Effect of Over-Aging Degree on Microstructures, Precipitation Kinetics, and Mechanical Properties of an Ultra-High-Strength Al-Zn-Mg-Cu Alloy
Abstract
1. Introduction
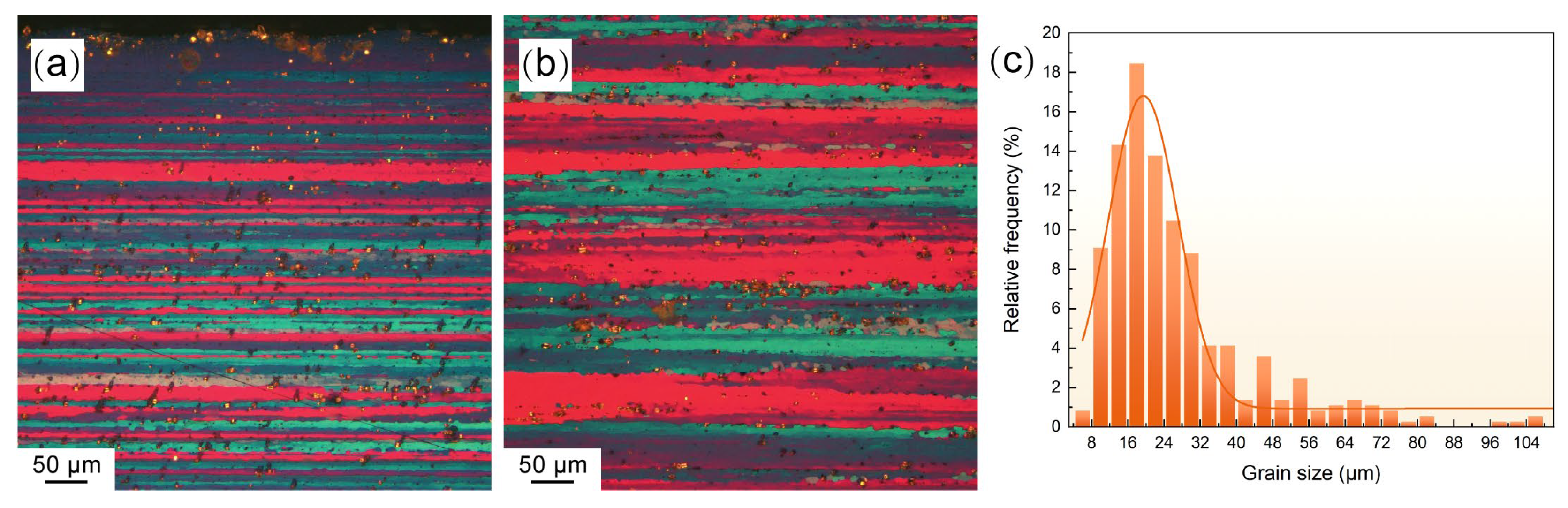
2. Materials and Methods
3. Results
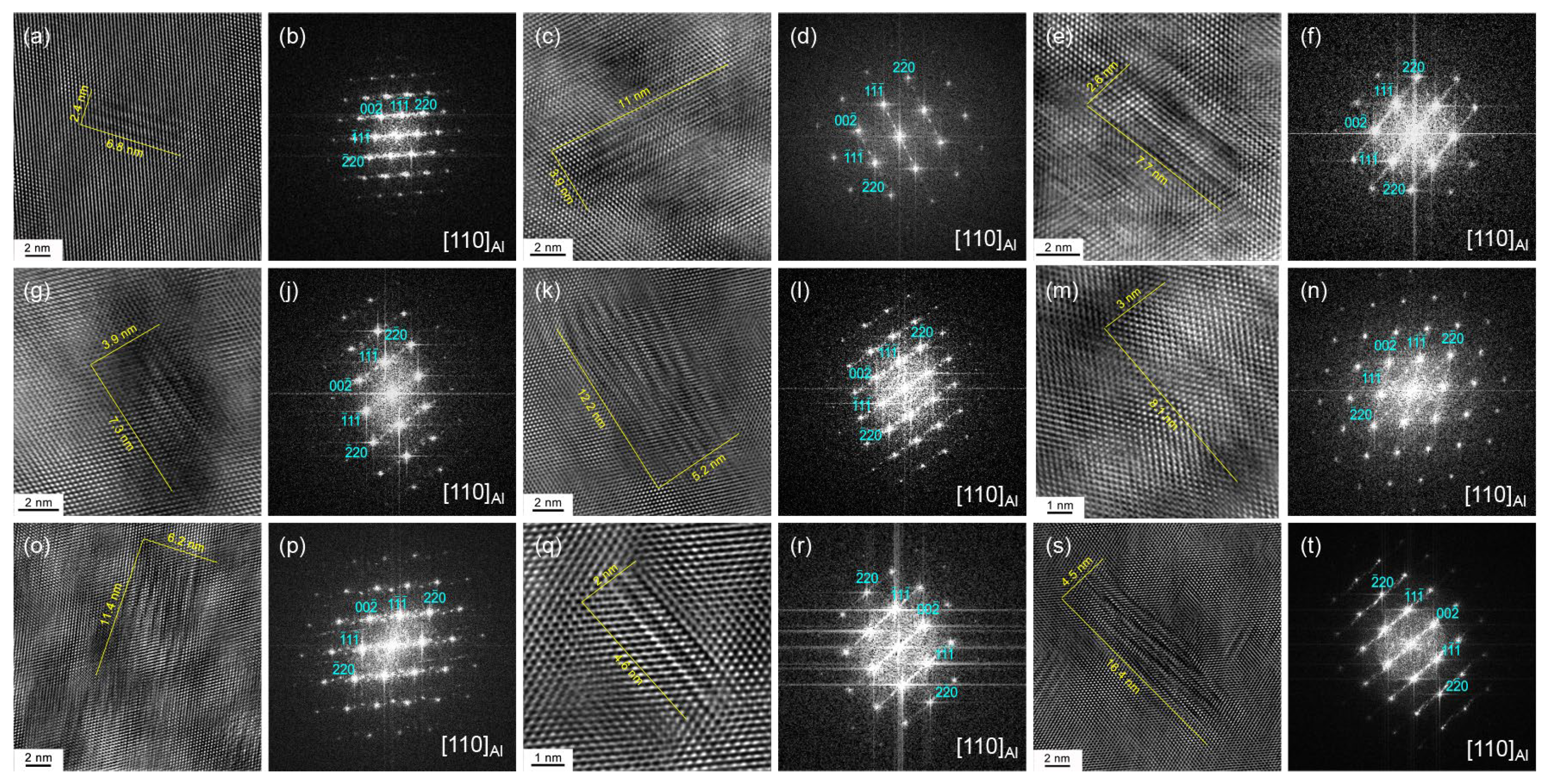
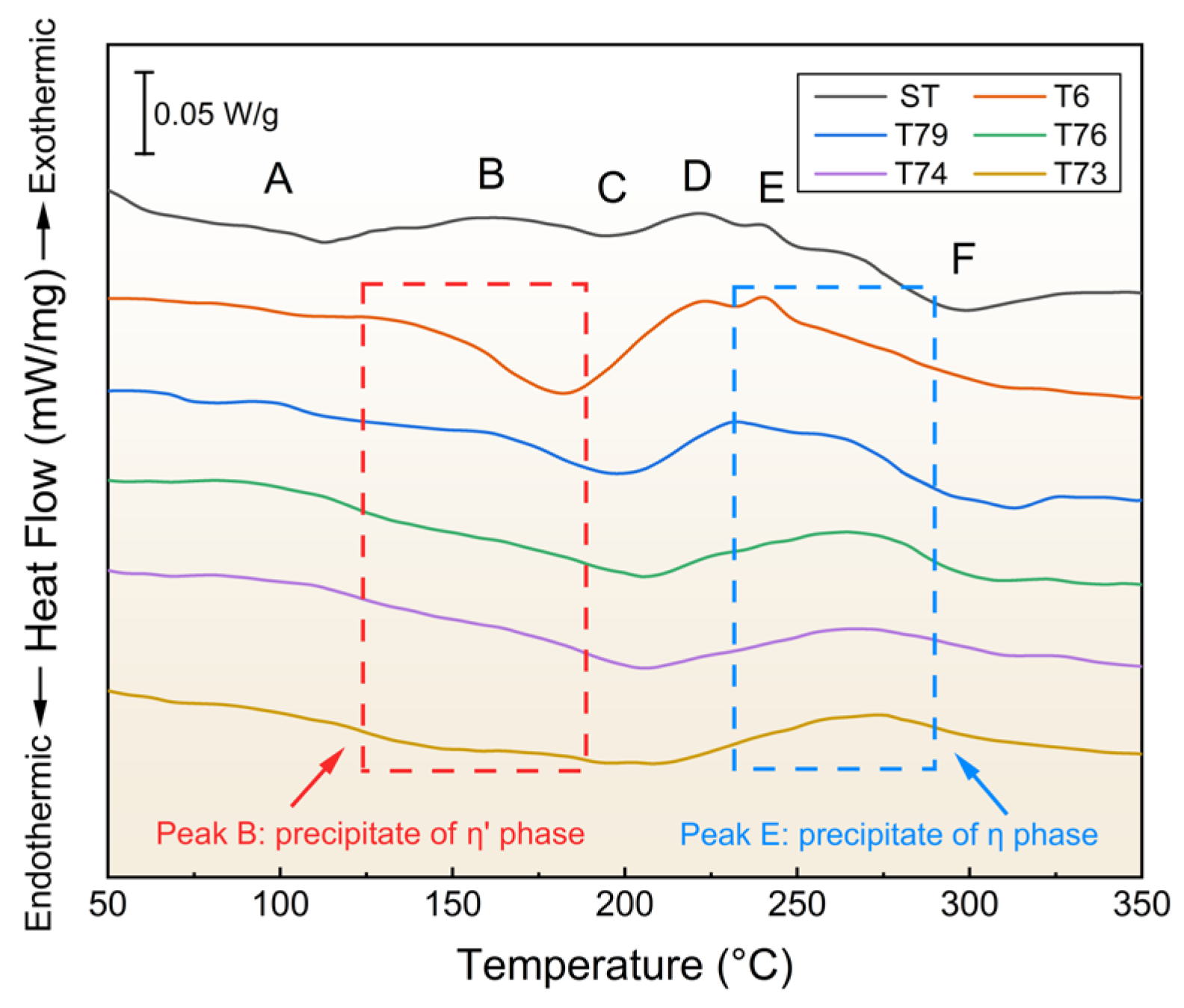
3.1. Aging Precipitation Behavior
3.2. Tensile Properties
4. Discussion
4.1. Effect of the Over-Aging Degree on the Precipitation Evolution of Matrix Precipitation
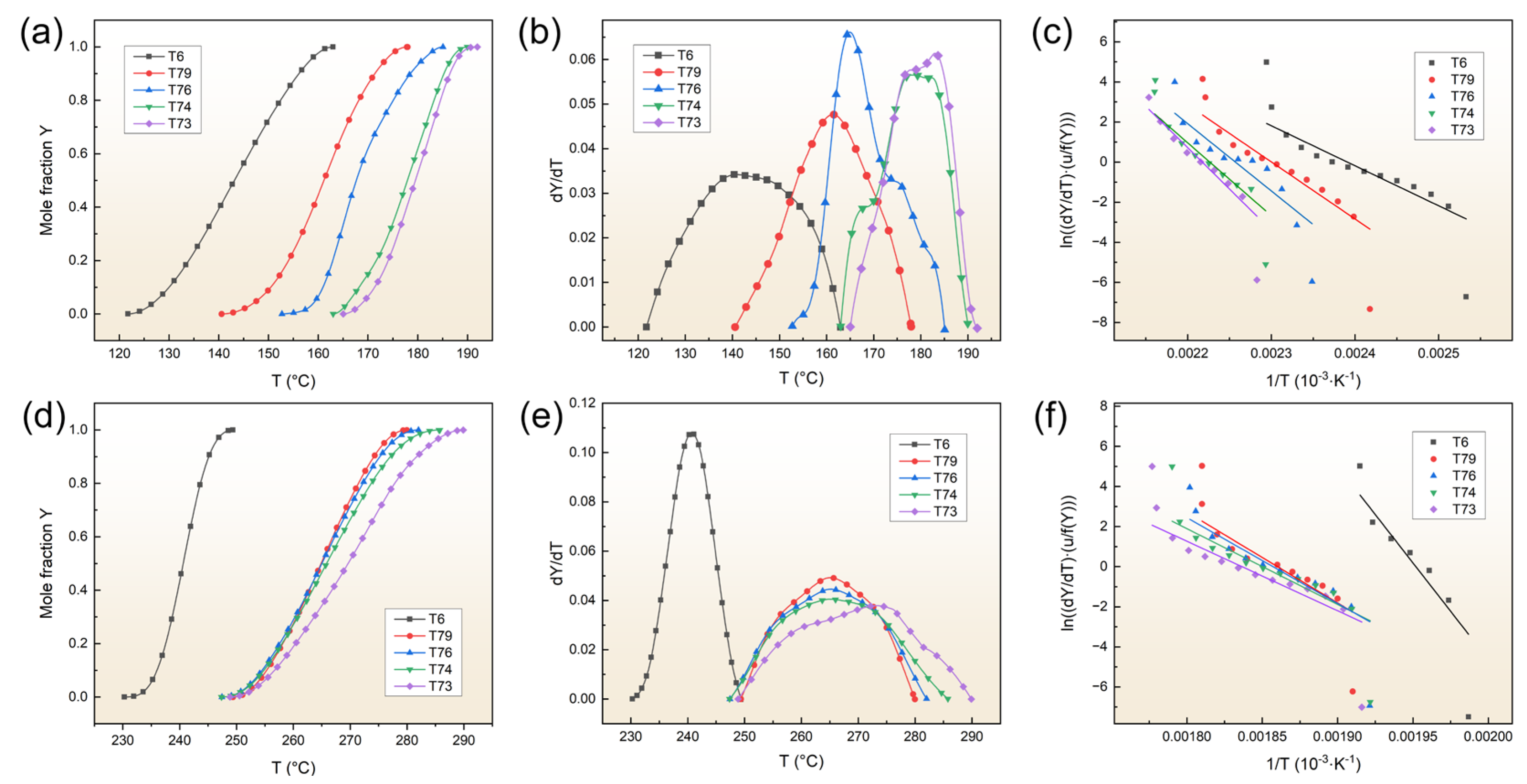
4.2. Effect of the Over-Aging Degree on the Precipitation Kinetics of Matrix Precipitation
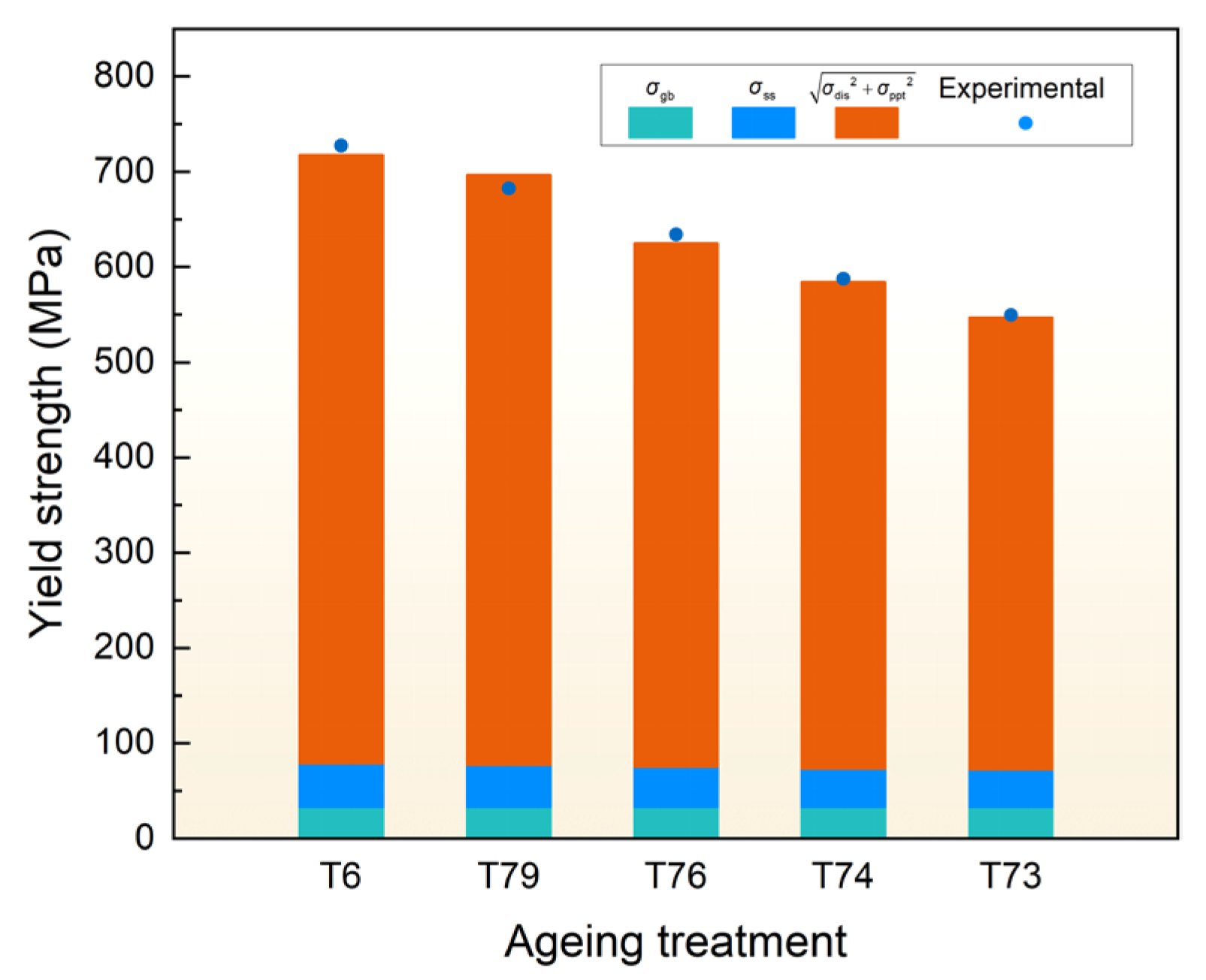
4.3. Strengthening Model
5. Conclusions
- Both the average size and volume fraction of matrix precipitates increased with the deepening of the over-aging;
- The static strengths of the alloy decreased with the deepening of over-aging, while elongation improved with increased over-aging;
- TEM confirms that the matrix precipitates are η′ phase precipitates in the T6 sample, and η′ and η phase precipitates in the T7X samples. The activation energies required to precipitate the η′ and η phase precipitates of the various aging samples were determined using the DSC and JMAK equations. The results indicate that the precipitation of η′ and η phases in all samples is controlled by the kinetics of the aging process. After the calculations of the JMAK equations, the activation energy required to precipitate the η′ phase precipitates ranging from 166.08 to 343.28 kJ/mol, and the activation energy required to precipitate the η phase precipitates ranging from 802.03 to 288.42 kJ/mol from the T6 to T73 conditions. Compared to the T7X treatments, the lowest activation energy required to precipitate the η′ phase precipitates, and the highest for η phase precipitates were calculated under the T6 treatment, suggesting that η phase precipitates are difficult to precipitate under the T6 treatment. Under T7X treatments, the activation energy required to precipitate η′ phase precipitation increased with increasing over-aging, while the opposite trend was observed for η phase precipitates, indicating that T7X treatments promote η phase precipitation;
