Scholar] [CrossRef]
- Quiñones, C.; Ayala, J.; Vallejo, W. Methylene blue photoelectrodegradation under UV irradiation on Au/Pd-modified TiO2 films. Appl. Surf. Sci. 2010, 257, 367–371. [Google Scholar] [CrossRef]
- Image.sc ImageJ. Available online: https://imagej.net/ij/ (accessed on 7 October 2024).
- Rajaramanan, T.; Velauthapillai, D.; Ravirajan, P.; Senthilnanthanan, M. A facile impregnation synthesis of Ni-doped TiO2 nanomaterials for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2023, 34, 916. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, X.; Chen, H.; Lin, J.; Liu, Y.; Chang, B.; Qiu, F.; Han, S.; Zhang, F. A high performance solid-state asymmetric supercapacitor based on Anderson-type polyoxometalate-doped graphene aerogel. Res. Chem. Intermed. 2019, 45, 3237–3250. [Google Scholar] [CrossRef]
- Ekoi, E.J.; Gowen, A.; Dorrepaal, R.; Dowling, D.P. Characterisation of titanium oxide layers using Raman spectroscopy and optical profilometry: Influence of oxide properties. Results Phys. 2019, 12, 1574–1585. [Google Scholar] [CrossRef]
- Radha, E.; Komaraiah, D.; Sayanna, R.; Sivakumar, J. Photoluminescence and photocatalytic activity of rare earth ions doped anatase TiO2 thin films. J. Lumin. 2022, 244, 118727. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi Basic Res. 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Volkringer, C.; Loiseau, T.; Tejeda, A.; Hureau, M.; Moissette, A. Band gap analysis in MOF materials: Distinguishing direct and indirect transitions using UV–vis spectroscopy. Appl. Mater. Today 2024, 37, 102094. [Google Scholar] [CrossRef]
- Liu, R.; Sun, Z.; Zhang, Y.; Xu, L.; Li, N. Polyoxometalate-modified TiO2 nanotube arrays photoanode materials for enhanced dye-sensitized solar cells. J. Phys. Chem. Solids 2017, 109, 64–69. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, R.; Li, J.; Tang, M.; Yang, L.; Gu, H.; Wang, X.; Sun, T. Recent Advances in Polyoxometalate Based Nanoplatforms Mediated Reactive Oxygen Species Cancer Therapy. Chem. Asian J. 2023, 18, e202300749. [Google Scholar] [CrossRef]
- Singh, G.; Mandal, D. Modulation of the Band Gap and Redox Properties by Mixed Addenda in Sandwich Polyoxometalates. Inorg. Chem. 2023, 62, 19648–19663. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, Z.; Lin, H.; Tian, A.; Liu, G.; Zhang, J. Assembly and photocatalysis of two novel 3D Anderson-type polyoxometalate-based metal–organic frameworks constructed from isomeric bis(pyridylformyl)piperazine ligands. Dalt. Trans. 2014, 43, 12272–12278. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chao, P.Y. Efficient photocatalytic hydrogen production by doped ZnS grown on Ni foam as porous immobilized photocatalysts. Int. J. Hydrogen Energy 2019, 44, 20805–20814. [Google Scholar] [CrossRef]
