Microstructure and Oxidation Behaviors of (TiVCr)2AlC MAX-Phase Coatings Prepared by Magnetron Sputtering
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
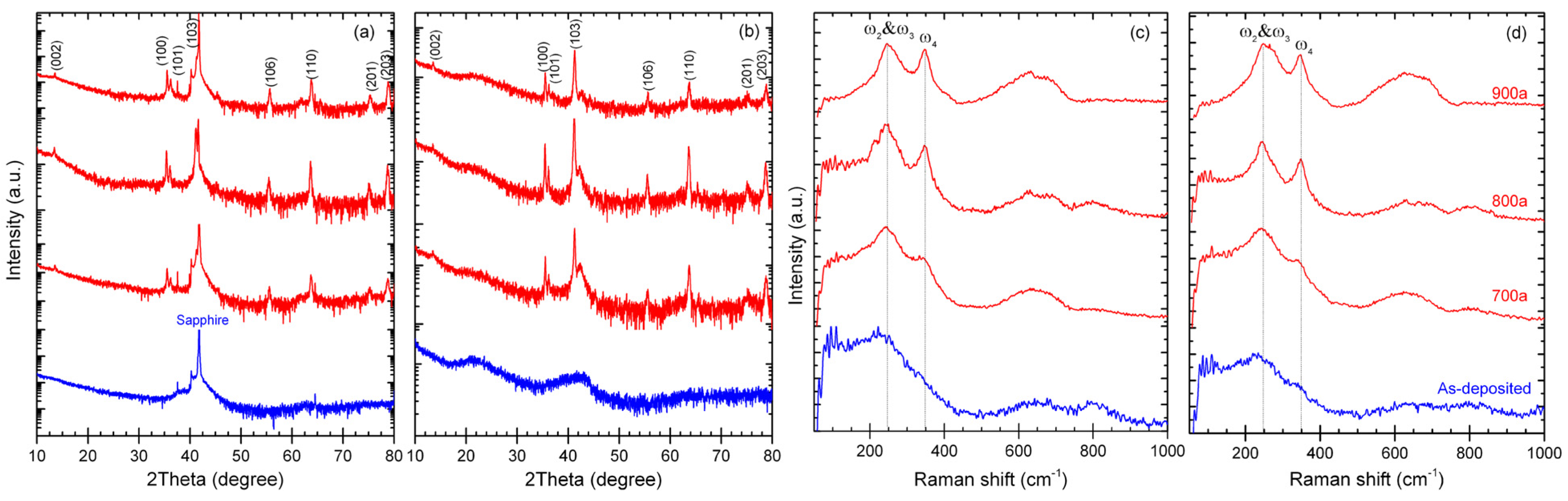
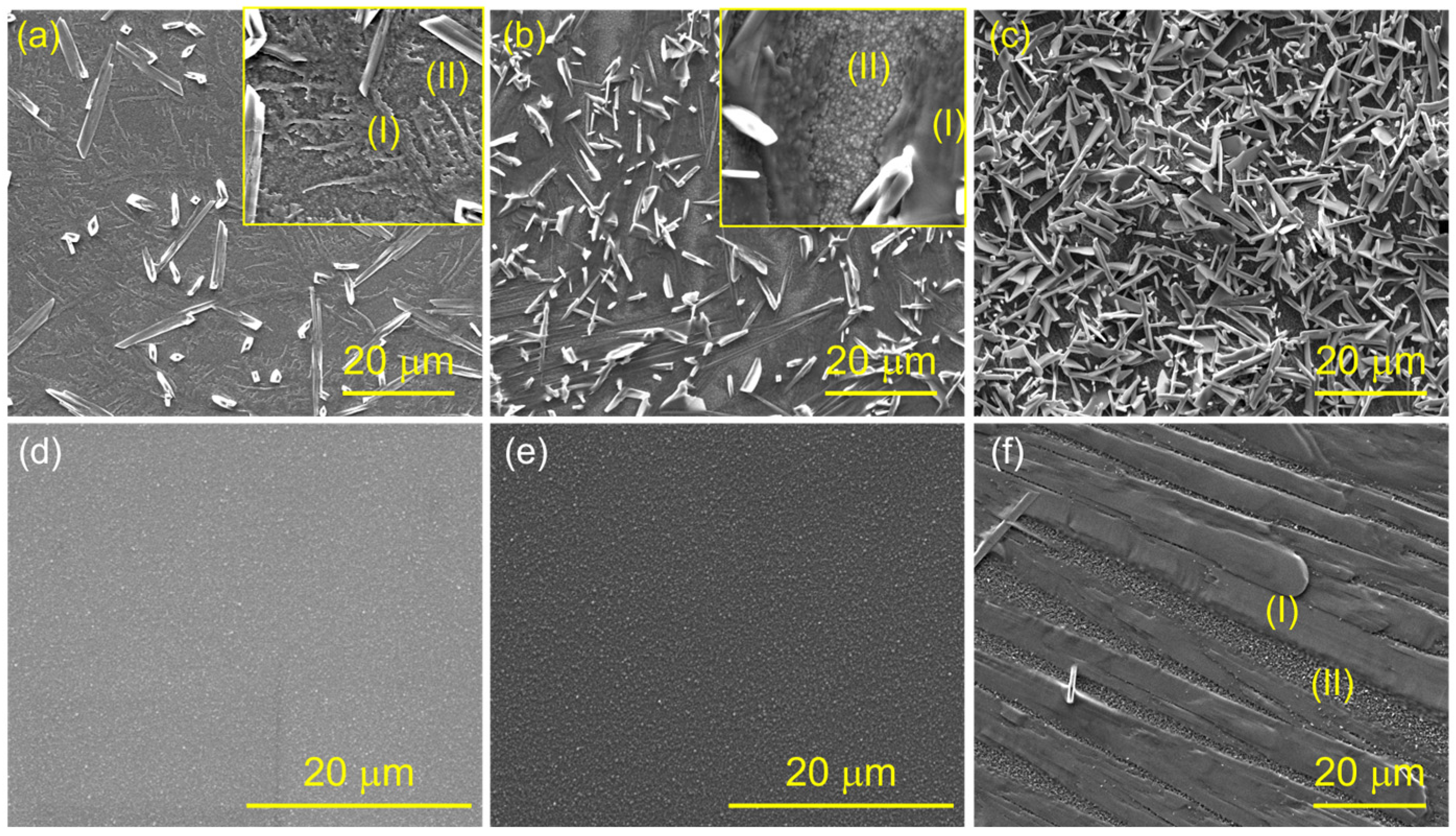
