Extended Lifetime of Dual-Layer Yttria-Stabilized Zirconia APS/Gadolinium Zirconate SPS Thermal Barrier Coatings in Furnace Cycle Tests
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials of Ceramic Coatings
2.2. Sample Preparation
2.3. Characterization
2.4. Furnace Cycling Tests
3. Results and Discussion
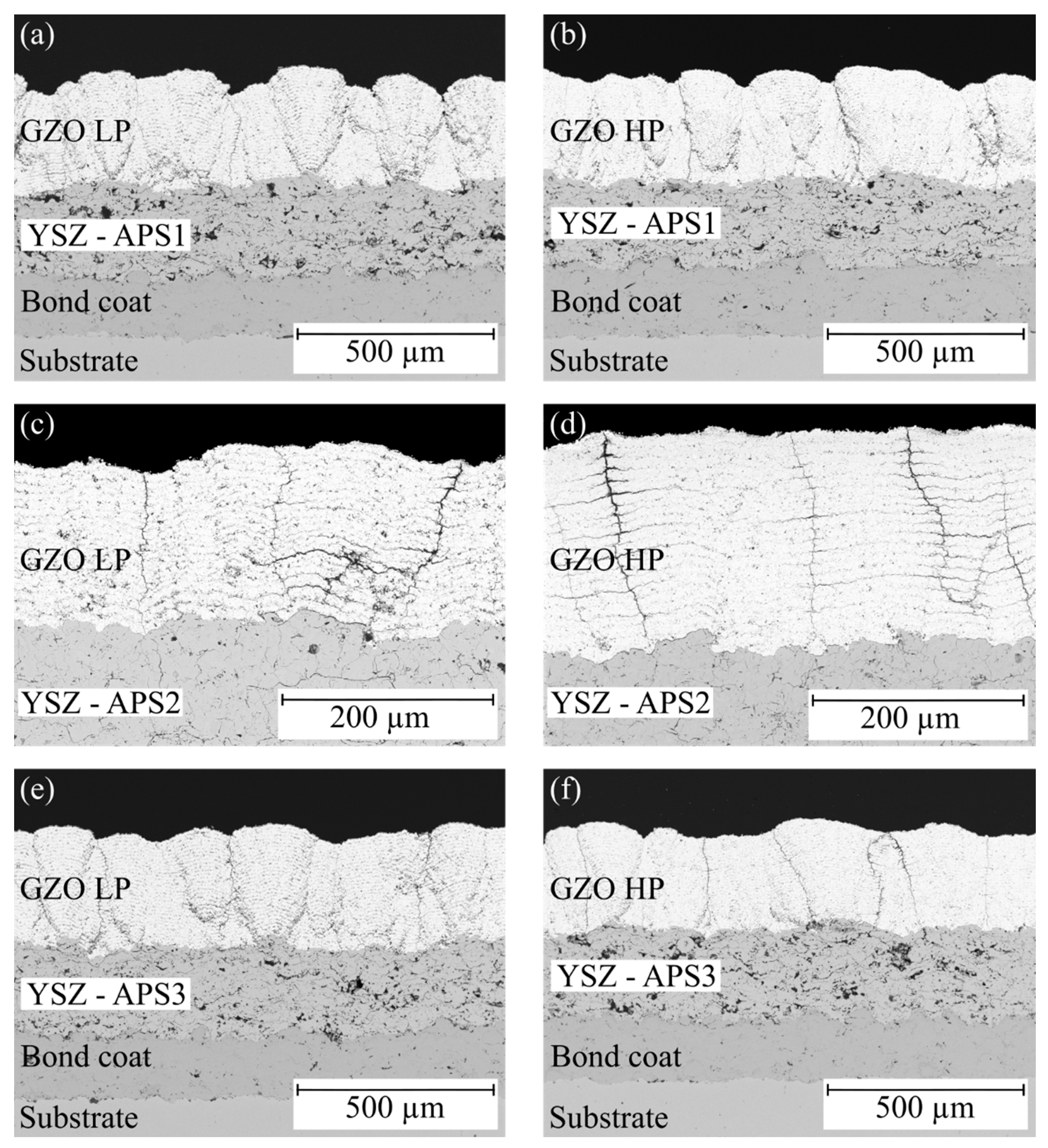
