The Influence of Nano-Silicon Carbide on the Properties of Aluminum Alloy Under Salt Dry–Wet Alternations
Abstract
1. Introduction
2. Experimental
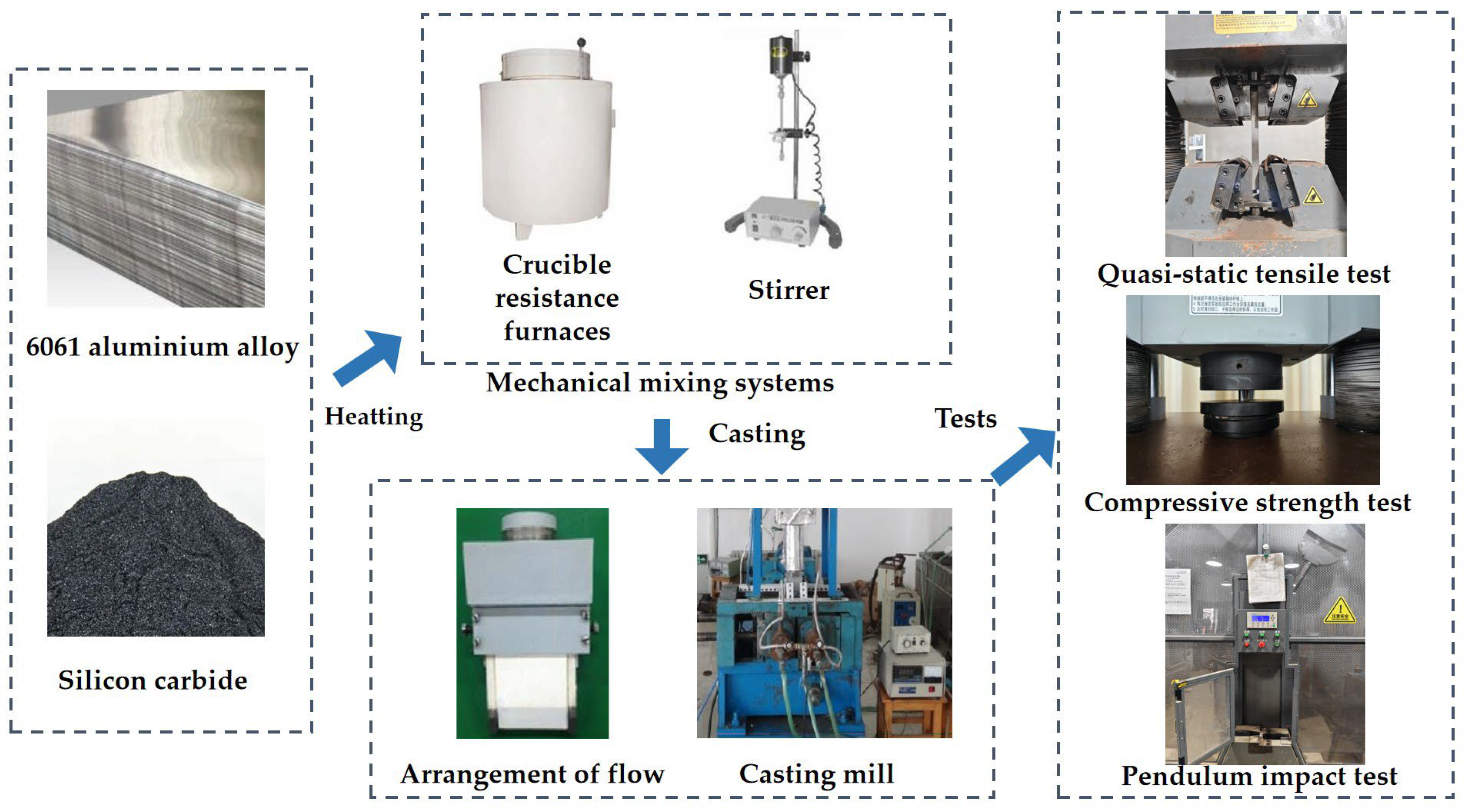
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Measurement Methods
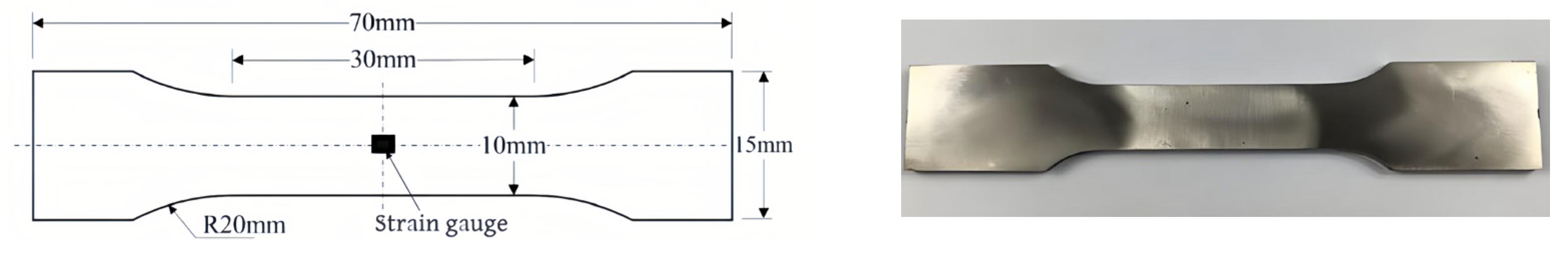
2.3.1. Quasi-Static Tensile Test
2.3.2. Compressive Strength Test
2.3.3. Pendulum Impact Test
2.3.4. Tensile Toughness Test
2.3.5. Dry–Wet Alternations of NaCl and Na2SO4
2.3.6. The Mass Loss Rate
2.3.7. Experiments to Measure Microscopic Properties
3. Results and Discussions
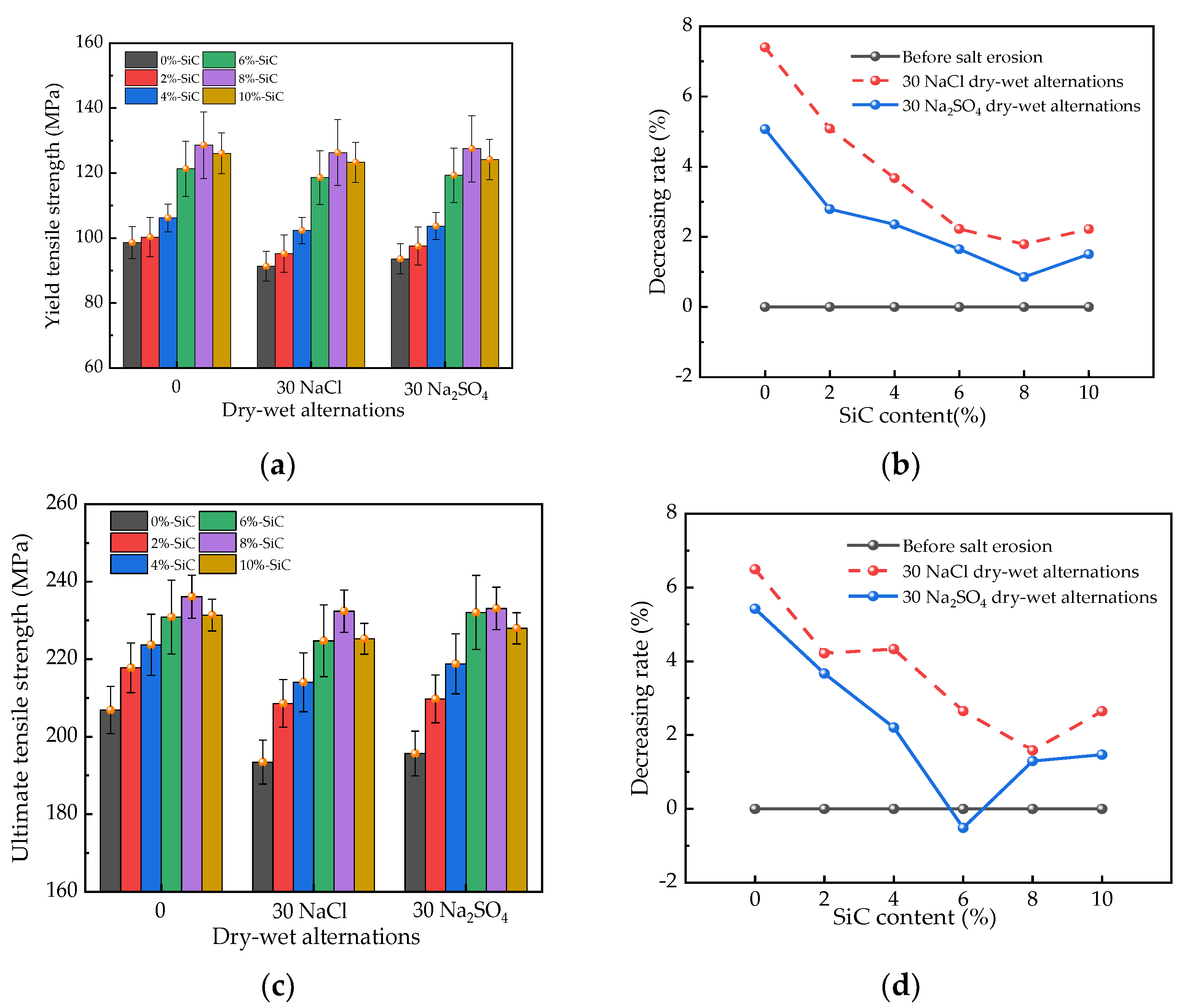
3.1. Mechanical Strengths
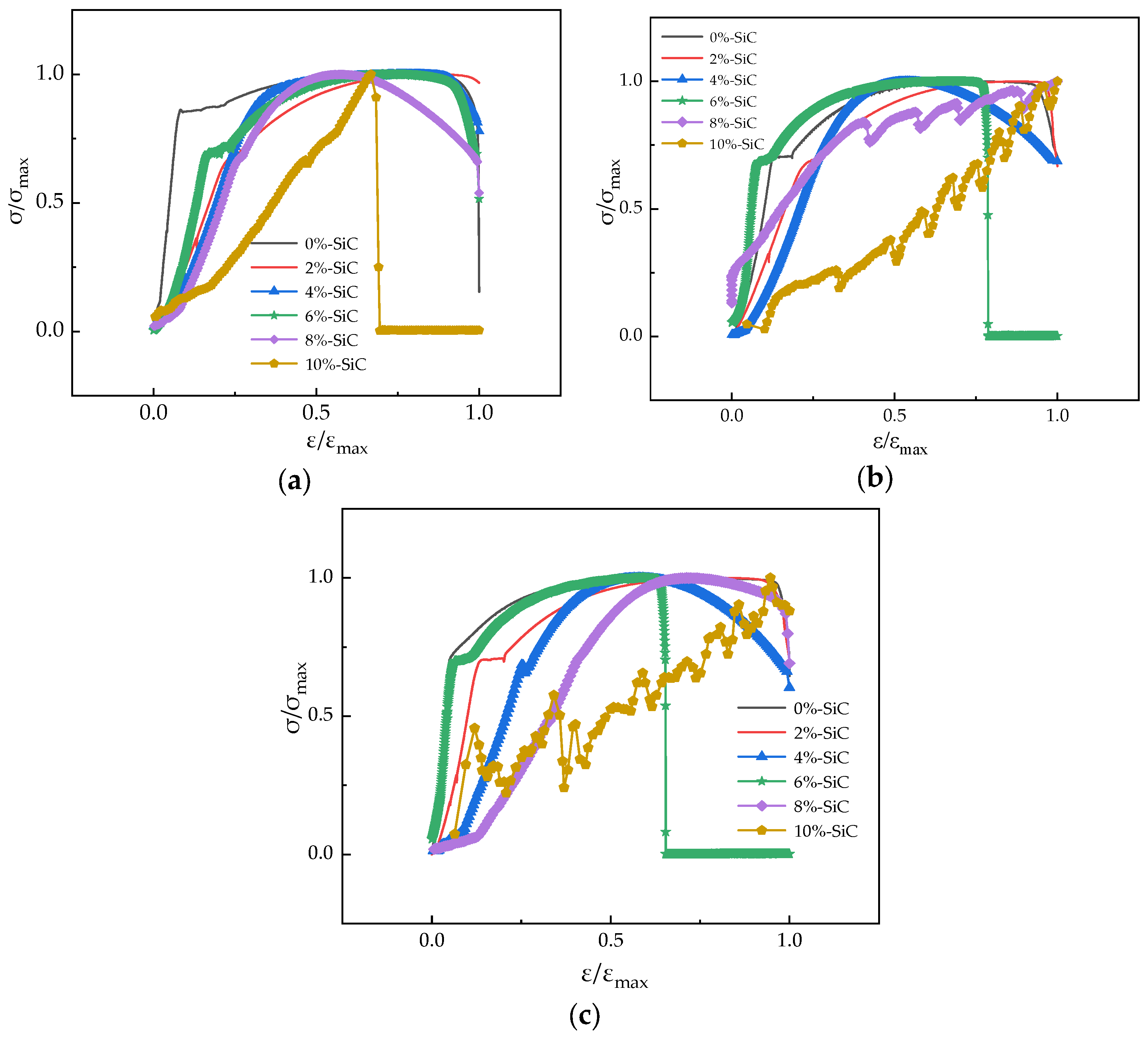
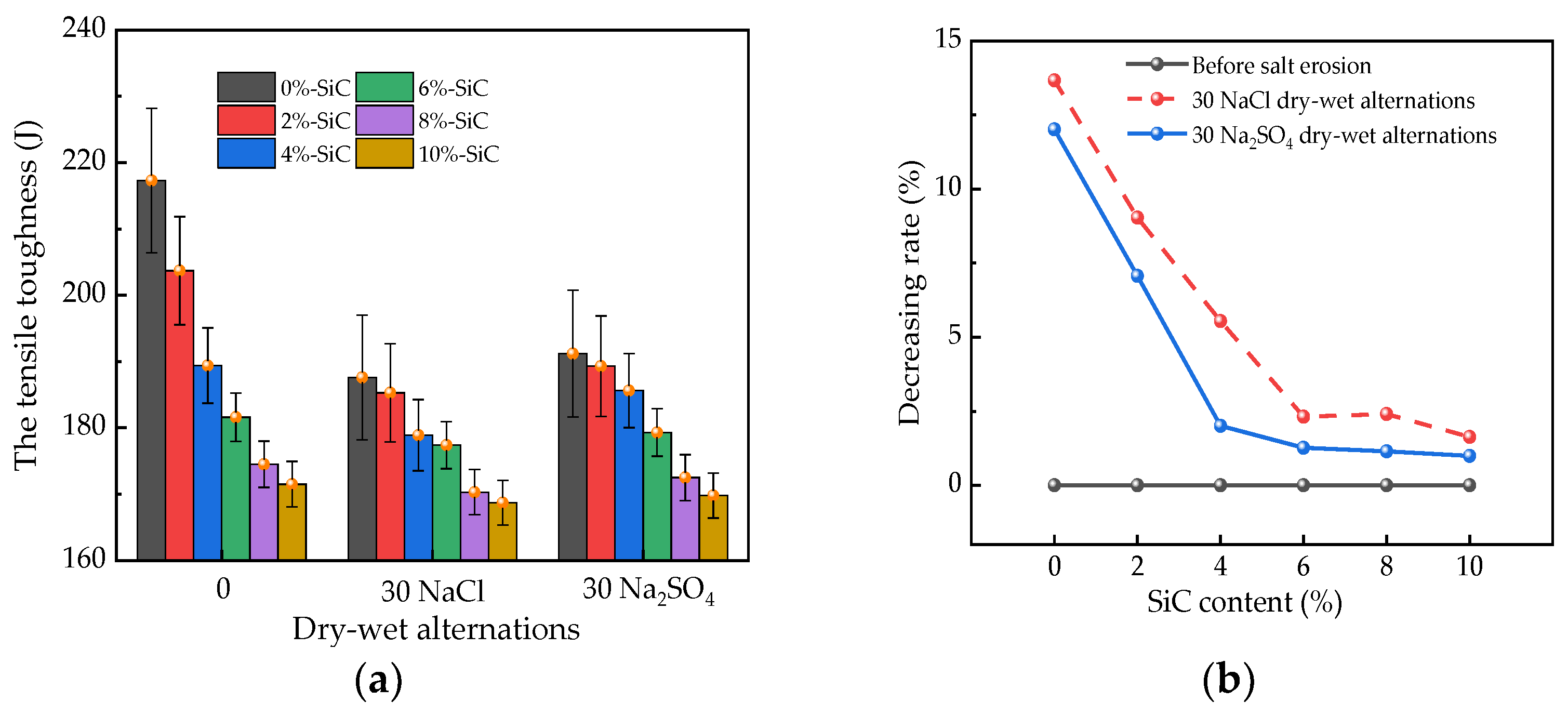
3.2. Toughness
3.3. Mass Loss Rates
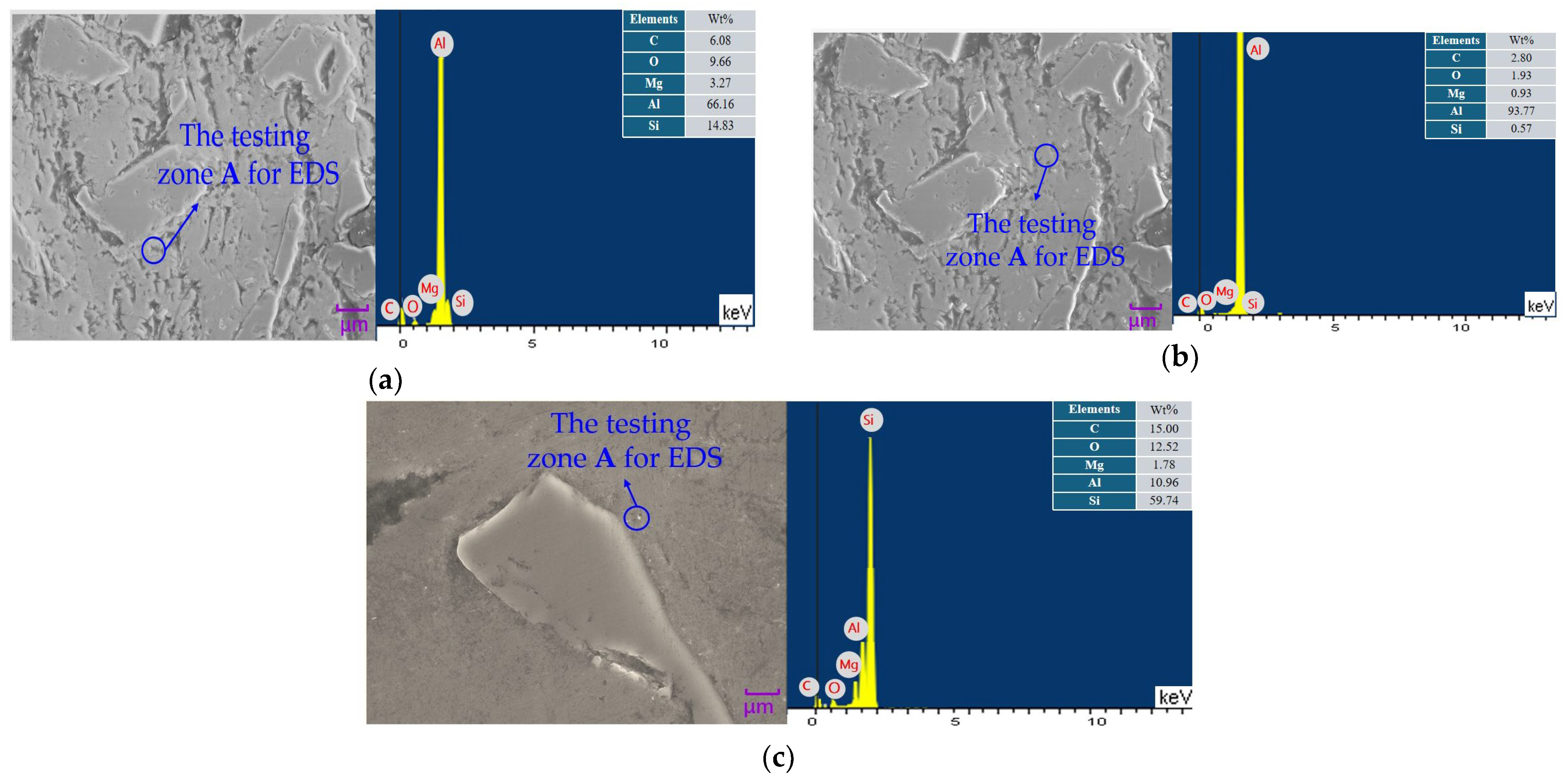
3.4. Scanning Electron Microscope Photos
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Li, J.; Sun, K.; Fan, J. Graphene-Reinforced Metal Matrix Composites: Fabrication, Properties, and Challenges. Int. J. Adv. Manuf. Technol. 2023, 125, 2925–2965. [Google Scholar] [CrossRef]
- Lei, X.-F.; Ding, W.-F.; Zhao, B.; Qian, C.; Liu, Z.-A.; Liu, Q.; Xu, D.-D.; Zhao, Y.-J.; Zhu, J.-H. Grinding of Particle-Reinforced Metal Matrix Composite Materials: Current Status and Prospects. Adv. Manuf. 2024, 1–26. [Google Scholar] [CrossRef]
- Vu, A.Q.; Vuillemin, B.; Oltra, R.; Allély, C. Cut-edge corrosion of a Zn− 55Al-coated steel: A comparison between sulphate and chloride solutions. Corros. Sci. 2011, 53, 3016–3025. [Google Scholar] [CrossRef]