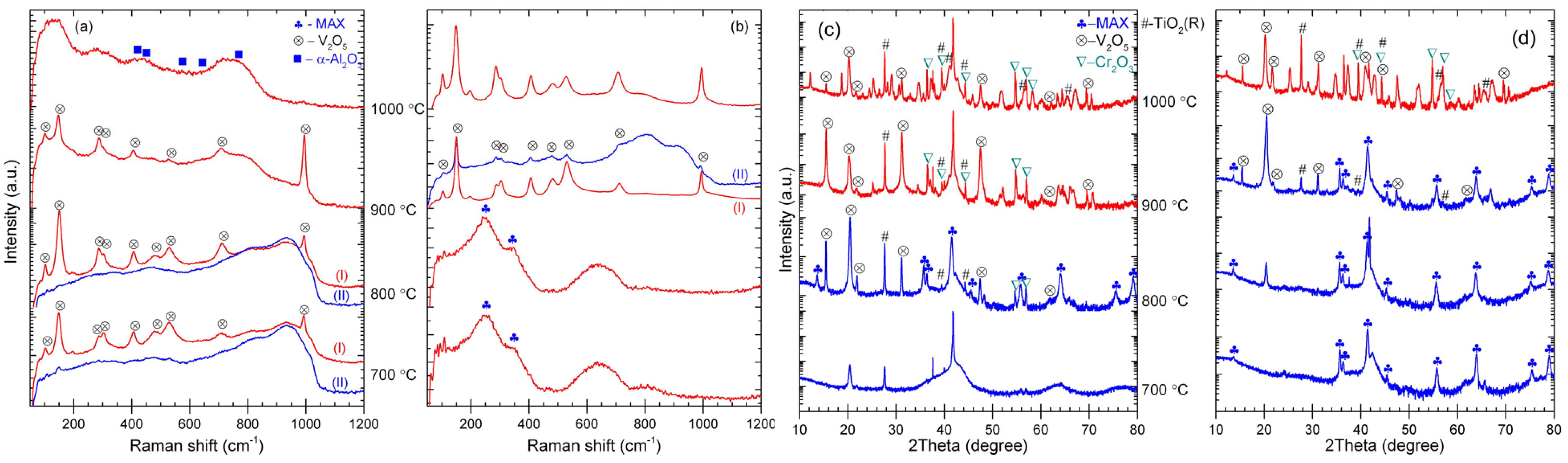
3.1. Microstructure
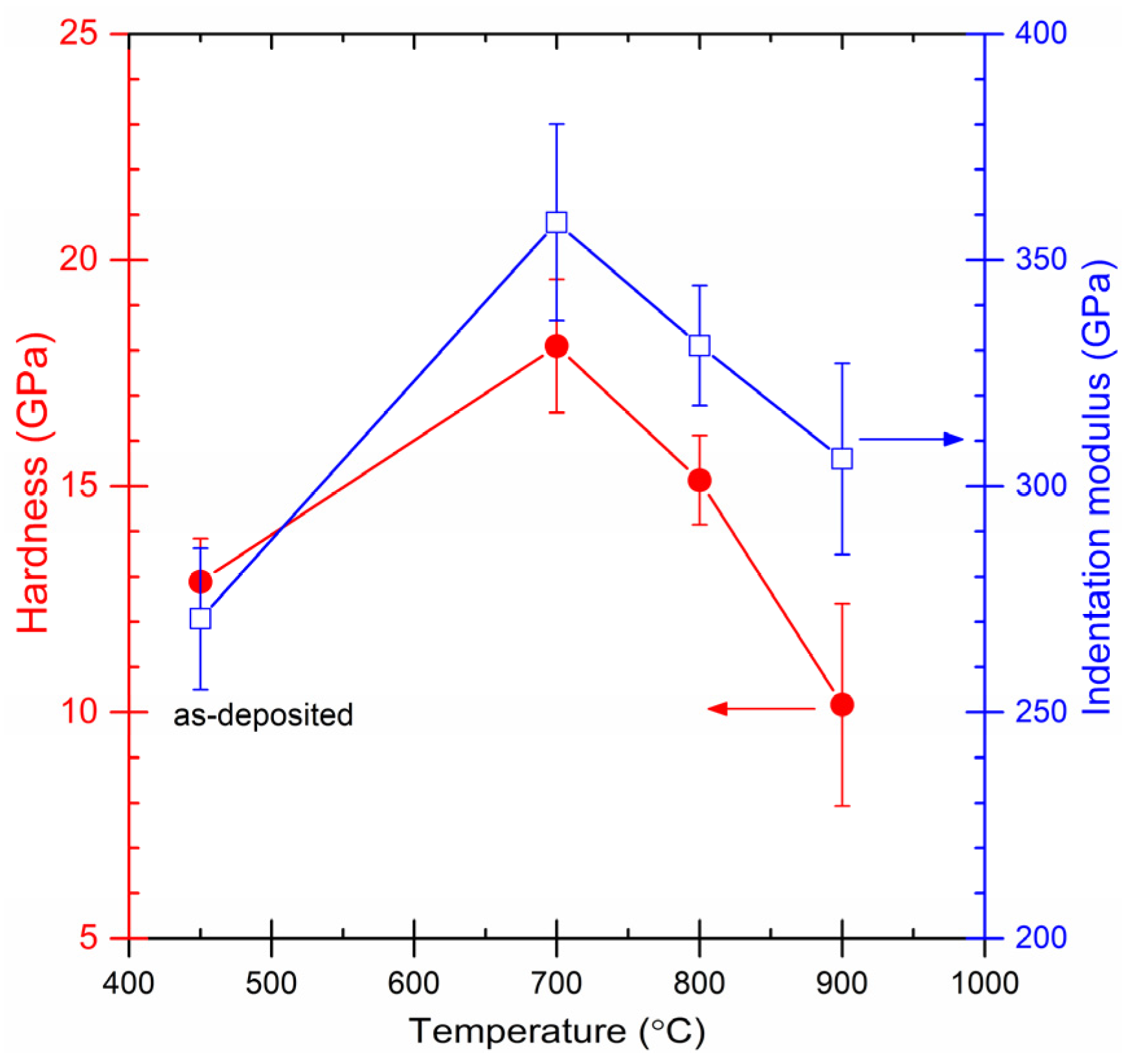
3.2. Mechanical Properties
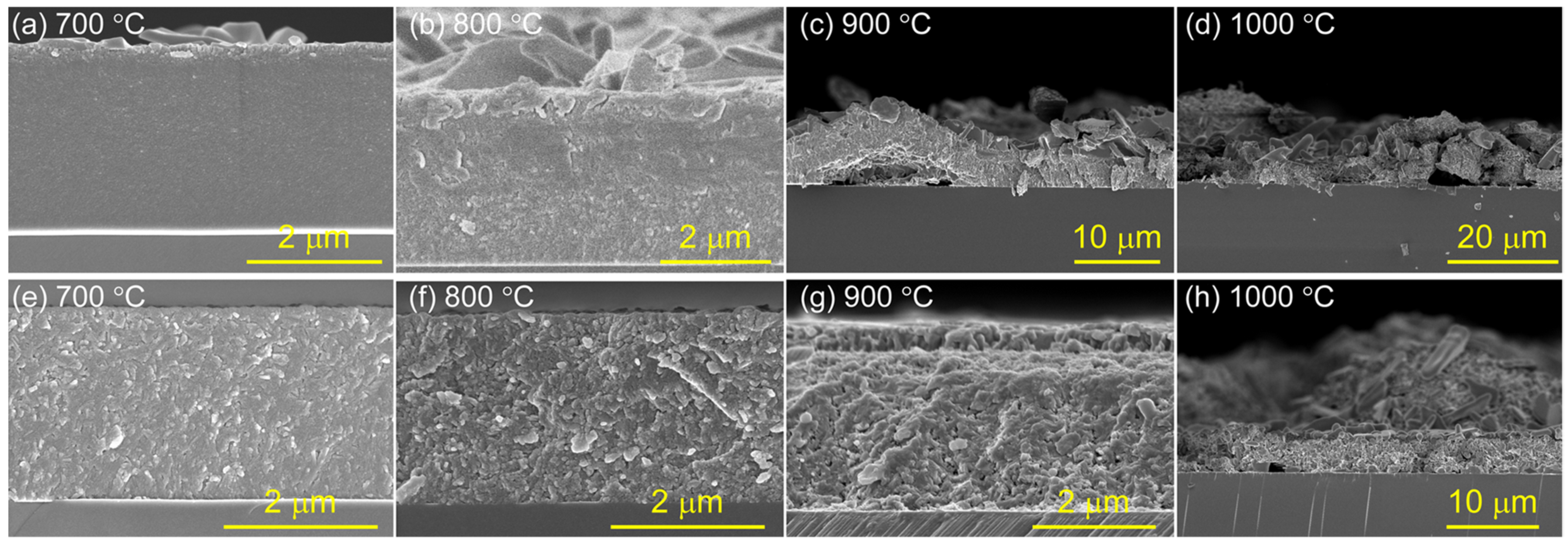
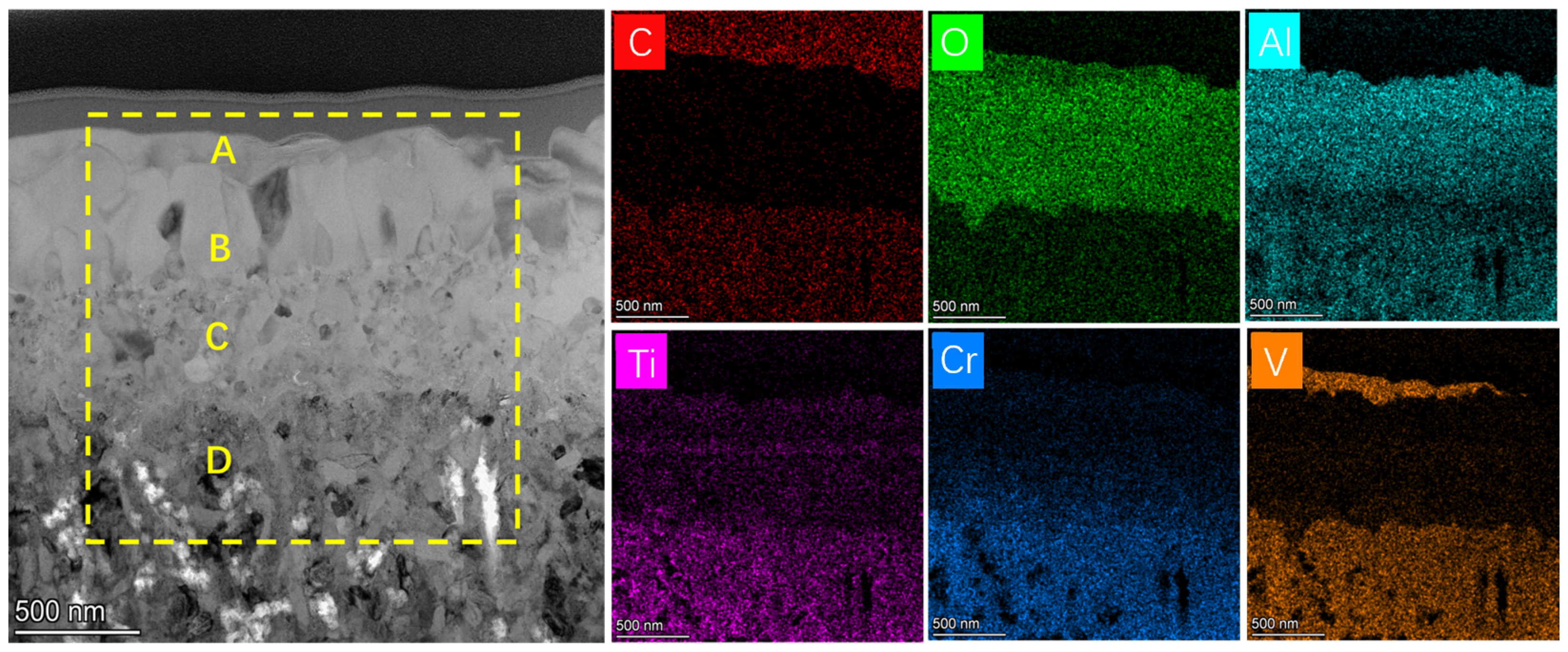
3.3. Oxidation Behaviors
4. Conclusions
- The M-site medium-entropy (TiVCr)2AlC MAX-phase can be achieved through vacuum annealing the amorphous counterpart at 700 °C, and the crystallinity increased with the temperature. However, higher temperatures of ≥800 °C led to pits emerging on the coating surface.
- After 700 °C annealing, the crystalline (TiVCr)2AlC medium-entropy-alloy MAX-phase coating exhibited its maximum hardness, reaching 18 GPa, significantly higher than that of the as-deposited coating.