- Chang, C.J.; Wei, Y.H.; Kuo, W.S. Free-standing CuS–ZnS decorated carbon nanotube films as immobilized photocatalysts for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 30553–30562. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Shaikh, S.K.; Rajpure, K.Y. Thin film photocatalysis for environmental remediation: A status review. Curr. Appl. Phys. 2020, 20, 931–952. [Google Scholar] [CrossRef]
- Petit, M.; Michez, L.; Raimundo, J.M.; Malinowski, T.; Dumas, P. An introduction to photocatalysis through methylene blue photodegradation. Eur. J. Phys. 2016, 37, 065808. [Google Scholar] [CrossRef]
- Zelinski, D.W.; dos Santos, T.P.M.; Takashina, T.A.; Leifeld, V.; Igarashi-Mafra, L. Photocatalytic Degradation of Emerging Contaminants: Artificial Sweeteners. Water Air Soil Pollut. 2018, 229, 207. [Google Scholar] [CrossRef]
- Fabre, B.; Falaise, C.; Cadot, E. Polyoxometalates-Functionalized Electrodes for (Photo)Electrocatalytic Applications: Recent Advances and Prospects. ACS Catal. 2022, 12, 12055–12091. [Google Scholar] [CrossRef]
- Cherevan, A.S.; Nandan, S.P.; Eder, D.; Roger, I.; Liu, R.; Streb, C.; Cherevan, A.S.; Nandan, S.P.; Roger, I.; Liu, R.; et al. Polyoxometalates on Functional Substrates: Concepts, Synergies, and Future Perspectives. Adv. Sci. 2020, 7, 1903511. [Google Scholar] [CrossRef]
- Wardhani, S.; Purwonugroho, D.; Fitri, C.W.; Prananto, Y.P. Effect of pH and irradiation time on TiO2-chitosan activity for phenol photo-degradation. AIP Conf. Proc. 2018, 2021, 50009. [Google Scholar]
- Ben Ameur, S.; BelHadjltaief, H.; Duponchel, B.; Leroy, G.; Amlouk, M.; Guermazi, H.; Guermazi, S. Enhanced photocatalytic activity against crystal violet dye of Co and In doped ZnO thin films grown on PEI flexible substrate under UV and sunlight irradiations. Heliyon 2019, 5, e01912. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Nguyen-Huy, C.; Lee, H.J.; Nguyen-Phan, T.D.; Son, T.H.; Kim, C.K.; Shin, E.W. Cu-doped TiO2/reduced graphene oxide thin-film photocatalysts: Effect of Cu content upon methylene blue removal in water. Ceram. Int. 2015, 41, 11184–11193. [Google Scholar] [CrossRef]
- Xie, W.; Zhong, L.; Wang, Z.; Liang, F.; Tang, X.; Zou, C.; Liu, G. Photocatalytic performance of Bi2VO5.5/Bi2O3 laminated composite films under simulated sunlight irradiation. Solid State Sci. 2019, 94, 1–7. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, J.E.; Kim, K.S.; Kang, J.M.; Lee, J.H.; Kim, K.H.; Cho, M.; Lee, S.G. Photocatalytic degradation of methylene blue under UV and visible light by brookite–rutile bi-crystalline phase of TiO2. New J. Chem. 2021, 45, 3485–3497. [Google Scholar] [CrossRef]