- The strengthening components of the ultra-high strength Al-Zn-Mg-Cu alloy under various over-aging degrees are systematically calculated through quantitative microstructure analysis. A high-precision model suitable for evaluating the yield strength of the current alloy under over-aging conditions was established.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| the volume fraction of precipitates | |
| the density of precipitates | |
| the thickness of precipitates | |
| the diameter of platelet-shaped precipitates | |
| the driving force for precipitation | |
| the atomic volume | |
| the Boltzmann constant for the driving force for precipitation () | |
| the current aging temperature | |
| refer to the solute concentrations | |
| the critical nucleation radius | |
| the surface energy | |
| the average precipitate radius | |
| the initial radius at the aging time | |
| is the diffusion coefficient | |
| the precipitate volume fraction for the JMAK equations | |
| the nucleation type constant for the JMAK equations | |
| the growth mode constant for the JMAK equations | |
| the activation energy | |
| the absolute temperature for the JMAK equations | |
| the gas constant for the JMAK equations | |
| the heating rate | |
| the area fraction for DSC thermograms | |
| the contribution of grain boundary strengthening | |
| the frictional stress of pure aluminum | |
| the Hall–Petch coefficient | |
| the average grain size | |
| the contribution of solid solution strengthening | |
| the initial contribution strength from the solid solution of the samples | |
| is the hardening constant for element | |
| the contribution of dislocation work hardening | |
| the average spacing of precipitation | |
| the average radius of precipitates | |