- Grandhi, S.; Oh, M.S. Investigated the effect of the Ca content on the microstructures and electrochemical properties of hot-dip aluminizing coatings on commercial quality steel. Mater. Lett. 2023, 353, 135278. [Google Scholar] [CrossRef]
- Rativa-Parada, W.; Nilufar, S. Nanocarbon-Infused Metal Matrix Composites: A Review. JOM 2023, 75, 4009–4023. [Google Scholar] [CrossRef]
- Taylor, A.; Klimša, L.; Kopeček, J.; Remeš, Z.; Vronka, M.; Čtvrtlík, R.; Tomáštík, J.; Mortet, V. Synthesis and Properties of Diamond - Silicon Carbide Composite Layers. J. Alloys Compd. 2019, 800, 327–333. [Google Scholar] [CrossRef]
- Ren, Q.; Yue, Z.; Soyarslan, C.; Tan, Z.; Yuan, F.; Li, Z. A Coupled Ductile Damage Model for Metal Matrix Composites: Development and Application. Compos. Part B Eng. 2024, 272, 111229. [Google Scholar] [CrossRef]
- Maity, J. An Analytical Model to Ascertain A.C. Electrical Conductivity of Metal Matrix Composites. Philos. Mag. 2024, 104, 1052–1075. [Google Scholar] [CrossRef]
- Yan, A.; Zhang, H.; Deng, B.; Peng, F.; Yan, R.; Cui, F. Analytical Modeling of Subsurface Damage in Laser-Assisted Machining of Metal Matrix Composites Based on the Reinforcement Fracture Probability. J. Manuf. Process. 2023, 109, 300–312. [Google Scholar] [CrossRef]
- Tajdary, P.; Dorhmi, K.; Morin, L.; Derrien, K.; Hadjem-Hamouche, Z.; Braham, C.; Chevalier, J.-P. Characterization and Modeling of the Damage Mechanisms in Ductile Steel Metal-Matrix Composites: Application to Virtual Forming. Mech. Mater. 2023, 184, 104741. [Google Scholar] [CrossRef]