- Compared to the as-prepared amorphous TiVCrAlC coating, the high-temperature oxidation resistance of the MAX coating was significantly improved; mixed oxides formed on the amorphous sample, while only a dense continuous AlOx-rich oxide formed on the MAX coating. This characteristic ensures that the crystalline (TiVCr)2AlC coating is expected to play a role in practical applications such as aerospace and nuclear power generation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Meng, F.; Ge, F.; Huang, F. Improved oxidation resistance through an in-situ formed diffusion barrier: Oxidation behavior of amorphous multi-component FeCrAlMoSiY-coated Zr in high-temperature steam. Corros. Sci. 2021, 189, 109566. [Google Scholar] [CrossRef]
- Du, Q.; Wei, D.; Wang, Y.; Li, B.; Zhou, Y. Microstructure and surface performance of hydroxyapatite-modified multilayer amorphous coating on Ti-rich TiNbZrSn medium entropy alloy: A comparative study. Surf. Interfaces 2023, 41, 103288. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Zhang, Y.; Chen, S.; Luo, Z.; Wu, J.; Zhu, L.; Lei, J. Improving wear and corrosion resistance of LDEDed CrFeNi MEA through addition of B and Si. J. Alloys Compd. 2023, 968, 172223. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, S.; Chen, T.; Fu, Q.; Wei, R.; Chen, C.; Wang, T.; Cai, Y.; Li, F. Microstructure and corrosion behavior of Co-free FeCrNiSi0.4 medium entropy alloy coating fabricated by laser cladding. Intermetallics 2023, 162, 108024. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, D.; Wang, Y.; Wang, K.; Huang, J.; Jin, X.; Kong, D.; Wang, M.; Yamaguchi, T.; Wang, H. Optimized wear behaviors and related wear mechanisms of medium entropy alloy-based composite coatings. J. Mater. Res. Technol. 2024, 29, 12–27. [Google Scholar] [CrossRef]
- Meng, A.; Liang, F.; Mao, Q.; Fan, Y.; Lin, Y.; Chen, X.; Zhao, Y. Tribo-induced microstructural changes and associated wear mechanisms of CoFeNi2 medium entropy alloy at elevated temperatures. Tribol. Int. 2023, 189, 108892. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Tan, N.; Mou, H.; Tong, Y.; Xing, Z.; Cai, Z.; Wang, H. Influence of cBN on the microstructure and tribology properties of (CoCrNi)94Al3Ti3 medium-entropy alloy coating prepared by high-speed laser cladding: The evolution and strengthening mechanism of cBN. Ceram. Int. 2024, 50, 22041–22049. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, X.; Miao, J.; Fu, F.; Shen, X. Friction and Wear Performance of CoCrFeMnNiW Medium-Entropy Alloy Coatings by Plasma-Arc Surfacing Welding on Q235 Steel. Coatings 2021, 11, 715. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, K.; Wang, Z.; Zhou, H.; Shi, C.; Qin, S.; Liu, J.; Lv, T.; Xu, J. Thermal Deformation Behavior and Processing Map of a Novel CrFeNiSi0.15 Medium Entropy Alloy. Acta Metall. Sin. (Engl. Lett.) 2023, 36, 1870–1882. [Google Scholar] [CrossRef]