- Che Ramli, Z.A.; Asim, N.; Isahak, W.N.R.W.; Emdadi, Z.; Ahmad-Ludin, N.; Yarmo, M.A.; Sopian, K. Photocatalytic Degradation of Methylene Blue under UV Light Irradiation on Prepared Carbonaceous TiO2. Sci. World J. 2014, 2014, 415136. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
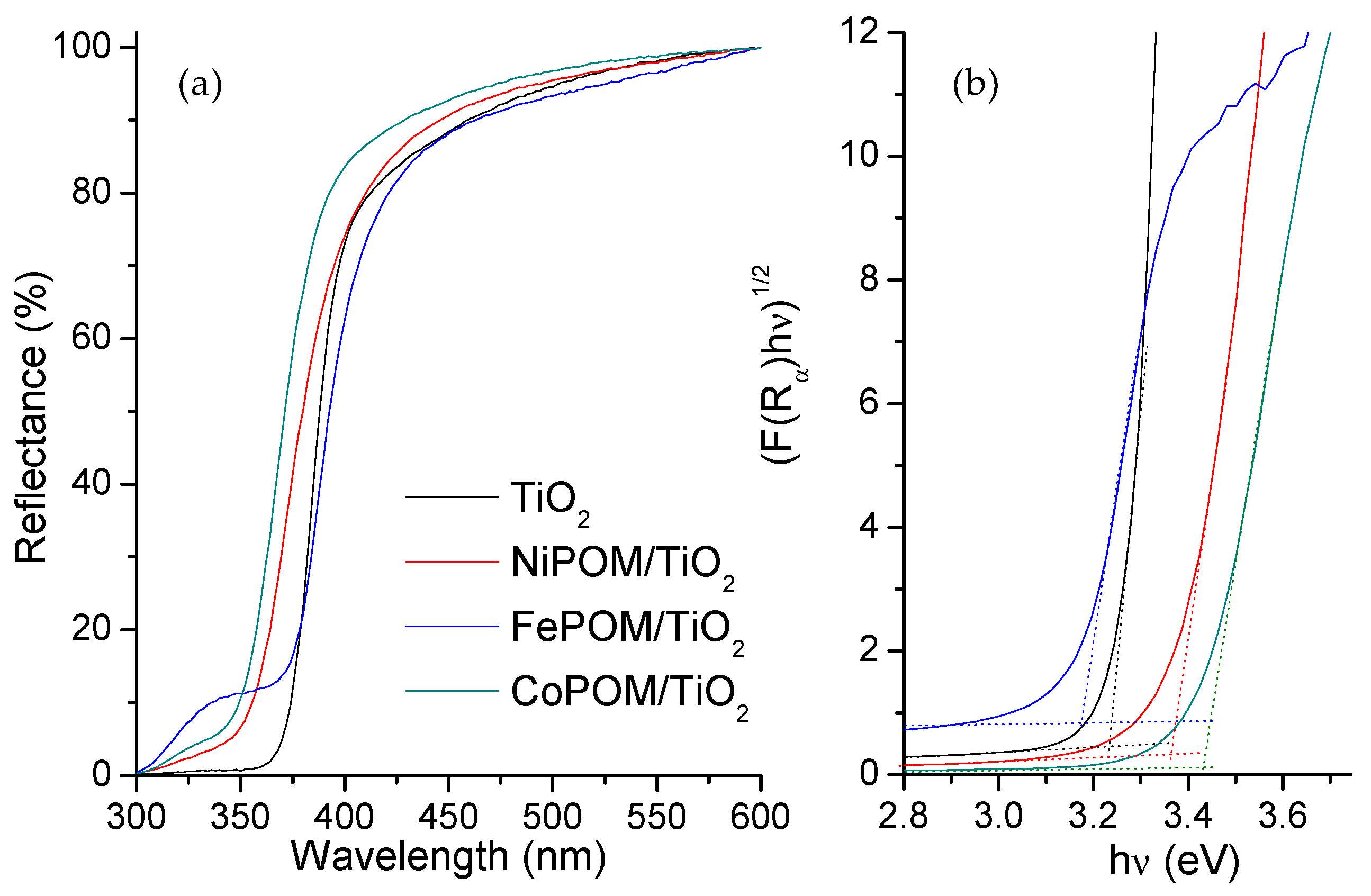


| Compound | Energy Band Gap 1 (eV) | Pore Area (μm) |
|---|---|---|
| TiO2 | 3.22 | 210 |
| FePOM/TiO2 | 3.17 | 900 |
| CoPOM/TiO2 | 3.30 | 555 |
| NiPOM/TiO2 | 3.41 | 351 |
| Element | CoPOM/TiO2 | NiPOM/TiO2 | FePOM/TiO2 |
|---|---|---|---|
| Atomic (%) 1 | Atomic (%) | Atomic (%) | |
| O | 78.01 | 64.1 | 71.1 |
| Ti | 8.13 | 24.90 | 11.3 |
| Mo | 0.28 | 1.19 | 0.69 |
| Co | 0.05 | 0.0 | 0.0 |
| Ni | 0.0 | 0.2 | 0.0 |
| Fe | 0.0 | 0.0 | 0.13 |
| C | 12.8 | 7.8 | 12.2 |
| Compound | Photocatalytic Efficiency (%) | k (min−1) × 10−4 | R2 | k/kTiO2 |
|---|---|---|---|---|
| TiO2 | 18.3 | 6.5 | 0.972 | - |
| FePOM/TiO2 | 46.2 | 22 | 0.969 | 3.4 |
| CoPOM/TiO2 | 50.1 | 24 | 0.988 | 3.7 |
| NiPOM/TiO2 | 49.4 | 23 | 0.975 | 3.5 |
| Semiconductor | Pollutant/Time Irradiation | Degradation Efficiency (%) |
|---|---|---|
| TiO2-chitosan/TF ¨ [51] | Phenol/300 min | 35 |
| ZnO:Co/TF [52] | CV +*/210 min | 90 |
| Cu:TiO2/reduced GO */TF [53] | MB **/180 min | 63 |
| Bi2VO5.5/Bi2O3/TF [54] | MB/300 min | 90 |
| BCTi +/powder [55] | MB/180 min | 90 |
| CTi ++/powder [56] | MB/120 min | 98 |
| CoPOM/TiO2 (this work) | MB/300 min | 50.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo, W.; Corzo, G.; Berrio, R.; Diaz-Uribe, C.; Duran, F.; Zarate, X.; Schott, E. Synthesis and Characterization of TiO2 Thin Films Modified with Anderson-Type Polyoxometalates (Ni, Co, and Fe). Coatings 2024, 14, 1362. https://doi.org/10.3390/coatings14111362
Vallejo W, Corzo G, Berrio R, Diaz-Uribe C, Duran F, Zarate X, Schott E. Synthesis and Characterization of TiO2 Thin Films Modified with Anderson-Type Polyoxometalates (Ni, Co, and Fe). Coatings. 2024; 14(11):1362. https://doi.org/10.3390/coatings14111362
Chicago/Turabian StyleVallejo, William, Gabriel Corzo, Ricardo Berrio, Carlos Diaz-Uribe, Freider Duran, Ximena Zarate, and Eduardo Schott. 2024. "Synthesis and Characterization of TiO2 Thin Films Modified with Anderson-Type Polyoxometalates (Ni, Co, and Fe)" Coatings 14, no. 11: 1362. https://doi.org/10.3390/coatings14111362
APA StyleVallejo, W., Corzo, G., Berrio, R., Diaz-Uribe, C., Duran, F., Zarate, X., & Schott, E. (2024). Synthesis and Characterization of TiO2 Thin Films Modified with Anderson-Type Polyoxometalates (Ni, Co, and Fe). Coatings, 14(11), 1362. https://doi.org/10.3390/coatings14111362