- Kosaba, T.; Muto, I.; Sugawara, Y. Effect of anodizing on galvanic corrosion resistance of Al coupled to Fe or type 430 stainless steel in diluted synthetic seawater. Corros. Sci. 2021, 179, 109145. [Google Scholar] [CrossRef]
- Bai, L.-J.; Kou, G.; Zhao, K.; Chen, G.-T.; Yan, F.-X. Effect of in-situ micro-arc oxidation coating on the galvanic corrosion of AZ31Mg coupled to aluminum alloys. J. Alloys Compd. 2019, 775, 1077–1085. [Google Scholar] [CrossRef]
- Bagheri, M.; Alizadeh, M.; Ahmadi, A.; Hajizamani, M. A Quantitative Hot Tearing Criterion for Aluminum Alloys. Eng. Res. Express 2024, 6, 035545. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Li, R.; Tang, Z.; Li, J.; Chen, D. Collision Study on New Aluminum Alloy W-Beam Guardrail. Appl. Sci. 2024, 14, 5266. [Google Scholar] [CrossRef]
- Han, F.; Li, C.; Wang, Y.; Pai, Z.; Meng, Y.; Cao, M.; Liu, Y.; He, P.; Ma, X.; Xue, L.; et al. Comparative Study on Corrosion Property of 2219 Aluminum Alloy Sheet and Additively Manufactured 2319 Aluminum Alloy. J. Mater. Res. Technol. 2024, 30, 3178–3185. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M. Experimental Investigations on Effect of Silicon Carbide on Microstructure and Mechanical Properties in Mg-3 Wt% Al Alloy Matrix Using Powder Metallurgy. Silicon 2022, 14, 9163–9173. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Y.; Liu, L.; Lin, T.; Liu, X. Microstructure and Mechanical Properties of SiC/SiC Joints Reinforced by in-Situ Growth SiC Nanowires. Mater. Charact. 2021, 179, 111315. [Google Scholar] [CrossRef]