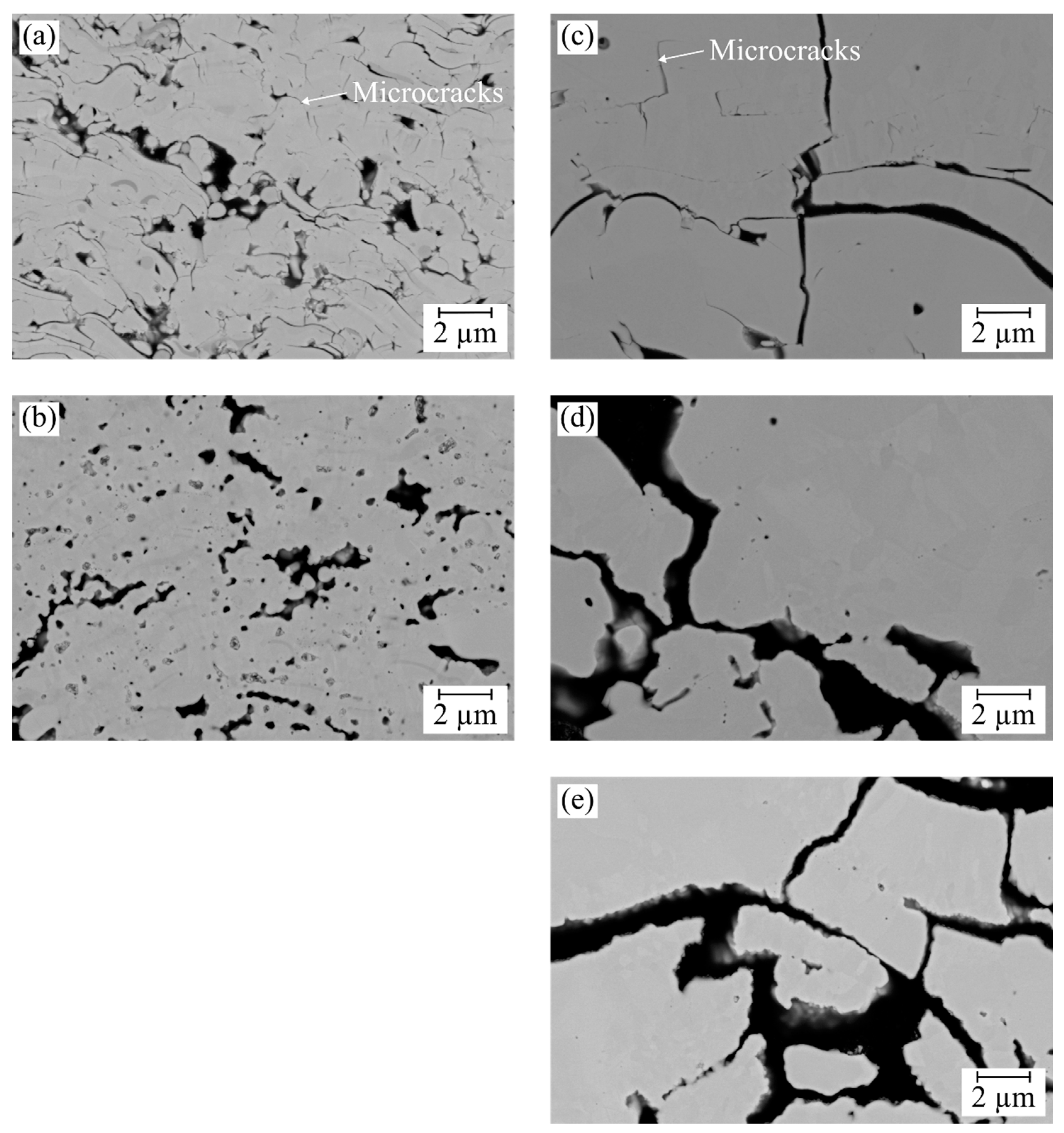
3.1. Microstructure of As-Sprayed Double-Layer Systems
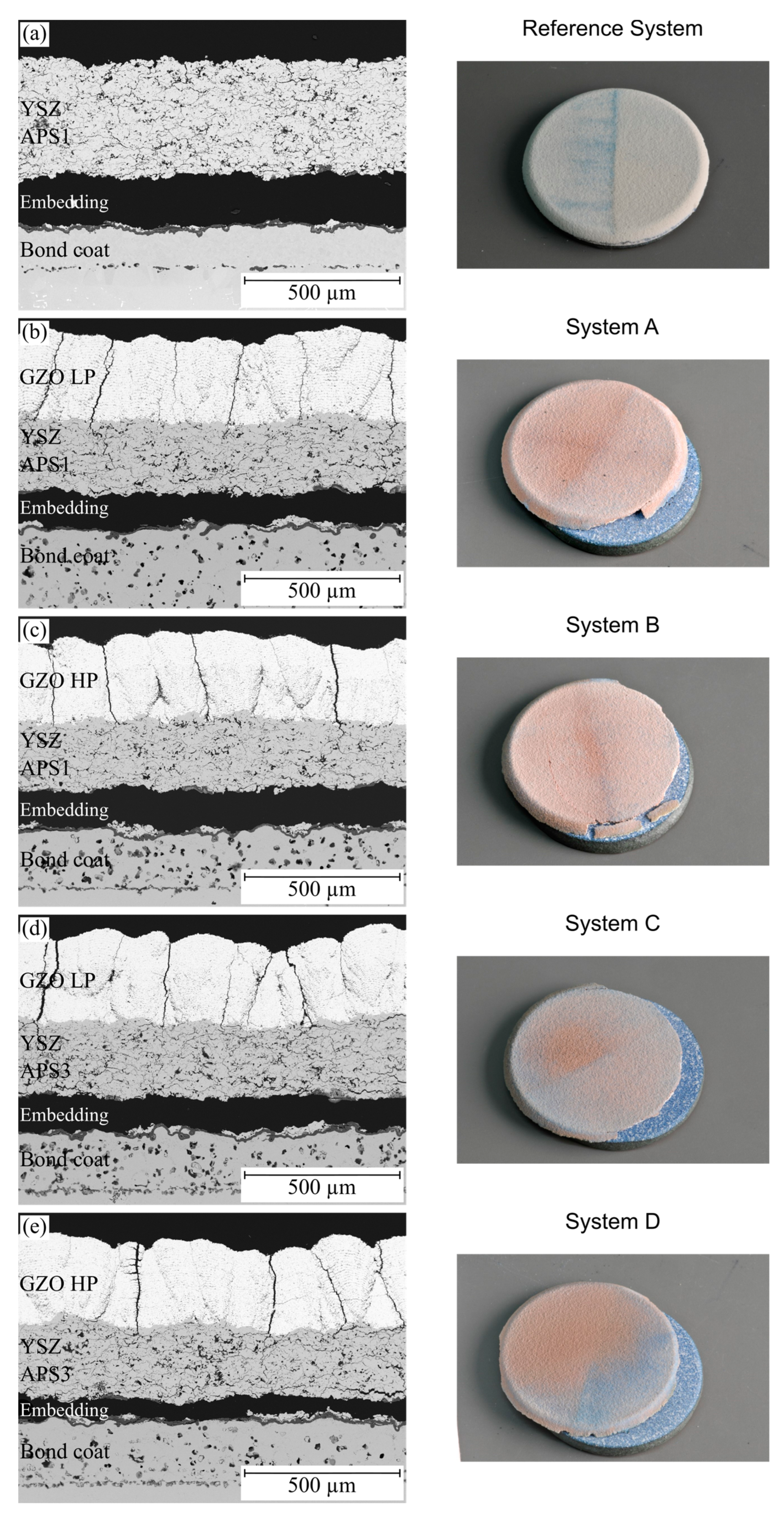
3.2. Microstructure of Thermal Cycling Samples in the As-Sprayed Condition
3.3. Furnace Cycling
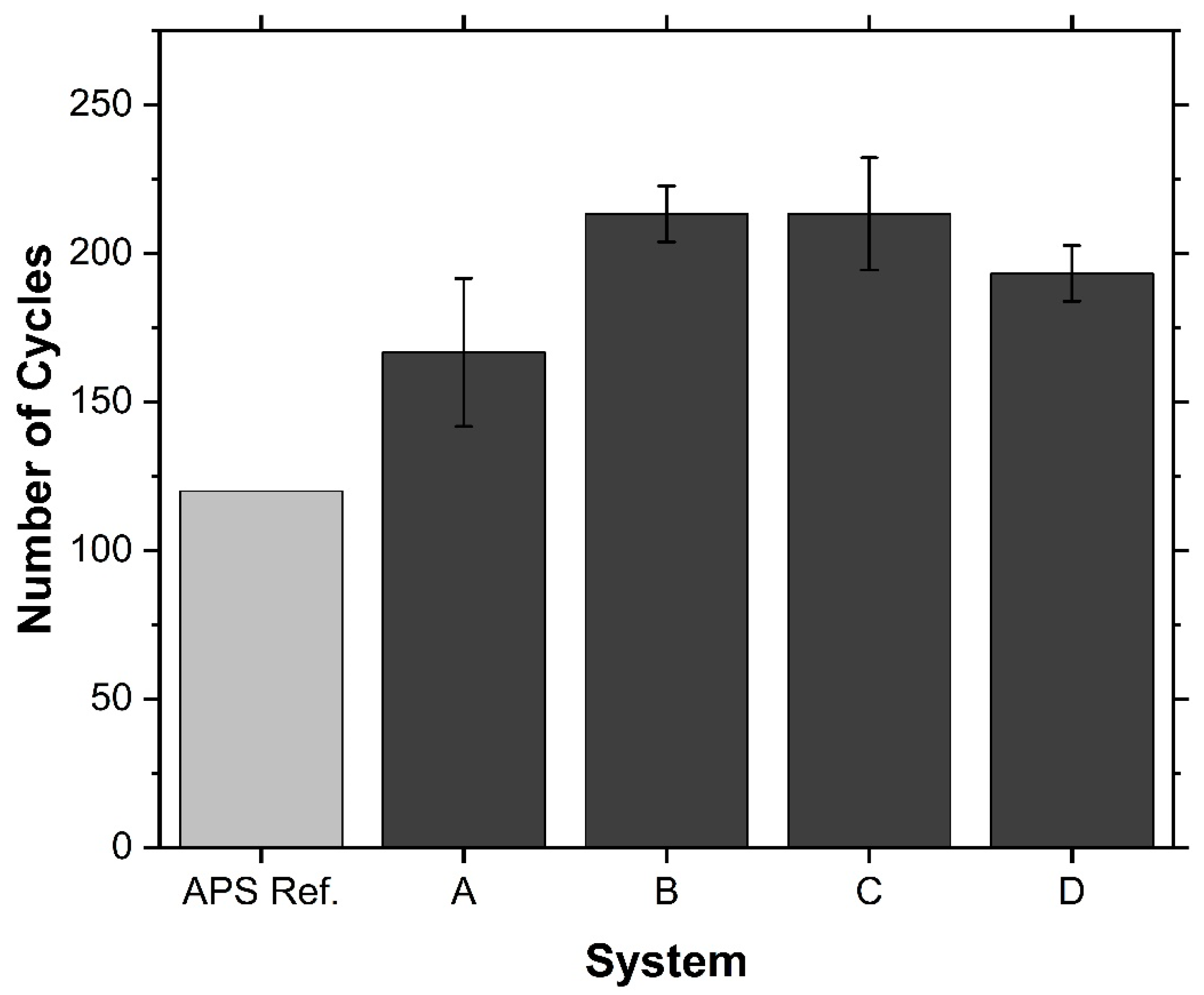
3.3.1. Coating Lifetime
3.3.2. Failure Mode