| the critical shear radius | |
| the contribution of the precipitation strengthening | |
| the shear modulus of the aluminum matrix | |
| the Burgers vector | |
| the coefficient | |
| thecoefficient | |
| the Taylor factor |
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. (1980–2015) 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Froes, F. Advanced metals for aerospace and automotive use. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 1994, 184, 119–133. [Google Scholar] [CrossRef]
- Mouritz, A.P. Introduction to Aerospace Materials; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Azarniya, A.; Taheri, A.K.; Taheri, K.K. Recent advances in ageing of 7xxx series aluminum alloys: A physical metallurgy perspective. J. Alloy. Compd. 2019, 781, 945–983. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys—Some recent developments. Trans. Nonferrous Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Hadjadj, L.; Amira, R.; Hamana, D.; Mosbah, A. Characterization of precipitation and phase transformations in Al–Zn–Mg alloy by the differential dilatometry. J. Alloy. Compd. 2008, 462, 279–283. [Google Scholar] [CrossRef]
- Berg, L.; Gjønnes, J.; Hansen, V.; Li, X.; Knutson-Wedel, M.; Schryvers, D.; Wallenberg, L. GP-zones in Al–Zn–Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Yang, X.; Ren, S.; Wu, C.; Xu, H.; Zou, J. Revisiting the precipitation sequence in Al–Zn–Mg-based alloys by high-resolution transmission electron microscopy. Scr. Mater. 2010, 63, 1061–1064. [Google Scholar] [CrossRef]
- Rao, A.U.; Vasu, V.; Govindaraju, M.; Srinadh, K.S. Stress corrosion cracking behaviour of 7xxx aluminum alloys: A literature review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar] [CrossRef]
- Speidel, M.O. Stress corrosion cracking of aluminum alloys. Metall. Trans. A 1975, 6, 631–651. [Google Scholar] [CrossRef]