- Agustianingrum, M.P.; Lee, U.; Park, N. High-temperature oxidation behaviour of CoCrNi medium-entropy alloy. Corros. Sci. 2020, 173, 108755. [Google Scholar] [CrossRef]
- Kai, W.; Jiang, Z.Y.; Chen, G.T.; Lee, I.H.; Lin, H.J.; Hsieh, H.H.; Lin, W.T.; Kai, J.J. High-temperature air-oxidation of NiCoCrAlx medium-entropy alloys. Corros. Sci. 2021, 192, 109858. [Google Scholar] [CrossRef]
- Lin, S.; Lai, W.; Vogel, F.; Tong, X.; You, D.; Li, W.; Wang, X. Mechanical and corrosion properties of biomedical (TiZr)90-xNbxTa5Mo5 medium entropy alloys. Int. J. Refract. Met. Hard Mater. 2023, 116, 106361. [Google Scholar] [CrossRef]
- Azina, C.; Poll, M.; Holzapfel, D.M.; Tailleur, E.; Zuber, A.; Dubois, S.; Eklund, P.; Gonzalez-Julian, J. Microstructural and compositional design of Cr2AlC MAX phases and their impact on oxidation resistance. J. Eur. Ceram. Soc. 2024, 44, 4895–4904. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Barsoum, M.W.; Rosen, J. MAX phases–Past, present, and future. Mater. Today 2024, 72, 1–24. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Zhao, S.; Han, M.; Sun, Z.; Zhang, P.; Huang, Z.; Zhang, Y.; Wang, J.; Sun, Z.; et al. Progress in Structural Tailoring and Properties of Ternary Layered Ceramics. J. Inorg. Mater. 2023, 38, 845–884. [Google Scholar] [CrossRef]
- Berger, O.; Boucher, R.; Ruhnow, M. Part I. Mechanism of oxidation of Cr2AlC films in temperature range 700–1200 °C. Surf. Eng. 2015, 31, 373–385. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Zhang, X.; Chen, X. Research and Development on Cold-Sprayed MAX Phase Coatings. Coatings 2023, 13, 869. [Google Scholar] [CrossRef]
- Azina, C.; Mráz, S.; Greczynski, G.; Hans, M.; Primetzhofer, D.; Schneider, J.M.; Eklund, P. Oxidation behaviour of V2AlC MAX phase coatings. J. Eur. Ceram. Soc. 2020, 40, 4436–4444. [Google Scholar] [CrossRef]
- Wang, Q.; Garkas, W.; Renteria, A.F.; Leyens, C.; Sun, C.; Kim, K. Oxidation behaviour of a Ti2AlN MAX-phase coating. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 082025. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Li, Z.; Wang, Z.; Ke, P.; Wang, A. Electrochemical Corrosion Inhibition of Cr2AlC MAX Phase Coatings via Mo Solid Solution: Comprehensive Experimental and Simulation Study. J. Phys. Chem. C 2024, 128, 3916–3923. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, Y.; Wang, A.; Ke, P. M-site solid solution of vanadium enables the promising mechanical and high-temperature tribological properties of Cr2AlC coating. Mater. Des. 2022, 222, 111060. [Google Scholar] [CrossRef]