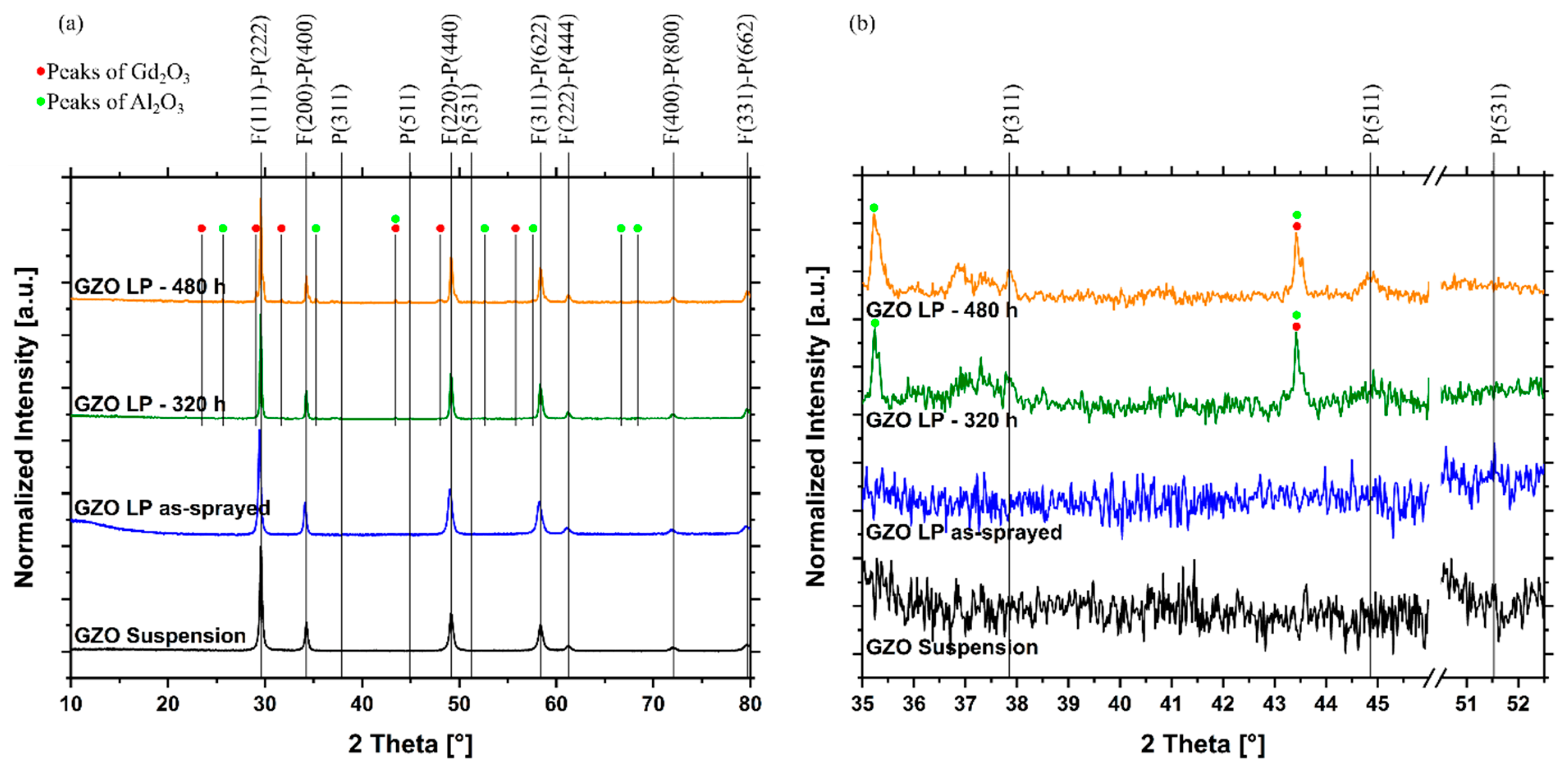
3.4. Phase Composition
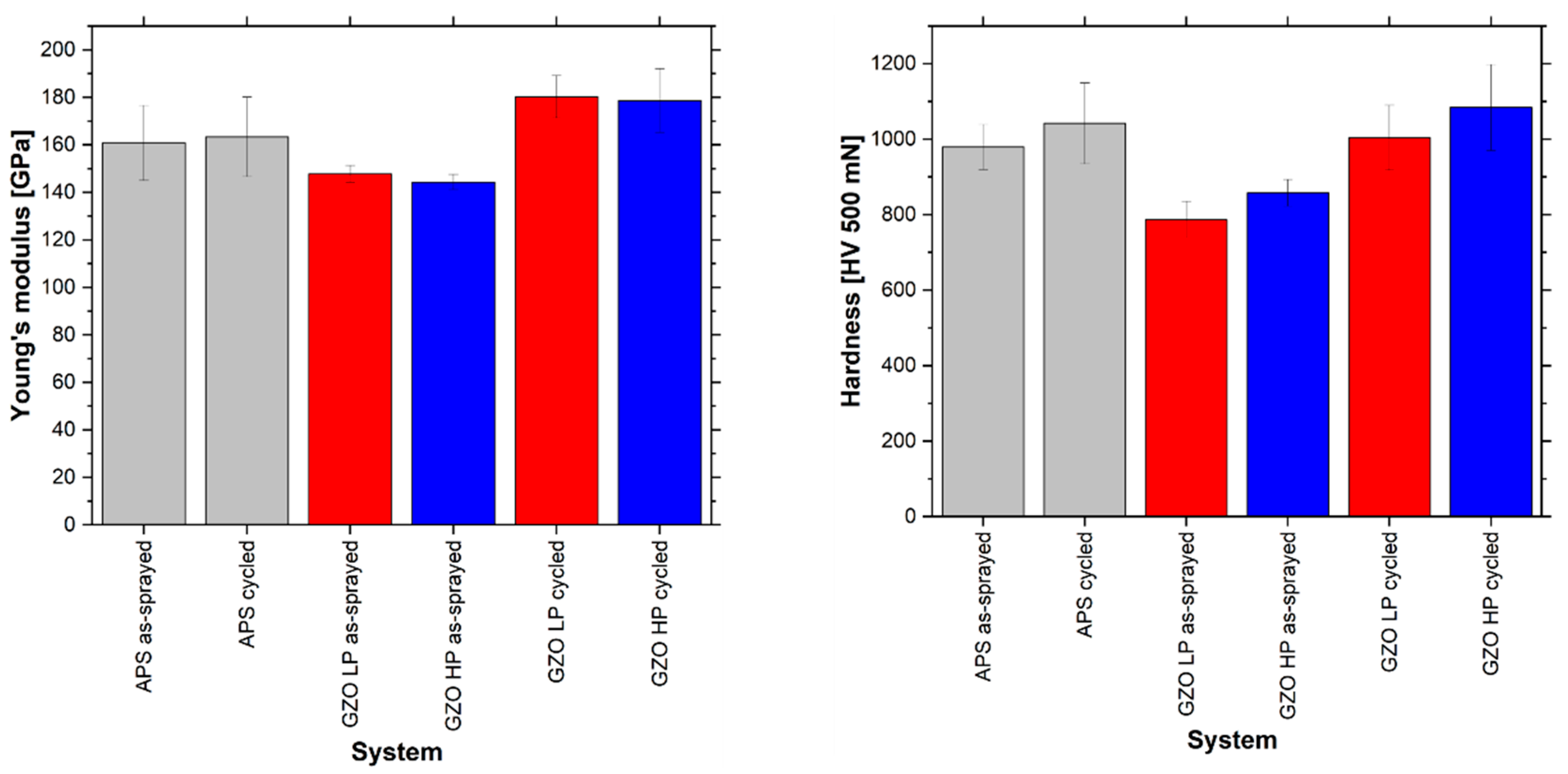
3.5. Change in Microstructural and Mechanical Properties by Thermal Exposure
4. Conclusions
- The developed GZO suspension and the radial injection of the suspension by nozzles without atomization allow for the manufacture of a double-layer system with a single plasma torch in one facility. Different microstructures can be produced in this way. The APS-YSZ layers were deposited with a porous lamellar structure, and the SPS-GZO layers were deposited with a strain-tolerant columnar and vertically cracked microstructure. In the future, it may be possible to produce such double-layer systems in a single coating run without modifying the hardware, which is interesting from an economic point of view. In addition, the performance of gas turbines can be improved with the developed coating systems. The GZO top coat allows for very high combustion temperatures due to the high phase stability, and the combination of microstructures enables flexible operation of the turbines with fast-changing loads.
- The columnar and vertically cracked GZO top layers showed good cyclability and, in the best case, doubled the lifetime compared to the reference system. This is due to the high strain tolerance of the top layer combined with the high fracture toughness of the ceramic interlayer.