- Su, R.; Liu, T.; Qu, Y.; Bai, G.; Li, R. Mechanical properties and corrosion behavior of spray-formed 7075 alloy with one-stage aging. J. Mater. Eng. Perform. 2019, 28, 2212–2220. [Google Scholar] [CrossRef]
- Cheng, Q.; Ye, L.; Huang, Q.; Dong, Y.; Liu, S. Effect of two-stage overaging on microstructure and corrosion properties of an Al–Zn–Mg–Cu alloy. J. Mater. Res. Technol.-JMRT 2022, 20, 3185–3194. [Google Scholar] [CrossRef]
- Baydogan, M.; Cimenoglu, H.; Sabri Kayali, E.; Rasty, J. Improved resistance to stress-corrosion-cracking failures via optimized retrogression and reaging of 7075-T6 aluminum sheets. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2008, 39, 2470–2476. [Google Scholar] [CrossRef]
- Yang, W.; Ji, S.; Zhang, Q.; Wang, M. Investigation of mechanical and corrosion properties of an Al–Zn–Mg–Cu alloy under various ageing conditions and interface analysis of η′ precipitate. Mater. Des. 2015, 85, 752–761. [Google Scholar] [CrossRef]
- Cao, F.; Qin, Z. Effect of two-stage aging on hardness and electrical conductivity of as-extruded 7075 aAluminum Alloy. J. Phys. Conf. Ser. 2021, 1885, 042035. [Google Scholar] [CrossRef]
- Cai, S.; He, Y.; Song, R. Study on the strengthening mechanism of two-stage double-peaks aging in 7075 aluminum alloy. Trans. Indian Inst. Met. 2020, 73, 109–117. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, G.; Tian, N. Two-step Aging Treatment in Ultra-High-Strength Al-Zn-Mg-Cu Aluminum Alloy. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; p. 05020. [Google Scholar]
- Lin, L.; Liu, Z.; Ying, P.; Liu, M. Improved stress corrosion cracking resistance and strength of a two-step aged Al-Zn-Mg-Cu alloy using Taguchi method. J. Mater. Eng. Perform. 2015, 24, 4870–4877. [Google Scholar] [CrossRef]
- Puiggali, M.; Zielinski, A.; Olive, J.; Renauld, E.; Desjardins, D.; Cid, M. Effect of microstructure on stress corrosion cracking of an Al-Zn-Mg-Cu alloy. Corros. Sci. 1998, 40, 805–819. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, K.; Ren, C.; Naseem, S. Precipitation sequence and hardening effect in 7A85 aluminum alloy. J. Alloys Compd. 