- Huang, F.; Ge, F.; Zhu, P.; Wang, H.; Meng, F.; Li, S. Superhard V-Si-N coatings (>50 GPa) with the cell-like nanostructure prepared by magnetron sputtering. Surf. Coat. Technol. 2013, 232, 600–605. [Google Scholar] [CrossRef]
- Meng, F.; Wang, B.; Ge, F.; Huang, F. Microstructure and mechanical properties of Ni-alloyed SiC coatings. Surf. Coat. Technol. 2012, 213, 77–83. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrück, M.; Klimenkov, M.; Jäntsch, U.; Seifert, H.J.; Ulrich, S.; Stüber, M. Textured growth of polycrystalline MAX phase carbide coatings via thermal annealing of M/C/Al multilayers. J. Vac. Sci. Technol. A 2020, 38, 013401. [Google Scholar] [CrossRef]
- Walter, C.; Sigumonrong, D.P.; El-Raghy, T.; Schneider, J.M. Towards large area deposition of Cr2AlC on steel. Thin Solid Film. 2006, 515, 389–393. [Google Scholar] [CrossRef]
- Leaffer, O.D.; Gupta, S.; Barsoum, M.W.; Spanier, J.E. On Raman scattering from selected M2AC compounds. J. Mater. Res. 2011, 22, 2651–2654. [Google Scholar] [CrossRef]
- Spanier, J.E.; Gupta, S.; Amer, M.; Barsoum, M.W. Vibrational behavior of theMn+1AXnphases from first-order Raman scattering(M=Ti,V,Cr,A=Si,X=C,N). Phys. Rev. B 2005, 71, 012103. [Google Scholar] [CrossRef]
- Tang, C.; Klimenkov, M.; Jaentsch, U.; Leiste, H.; Rinke, M.; Ulrich, S.; Steinbrück, M.; Seifert, H.J.; Stueber, M. Synthesis and characterization of Ti2AlC coatings by magnetron sputtering from three elemental targets and ex-situ annealing. Surf. Coat. Technol. 2017, 309, 445–455. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Shuai, J.; Xu, B.; Wang, A.; Ke, P. A high oxidation resistance Ti2AlC coating on Zirlo substrates for loss-of-coolant accident conditions. Ceram. Int. 2019, 45, 13912–13922. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, X.; Wang, Z.; Wang, L.; Wu, X.; Ke, P.; Wang, A. Fabrication and mechanical properties of high purity of Cr2AlC coatings by adjustable Al contents. J. Alloys Compd. 2018, 753, 11–17. [Google Scholar] [CrossRef]
- Zhao, G.; Ge, F.; Cheng, X.; Huang, F. Effects of Bombarding Ions Energy on Structure and Mechanical Properties of V2AlC MAX-phase Coatings. China Surface Eng. 2019, 32, 80–87. [Google Scholar] [CrossRef]
- He, G.; Zhang, Y.; Yao, P.; Li, X.; Ma, K.; Zuo, J.; Li, M.; Liu, C.; Xu, J. A novel medium-entropy (TiVNb)2AlC MAX phase: Fabrication, microstructure, and properties. J. Mater. Sci. Technol. 2023, 137, 91–99. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, P.; Meng, F.; Xue, Q.; Huang, F. Achieving very low wear rates in binary transition-metal nitrides: The case of magnetron sputtered dense and highly oriented VN coatings. Surf. Coat. Technol. 2014, 248, 81–90. [Google Scholar] [CrossRef]