- Özdemir, İ.; Cöcen, Ü.; Önel, K. The Effect of Forging on the Properties of Particulate-SiC-Reinforced Aluminium-Alloy Composites. Compos. Sci. Technol. 2000, 60, 411–419. [Google Scholar] [CrossRef]
- Sornakumar, T.; Kathiresan, M. Machining Studies of Die Cast Aluminum Alloy-Silicon Carbide Composites. Int. J. Miner. Metall. Mater. 2010, 17, 648–653. [Google Scholar] [CrossRef]
- Zuo, L.; Zhao, X.; Li, Z.; Zuo, D.; Wang, H. A Review of Friction Stir Joining of SiCp/Al Composites. Chin. J. Aeronaut. 2020, 33, 792–804. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Du, A.; Zhao, X.; Fan, Y.; Ma, R.; Cao, X. Excellent Thermal Shock Resistance of Al Alloy-Infiltrated SiC Composites. J. Mater. Process. Technol. 2019, 269, 16–25. [Google Scholar] [CrossRef]
- Huang, S.; Yu, X. A Study of Grinding Forces of SiCp/Al Composites. Int. J. Adv. Manuf. Technol. 2017, 94, 3633–3639. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Sharma, S.C.; Rao, P.R.; Girish, B.M. Mechanical Properties of As-Cast and Heat-Treated ZA-27/Silicon Carbide Particulate Composites. Mater. Des. 1995, 16, 277–281. [Google Scholar] [CrossRef]
- Winiczenko, R.; Skibicki, A.; Skoczylas, P. The Experimental and FEM Studies of Friction Welding Process of Tungsten Heavy Alloy with Aluminium Alloy. Appl. Sci. 2024, 14, 2038. [Google Scholar] [CrossRef]