2021, 875, 159950. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, S.; Jiang, D. Influence of repetitious non-isothermal aging on microstructure and strength of Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2016, 689, 632–640. [Google Scholar] [CrossRef]
- Yang, W.; Ji, S.; Wang, M.; Li, Z. Precipitation behaviour of Al–Zn–Mg–Cu alloy and diffraction analysis from η′ precipitates in four variants. J. Alloys Compd. 2014, 610, 623–629. [Google Scholar] [CrossRef]
- Luo, J.; Luo, H.; Li, S.; Wang, R.; Ma, Y. Effect of pre-ageing treatment on second nucleating of GPII zones and precipitation kinetics in an ultrafine grained 7075 aluminum alloy. Mater. Des. 2020, 187, 108402. [Google Scholar] [CrossRef]
- Park, S.; Kayani, S.H.; Euh, K.; Seo, E.; Kim, H.; Park, S.; Yadav, B.N.; Park, S.J.; Sung, H. High strength aluminum alloys design via explainable artificial intelligence. J. Alloys Compd. 2022, 903, 163828. [Google Scholar] [CrossRef]
- Dumont, M.; Lefebvre, W.; Doisneau-Cottignies, B.; Deschamps, A. Characterisation of the composition and volume fraction of η′ and η precipitates in an Al–Zn–Mg alloy by a combination of atom probe, small-angle X-ray scattering and transmission electron microscopy. Acta Mater. 2005, 53, 2881–2892. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al–Zn–Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Aaronson, H.; Kinsman, K.; Russell, K. The volume free energy change associated with precipitate nucleation. Scr. Metall. 1970, 4, 101–106. [Google Scholar] [CrossRef]
- Deschamps, A.; Brechet, Y. Influence of predeformation and ageing of an Al–Zn–Mg alloy—II. Modeling of precipitation kinetics and yield stress. Acta Mater. 1998, 47, 293–305. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Chen, L. Coarsening dynamics of self-accommodating coherent patterns. Acta Mater. 2002, 50, 13–21. [Google Scholar] [CrossRef]