- A change in the microstructure and mechanical properties due to the heat treatment in the furnace test can be observed especially in the GZO layer, which should have a higher sintering resistance than the APS-YSZ layer. This can be explained by the different microstructures produced by the two processes. The much finer distributed porosity in the SPS-GZO layer seems to have a significant influence on the material change during heat treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, A.; Vasudev, H.; Singh, S. Recent developments in the designing of deposition of thermal barrier coatings—A review. Mater. Today Proc. 2020, 26, 1336–1342. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T.; Ashary, A.; Bolcavage, A.; Hitchman, N. Technical and Economical Aspects of Current Thermal Barrier Coating Systems for Gas Turbine Engines by Thermal Spray and EBPVD: A Review. J. Therm. Spray Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Maricocchi, A.; Barz, A.; Wortman, D. PVD TBC Experience on GE Aircraft Engines. In Thermal Barrier Coating Workshop; NASA Conference Publication: Leveland, OH, USA, 1995; pp. 79–90. [Google Scholar]
- Rigney, D.V.; Viguie, R.; Wortman, D.J.; Skelly, D.W. PVD thermal barrier coating applications and process development for aircraft engines. J. Therm. Spray Technol. 1997, 6, 167–175. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials Design for the Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Bernard, B.; Quet, A.; Bianchi, L.; Joulia, A.; Malié, A.; Schick, V.; Rémy, B. Thermal insulation properties of YSZ coatings: Suspension Plasma Spraying (SPS) versus Electron Beam Physical Vapor Deposition (EB-PVD) and Atmospheric Plasma Spraying (APS). Surf. Coat. Technol. 2017, 318, 122–128. [Google Scholar] [CrossRef]
- Stecura, S. Effects of Compositional Changes on the Performance of a Thermal Barrier Coating System. Technical Report, NASA TM-78976; National Aeronautics and Space Administration: Cleveland, OH, USA, 1978. [Google Scholar]
- Raghavan, S.; Wang, H.; Dinwiddie, R.B.; Porter, W.D.; Mayo, M.J. The effect of grain size, porosity and yttria content on the thermal conductivity of nanocrystalline zirconia. Scr. Mater. 1998, 39, 1119–1125. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Lawson, K.J.; Rickerby, D.S.; Morrell, P. Advanced Processing of TBC’s for Reduced Thermal Conductivity; Advisory Group for Aerospace Research & Development Conference Publication: Aalborg, Denmark, 1998; Available online: https://apps.dtic.mil/sti/pdfs/ADA344715.pdf (accessed on 1 December 2024).
- Nicholls, J.R.; Lawson, K.J.; Johnstone, A.; Rickerby, D. Low Thermal Conductivity EB-PVD Thermal Barrier Coatings. Mater. Sci. Forum 2001, 369–372, 595–606. [Google Scholar] [CrossRef]
- Vaßen, R.; Kerkhoff, G.; Stöver, D. Development of a micromechanical life prediction model for plasma sprayed thermal barrier coatings. Mater. Sci. Eng. A 2001, 303, 100–109. [Google Scholar] [CrossRef]
- Sakuma, T.; Yoshizawa, Y.-I.; Suto, H. The microstructure and mechanical properties of yttria-stabilized zirconia prepared by arc-melting. J. Mater. Sci. 1985, 20, 2399–2407. [Google Scholar] [CrossRef]
- Vaßen, R.; Bakan, E.; Mack, D.; Schwartz-Lückge, S.; Sebold, D.; Sohn, Y.J.; Zhou, D.; Guillon, O. Performance of YSZ and Gd2Zr2O7/YSZ double layer thermal barrier coatings in burner rig tests. J. Eur. Ceram. Soc. 2020, 40, 480–490. [Google Scholar] [CrossRef]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Li, C.; Zheng, Z.; Li, Q. Microstructural evolution and growth kinetics of thermally grown oxides in plasma sprayed thermal barrier coatings. Prog. Nat. Sci. Mater. Int. 2016, 26, 103–111. [Google Scholar] [CrossRef]
- Rätzer-Scheibe, H.-J.; Schulz, U. The effects of heat treatment and gas atmosphere on the thermal conductivity of APS and EB-PVD PYSZ thermal barrier coatings. Surf. Coat. Technol. 2007, 201, 7880–7888. [Google Scholar] [CrossRef]
- Trice, R.W.; Su, Y.J.; Mawdsley, J.R.; Faber, K.T.; de Arellano-López, A.R.; Wang, H.; Porter, W.D. Effect of heat treatment on phase stability, microstructure, and thermal conductivity of plasma-sprayed YSZ. J. Mater. Sci. 2002, 37, 2359–2365. [Google Scholar] [CrossRef]
- Choi, S.R.; Zhu, D.; Miller, R.A. Effect of Sintering on Mechanical and Physical Properties of Plasma-Sprayed Thermal Barrier Coatings. NASA/TM—2004-212625. Available online: https://ntrs.nasa.gov/api/citations/20040058073/downloads/20040058073.pdf (accessed on 1 December 2024).