- Fan, J.; Xu, S. Aluminum Oxide Particles/Silicon Carbide Whiskers’ Synergistic Effect on Thermal Conductivity of High-Density Polyethylene Composites. Iran. Polym. J. 2018, 27, 339–347. [Google Scholar] [CrossRef]
- Shen, D.; Zhan, Z.; Liu, Z.; Cao, Y.; Zhou, L.; Liu, Y.; Dai, W.; Nishimura, K.; Li, C.; Lin, C.-T.; et al. Enhanced Thermal Conductivity of Epoxy Composites Filled with Silicon Carbide Nanowires. Sci. Rep. 2017, 7, 2606. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Z.; Casey, N.; Korah, M.M.; Uppal, A.; Green, M.D.; Rykaczewski, K.; Wang, R.Y. High Thermal Conductivity in Multiphase Liquid Metal and Silicon Carbide Soft Composites. Adv. Mater. Interfaces 2021, 8, 2100069. [Google Scholar] [CrossRef]
- Amirkhanlou, S.; Ji, S.A. Review on High Stiffness Aluminum-Based Composites and Bimetallics. Crit. Rev. Solid State Mater. Sci. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- Sudherson, D.P.S.; Anandkumar, P.P.; Jinu, G.R.; Balasubramanian, K.A.; Vettivel, S.C. Experimental Investigation on Corrosion Behavior of Friction Surfaced Mild Steel with Aluminum Alloy 5083-Cadmium Composite. Mater. Res. Express 2019, 6, 086587. [Google Scholar] [CrossRef]
- Tong, Z.; Jiao, J.; Zhou, W.; Yang, Y.; Chen, L.; Liu, H.; Sun, Y.; Ren, X. Improvement in Cavitation Erosion Resistance of AA5083 Aluminium Alloy by Laser Shock Processing. Surf. Coat. Technol. 2019, 377, 124799. [Google Scholar] [CrossRef]