- Panigrahi, S.; Jayaganthan, R.; Pancholi, V.; Gupta, M. A DSC study on the precipitation kinetics of cryorolled Al 6063 alloy. Mater. Chem. Phys. 2010, 122, 188–193. [Google Scholar] [CrossRef]
- Birolia, G.; Cagliotiab, G.; Martini, L.; Riontino, G. Precipitation kinetics of AA4032 and AA6082: A comparison based on DSC and TEM. Scr. Mater. 1998, 39, 197–203. [Google Scholar] [CrossRef]
- Lang, P.; Wojcik, T.; Povoden-Karadeniz, E.; Falahati, A.; Kozeschnik, E. Thermo-kinetic prediction of metastable and stable phase precipitation in Al–Zn–Mg series aluminium alloys during non-isothermal DSC analysis. J. Alloy. Compd. 2014, 609, 129–136. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, J.; Pu, Q.; Li, K.; Luo, T.; Li, Y.; Xia, P.; Li, X.; Chen, D.; Sha, G. Achieving high performance by optimized heat treatment in a spray formed Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2024, 893, 146134. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, Q.; Yang, L.; Zhang, K.; Yuan, S.; Zhen, L. Non-isothermal ageing of an Al–8Zn–2Mg–2Cu alloy for enhanced properties. J. Mater. Process. Technol. 2016, 227, 110–116. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, W.; Yang, L.; Shao, W.; Yuan, S.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Satrink, M.; Zahra, A.-M. The kinetics of isothermal β′ precipitation in Al-Mg alloys. J. Mater. Sci. 1999, 34, 1117–1127. [Google Scholar] [CrossRef]
- Aouabdia, Y.; Boubertakh, A.; Hamamda, S. Precipitation kinetics of the hardening phase in two 6061 aluminium alloys. Mater. Lett. 2010, 64, 353–356. [Google Scholar] [CrossRef]
- Luo, A.; Lloyd, D.; Gupta, A.; Youdelis, W. Precipitation and dissolution kinetics in Al-Li-Cu-Mg alloy 8090. Acta Metall. Mater. 1993, 41, 769–776. [Google Scholar] [CrossRef]
- Ghosh, K.; Gao, N. Determination of kinetic parameters from calorimetric study of solid state reactions in 7150 Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 1199–1209. [Google Scholar] [CrossRef]