- Su, Q.; Liu, X.Q.; Ma, H.L.; Guo, Y.P.; Wang, Y.Y. Raman spectroscopic characterization of the microstructure of V2O5 films. J. Solid State Electrochem. 2008, 12, 919–923. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, T.F.; Xia, Q.; Lim, S.-H.; Wan, Z.; Lee, T.-W.; Kim, K.H.; Yun, J.M. Oxidation and Corrosion Behavior of Nanolaminated MAX-Phase Ti2AlC Film Synthesized by High-Power Impulse Magnetron Sputtering and Annealing. J. Nanomater. 2015, 2015, 213128. [Google Scholar] [CrossRef]
- Ougier, M.; Michau, A.; Lomello, F.; Schuster, F.; Maskrot, H.; Schlegel, M.L. High-temperature oxidation behavior of HiPIMS as-deposited Cr–Al–C and annealed Cr2AlC coatings on Zr-based alloy. J. Nucl. Mater. 2020, 528, 151855. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; Dahm, K.L.; Wang, F. Oxidation behaviour of sputter-deposited Ni–Cr–Al micro-crystalline coatings. Acta Mater. 1998, 46, 1691–1700. [Google Scholar] [CrossRef]


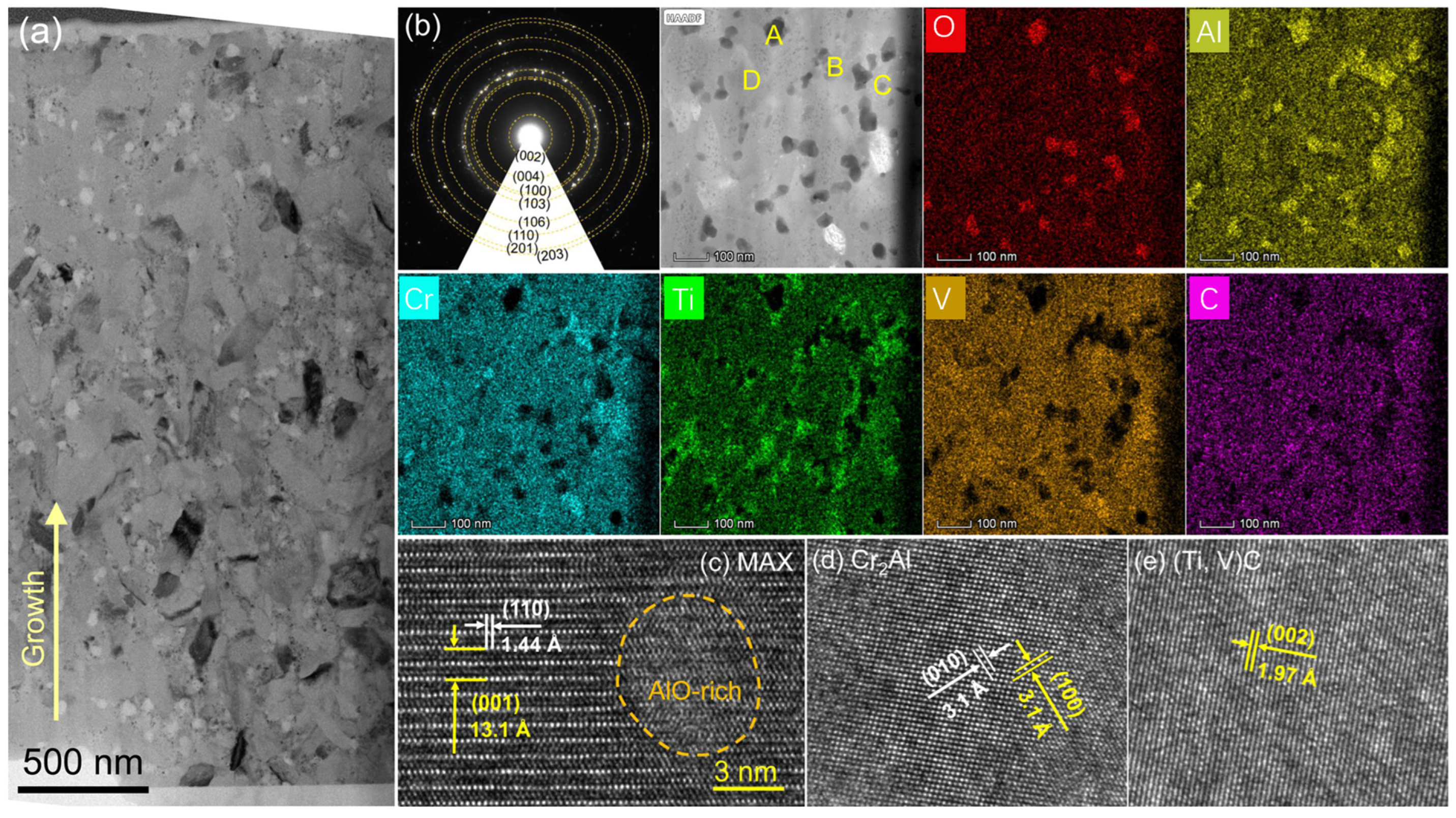

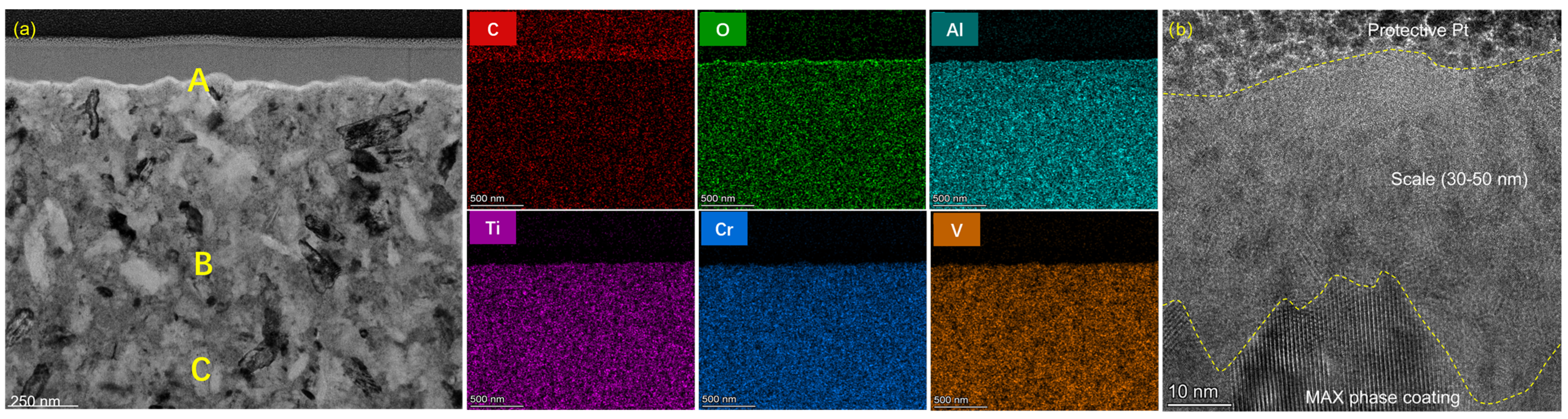
| Points | O | C | Al | Ti | V | Cr |
|---|---|---|---|---|---|---|
| A | 54.5 | 3.0 | 41.4 | 0.8 | 0.1 | 0.2 |
| B | 0.1 | 7.3 | 30.4 | 6.9 | 6.0 | 49.3 |
| C | 5.7 | 40.8 | 10.0 | 21.4 | 12.8 | 9.3 |
| D | 0 | 26.8 | 23.8 | 8.0 | 19.7 | 21.8 |
| Area | Ti | Cr | V | O | Al | C |
|---|---|---|---|---|---|---|
| A | 18.9 | 3.3 | 5.2 | 67.5 | 1.3 | 3.9 |
| B | 2.4 | 5.2 | 6.3 | 65.3 | 18.9 | 1.9 |
| C | 10.7 | 18.4 | 18.3 | 9.0 | 23.6 | 20.1 |
| Area | Ti | Cr | V | O | Al | C |
|---|---|---|---|---|---|---|
| A | 2.3 | 1.6 | 2.5 | 51.8 | 30.7 | 11.1 |
| B | 11.9 | 19.8 | 18.5 | 4.2 | 28.5 | 17.2 |
| C | 11.4 | 19.7 | 18.0 | 8.2 | 27.2 | 15.4 |
| Area | Ti | Cr | V | O | Al | C |
|---|---|---|---|---|---|---|
| A | 0.3 | 0.3 | 26.4 | 66.7 | 1.6 | 4.8 |
| B | 3.8 | 3.7 | 1.8 | 65.3 | 23.1 | 2.3 |
| C | 3.5 | 9.9 | 2.9 | 66.0 | 14.9 | 2.8 |
| D | 11.2 | 20.4 | 18.2 | 9.9 | 19.7 | 20.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zheng, Y.; Chen, K.; Huang, Q.; Meng, F. Microstructure and Oxidation Behaviors of (TiVCr)2AlC MAX-Phase Coatings Prepared by Magnetron Sputtering. Coatings 2024, 14, 1504. https://doi.org/10.3390/coatings14121504
Zhu Y, Zheng Y, Chen K, Huang Q, Meng F. Microstructure and Oxidation Behaviors of (TiVCr)2AlC MAX-Phase Coatings Prepared by Magnetron Sputtering. Coatings. 2024; 14(12):1504. https://doi.org/10.3390/coatings14121504
Chicago/Turabian StyleZhu, Yufeng, Yueqing Zheng, Ke Chen, Qing Huang, and Fanping Meng. 2024. "Microstructure and Oxidation Behaviors of (TiVCr)2AlC MAX-Phase Coatings Prepared by Magnetron Sputtering" Coatings 14, no. 12: 1504. https://doi.org/10.3390/coatings14121504
APA StyleZhu, Y., Zheng, Y., Chen, K., Huang, Q., & Meng, F. (2024). Microstructure and Oxidation Behaviors of (TiVCr)2AlC MAX-Phase Coatings Prepared by Magnetron Sputtering. Coatings, 14(12), 1504. https://doi.org/10.3390/coatings14121504