- Chandrasekar, P.; Nagaraju, D. Improvement of Bonding Strength at the Interfaces in Scrap Al Alloy Composites Using Electroless Ni-P Coated SiC. Silicon 2021, 14, 2941–2952. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, Y.; Wu, Y.; Zhang, S.; Mingers, A.M.; Ponge, D.; Gault, B.; Rohwerder, M.; Raabe, D. How Solute Atoms Control Aqueous Corrosion of Al-Alloys. Nat. Commun. 2024, 15, 561. [Google Scholar] [CrossRef] [PubMed]
- Zou, S. Galvanic corrosion behavior of aluminum alloy (2219 and ZL205A) coupled to carbon fiber-reinforced epoxy composites. Int. J. Electrochem. Sci. 2016, 11, 9625–9633. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, S.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- He, Q.-Q.; Zhou, M.-J.; Hu, J.-M. Electrodeposited Zn-Al layered double hydroxide films for corrosion protection of aluminum alloys. Electrochim. Acta 2020, 355, 136796. [Google Scholar] [CrossRef]
- Yi, G.; Sun, B.; Poplawsky, J.D.; Zhu, Y.; Free, M.L. Investigation of pre-existing particles in Al 5083 alloys. J. Alloys Compd. 2018, 740, 461–469. [Google Scholar] [CrossRef]
- Xiao, L.; Yun, H.; Shu, Z.; Xiao, W.; Ya, H.; Ming, L. Corrosion Behavior of 2219 Aluminum Alloy Base Metal and Welding Joints. Corros. Prot. 2016, 37, 618–622. [Google Scholar]
- Shariat, A.H.; Moghimi, H.; Giyahchi, M.; Ebrahim-Habibi, M.-B.; Tirandaz, H. Comprehensive Evaluation of Fungal-Induced Corrosion in Aluminum Alloys by Amorphotheca Resinae. Sci. Rep. 2024, 14, 19096. [Google Scholar] [CrossRef]
- GB/T 228-2002; Methods for Testing Tensile Strength of Metallic Materials. Standardization Administration of China: Beijing, China, 2002.