- Ghosh, K.; Das, K.; Chatterjee, U. Kinetics of solid-state reactions in Al-Li-Cu-Mg-Zr alloys from calorimetric studies. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2007, 38, 1965–1975. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, M.; Shankar, G. On the role of precipitates in controlling microstructure and mechanical properties of Ag and Sn added 7075 alloys during artificial ageing. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2018, 738, 399–411. [Google Scholar] [CrossRef]
- Hall, E. The deformation and ageing of mild steel: III discussion of results. Proc. Phys. Society. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]
- Starink, M.; Wang, S. A model for the yield strength of overaged Al–Zn–Mg–Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef]
- Ma, Z.; Robson, J. Understanding the effect of deformation combined with heat treatment on age hardening of Al–Zn–Mg–Cu alloy AA7075. Mater. Sci. Eng. A 2023, 878, 145212. [Google Scholar] [CrossRef]
- Esmaeili, S.; Lloyd, D.; Poole, W. A yield strength model for the Al-Mg-Si-Cu alloy AA6111. Acta Mater. 2003, 51, 2243–2257. [Google Scholar] [CrossRef]
- Shercliff, H.; Ashby, M. A process model for age hardening of aluminium alloys—I. The model. Acta Metall. Et Mater. 1990, 38, 1789–1802. [Google Scholar] [CrossRef]
- Dixit, M.; Mishra, R.; Sankaran, K. Structure–property correlations in Al 7050 and Al 7055 high-strength aluminum alloys. Mater. Sci. Eng. A 2008, 478, 163–172. [Google Scholar] [CrossRef]
- Ardell, A.J. Precipitation hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Hornbogen, E.; Zum Gahr, K.-H. Distribution of plastic strain in alloys containing small particles. Metallography 1975, 8, 181–202. [Google Scholar] [CrossRef]
- Yunlong, M.; Ben, L.; Xiao, Z.; Huang, Y. First-Principles Study of Mechanical and Electronic Properties of η and η' Phases Present in 7xxx Alloys. Phys. Met. Metallogr. 2021, 122, 1257–1263. [Google Scholar] [CrossRef]
- Kocks, U. Statistical treatment of penetrable obstacles. Can. J. Phys. 1967, 45, 737–755. [Google Scholar] [CrossRef]
- Kocks, U. A statistical theory of flow stress and work-hardening. Philos. Mag. 1966, 13, 541–566. [Google Scholar] [CrossRef]
- Duchaussoy, A.; Sauvage, X.; Deschamps, A.; De Geuser, F.; Renou, G.; Horita, Z. Complex interactions between precipitation, grain growth and recrystallization in a severely deformed Al-Zn-Mg-Cu alloy and consequences on the mechanical behavior. Materialia 2021, 15, 101028. [Google Scholar] [CrossRef]




| Elements | Zn | Mg | Cu | Zr | Fe | Si | Al |
|---|---|---|---|---|---|---|---|
| Content (wt.%) | 8.7 | 2.8 | 2.0 | 0.13 | 0.05 | 0.02 | Balance |
| Solid Solution Treatment Regimes | Aging Treatment Regimes | |
|---|---|---|
| T6 | 475 °C × 2 h | 120 °C × 24 h |
| T79 | 120 °C × 8 h + 165 °C × 3 h | |
| T76 | 120 °C × 8 h + 165 °C × 6 h | |
| T74 | 120 °C × 8 h + 165 °C × 10 h | |
| T73 | 120 °C × 8 h + 165 °C × 16 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhao, Z.; Wang, G. Effect of Over-Aging Degree on Microstructures, Precipitation Kinetics, and Mechanical Properties of an Ultra-High-Strength Al-Zn-Mg-Cu Alloy. Coatings 2024, 14, 1415. https://doi.org/10.3390/coatings14111415
Liu Y, Zhao Z, Wang G. Effect of Over-Aging Degree on Microstructures, Precipitation Kinetics, and Mechanical Properties of an Ultra-High-Strength Al-Zn-Mg-Cu Alloy. Coatings. 2024; 14(11):1415. https://doi.org/10.3390/coatings14111415
Chicago/Turabian StyleLiu, Yuyang, Zhihao Zhao, and Gaosong Wang. 2024. "Effect of Over-Aging Degree on Microstructures, Precipitation Kinetics, and Mechanical Properties of an Ultra-High-Strength Al-Zn-Mg-Cu Alloy" Coatings 14, no. 11: 1415. https://doi.org/10.3390/coatings14111415
APA StyleLiu, Y., Zhao, Z., & Wang, G. (2024). Effect of Over-Aging Degree on Microstructures, Precipitation Kinetics, and Mechanical Properties of an Ultra-High-Strength Al-Zn-Mg-Cu Alloy. Coatings, 14(11), 1415. https://doi.org/10.3390/coatings14111415