- GB/T 7314-2017; Methods for Ultrasonic Testing of Steel Plates. Standardization Administration of China: Beijing, China, 2017.
- GB/T 229-2020; Methods for Testing Impact Resistance of Metals. Standardization Administration of China: Beijing, China, 2020.
- ASTM E399; Standard Test Method for Linear-Elastic Plane-Strain Fracture Toughness KIc of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2019.
- Ruiz-Garcia, A.; Esquivel-Peña, V.; Godínez, F.A.; Montoya, R. Corrosion Modeling of Aluminum Alloys: A Brief Review. ChemElectroChem 2024, 11, e202300712. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Zhao, H.; Sun, W.; Zhang, Q.; Gault, B.; Hutchinson, C. Effect of Cluster Chemistry on the Strengthening of Al Alloys. Acta Mater. 2024, 269, 119809. [Google Scholar] [CrossRef]
- Wu, X.; Sun, M.; Hong, L.; Wen, S.; Wei, W.; Gao, K.; Rong, L.; Xiong, X.; Huang, H.; Nie, Z. Er-Containing Microalloyed Aluminum Alloys: A Review. J. Mater. Sci. 2024, 59, 9685–9696. [Google Scholar] [CrossRef]
- Xue, H.; Yang, C.; Zhang, P.; Wu, S.H.; Liu, G.; Sun, J. Heat-Resistant Al Alloys: Microstructural Design and Microalloying Effect. J. Mater. Sci. 2024, 59, 9749–9767. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Zheng, Y.; Zhao, Y.; Kang, H.; Gao, L. Development of TiB2 reinforced Aluminum foundry alloy based in situ composites: Part I: An improved halide salt route to fabricate Al-5 wt.% TiB2 master composite. Mater. Sci. Eng. A 2014, 605, 301–309. [Google Scholar] [CrossRef]
- Peng, L.; Yang, D.; Zhang, J.; Xin, Y.; Liu, Y.; Ai, M. Research on the Composite Performance of Aluminum Alloy Plate and Concrete-Based Material. Sustainability 2022, 14, 1999. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Liu, Z.; Liu, Z.; Song, T.; Zuo, X. Effect of B/Ti mass ratio on grain refining of low-titanium aluminum produced by electrolysis. Mater. Sci. Eng. A. 2006, 416, 312–316. [Google Scholar] [CrossRef]
- Gurevich, L.M.; Pronichev, D.V.; Kulevich, V.P.; Slautin, O.V.; Naumenko, V.A.; Kharlamov, V.O. Investigation of Aluminized Intermetallic Coatings on Fe–Cr–Al System Alloy Corrosion Resistance. Metallurgist 2023, 67, 70–78. [Google Scholar] [CrossRef]
- Sathees Kumar, S.; Kanagaraj, G. Effect of Graphite and Silicon Carbide Fillers on Mechanical Properties of PA6 Polymer Composites. J. Polym. Eng. 2017, 37, 547–557. [Google Scholar] [CrossRef]
- Feng, D.; Ren, Q.; Ru, H.; Wang, W.; Jiang, Y.; Ren, S.; Zhang, C. Effect of Oxygen Content on the Sintering Behaviour and Mechanical Properties of SiC Ceramics. Ceram. Int. 2019, 45, 23984–23992. [Google Scholar] [CrossRef]
- Liu, C.; Chen, B.; Li, X.; Zhao, Y. Effects of Proton Irradiation on the Microstructure and Mechanical Properties of Amosic-3 Silicon Carbide Minicomposites. J. Mater. Sci. Technol. 2019, 35, 2935–2941. [Google Scholar] [CrossRef]
- Faghihnasiri, M.; Rezvani, M.; Shabani, M.; Firouzian, A.H. The Temperature Effect on Mechanical Properties of Silicon Carbide Sheet Based on Density Functional Treatment. Solid State Commun. 2016, 227, 40–44. [Google Scholar] [CrossRef]
- Tao, H.; Cai, Q.; Chen, M.; Zhang, W.; Cao, G.; Zhang, H. Effect of heat treatment on the microstructure and corrosion performance of 2219 Al alloy cold sprayed coatings. Corros. Sci. 2024, 241, 112526. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Z.; Yang, L.; Lu, J.; Huang, J.; Tan, J.; Kuang, W. Superior corrosion resistance of a slurry FeAl coating on 316LN stainless steel in 550 °C liquid lead-bismuth eutectic. Corros. Sci. 2024, 227, 111757. [Google Scholar] [CrossRef]
- Bara, M.; Niedźwiedź, M.; Skoneczny, W.; Barylski, A. Nanostructure and morphology of the surface as well as micromechanical and sclerometric properties of Al2O3 layers subjected to thermo-chemical treatment. Materials 2022, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Bi, Y.; Li, H.; Chen, Y.; Jia, Q. Synthesis of SiC Whiskers via Catalytic Reaction Method in Self-Bonded SiC Composites. Ceram. Int. 2020, 46, 12975–12985. [Google Scholar] [CrossRef]
- Grandhi, S.; Oh, M.S. Influence of calcium on the morphology and corrosion performance of hot-dip Al–Si coatings. Mater. Lett. 2023, 353, 135278. [Google Scholar] [CrossRef]
- Yang, L.W.; Liu, H.T.; Cheng, H.F. Processing-Temperature Dependent Micro- and Macro-Mechanical Properties of SiC Fiber Reinforced SiC Matrix Composites. Compos. Part B Eng. 2017, 129, 152–161. [Google Scholar] [CrossRef]
- Li, S.; Fang, Y.; Cheng, L.; Li, Z.; Wang, J.; Tu, J. Porous Silicon Carbide Ceramics with Directional Pore Structures by CVI Combined with Sacrificial Template Method. Ceram. Int. 2022, 49, 8331–8338. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Li, L.; Shen, Q.; Li, H.; Li, K. Microstructure and Mechanical Properties of SiC Fibres after Exposure at Elevated Temperature. Adv. Appl. Ceram. 2016, 115, 449–456. [Google Scholar] [CrossRef]
- Nouri, M.; Li, D.Y. The Yttrium-Incorporated Aluminizing of Mg-3% Al Alloy for Improved Tribological and Corrosion Properties. J. Mater. Eng. Perform. 2022, 31, 3218–3227. [Google Scholar] [CrossRef]
- Huang, X.; Wang, D.; Dong, Y. Corrosion resistance phosphate coating formed by steam assisted curing on cast AlSi alloy. Surf. Coat. Technol. 2020, 382, 125242. [Google Scholar] [CrossRef]
- Pan, S.; Yuan, J.; Linsley, C.; Liu, J.; Li, X. Corrosion behavior of nano-treated AA7075 alloy with TiC and TiB2 nanoparticles. Corros. Sci. 2022, 206, 110479. [Google Scholar] [CrossRef]
- Shukla, A.K.; Mondal, D.P.; Madapana, D.; Majumdar, J.D. Surface degradation behavior of aluminium cenosphere composite foam developed by powder metallurgy route. Mater. Chem. Phys. 2023, 295, 127107. [Google Scholar] [CrossRef]





| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Mass percentage | 0.758 | 0.569 | 0.170 | 0.018 | 0.805 | 0.047 | 0.031 | 0.015 | 97.587 |
| Group | Silicon Carbide | 6061 Aluminum Alloy |
|---|---|---|
| C1 | 0% | 100% |
| C2 | 2% | 98% |
| C3 | 4% | 96% |
| C4 | 6% | 94% |
| C5 | 8% | 92% |
| C6 | 10% | 90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Liu, C.; Chen, W.; Wang, Z.; Wang, C.; Cao, Z.; Wang, H.; Shi, F. The Influence of Nano-Silicon Carbide on the Properties of Aluminum Alloy Under Salt Dry–Wet Alternations. Coatings 2024, 14, 1472. https://doi.org/10.3390/coatings14111472
Song S, Liu C, Chen W, Wang Z, Wang C, Cao Z, Wang H, Shi F. The Influence of Nano-Silicon Carbide on the Properties of Aluminum Alloy Under Salt Dry–Wet Alternations. Coatings. 2024; 14(11):1472. https://doi.org/10.3390/coatings14111472
Chicago/Turabian StyleSong, Shengpeng, Chuanyuan Liu, Wentao Chen, Zhen Wang, Chuanyin Wang, Zihao Cao, Hui Wang, and Feiting Shi. 2024. "The Influence of Nano-Silicon Carbide on the Properties of Aluminum Alloy Under Salt Dry–Wet Alternations" Coatings 14, no. 11: 1472. https://doi.org/10.3390/coatings14111472
APA StyleSong, S., Liu, C., Chen, W., Wang, Z., Wang, C., Cao, Z., Wang, H., & Shi, F. (2024). The Influence of Nano-Silicon Carbide on the Properties of Aluminum Alloy Under Salt Dry–Wet Alternations. Coatings, 14(11), 1472. https://doi.org/10.3390/coatings14111472