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Langjahr, P.A.; Oberacker, R.; Hoffmann, M.J. Long-Term Behavior and Application Limits of Plasma-Sprayed Zirconia Thermal Barrier Coatings. J. Am. Ceram. Soc. 2001, 84, 1301–1308. [Google Scholar] [CrossRef]
- Scott, H.G. Phase relationships in the zirconia-yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Vaßen, R. Entwicklung Neuer Oxidischer Wärmedämmschichten für Anwendungen in Stationären und Flug-Gasturbinen; Forschungszentrum Jülich: Jülich, Germany, 2004. [Google Scholar]
- Saruhan, B.; Francois, P.; Fritscher, K.; Schulz, U. EB-PVD processing of pyrochlore-structured La2Zr2O7-based TBCs. Surf. Coat. Technol. 2004, 182, 175–183. [Google Scholar] [CrossRef]
- Bansal, N.P.; Zhu, D.-M. Low-Thermal-Conductivity Pyrochlore Oxide Materials Developed for Advanced Thermal Barrier Coatings. Res. Technol. 2005. Available online: https://ntrs.nasa.gov/citations/20050217193 (accessed on 1 December 2024).
- Virkar, A.V.; Matsumoto, R.L.K. Ferroelastic Domain Switching as a Toughening Mechanism in Tetragonal Zirconia. J. Am. Ceram. Soc. 1986, 69, C-224–C-226. [Google Scholar] [CrossRef]
- Karaulov, A.G.; Zoz, E.I. Phase formation in the ZrO2—HfO2—Gd2O3 and ZrO2—HfO2—Yb2O3 systems. Refract. Ind. Ceram. 1999, 40, 479–483. [Google Scholar] [CrossRef]
- Lehmann, H.; Pitzer, D.; Pracht, G.; Vassen, R.; Stöver, D. Thermal Conductivity and Thermal Expansion Coefficients of the Lanthanum Rare-Earth-Element Zirconate System. J. Am. Ceram. Soc. 2003, 86, 1338–1344. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.; Peng, H.; Gong, S. Thermophysical properties of Yb2O3 doped Gd2Zr2O7 and thermal cycling durability of (Gd0.9Yb0.1)2Zr2O7/YSZ thermal barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 1255–1263. [Google Scholar] [CrossRef]
- Bast, U.; Schumann, E. Development of Novel Oxide Materials for TBC’s. In Advances in Ceramic Coatings and Ceramic-Metal Systems: Ceramic Engineering and Science Proceedings; Zhu, D., Plucknett, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 26, pp. 525–532. [Google Scholar]
- Lian, J.; Wang, L.; Chen, J.; Sun, K.; Ewing, R.C.; Farmer, J.M.; Boatner, L.A. The order–disorder transition in ion-irradiated pyrochlore. Acta Mater. 2003, 51, 1493–1502. [Google Scholar] [CrossRef]
- Wuensch, B. Connection between oxygen-ion conductivity of pyrochlore fuel-cell materials and structural change with composition and temperature. Solid. State Ion. 2000, 129, 111–133. [Google Scholar] [CrossRef]
- Leckie, R.M.; Krämer, S.; Rühle, M.; Levi, C.G. Thermochemical compatibility between alumina and ZrO2–GdO3/2 thermal barrier coatings. Acta Mater. 2005, 53, 3281–3292. [Google Scholar] [CrossRef]
- Zhou, D.; Mack, D.E.; Bakan, E.; Mauer, G.; Sebold, D.; Guillon, O.; Vaßen, R. Thermal cycling performances of multilayered yttria-stabilized zirconia/gadolinium zirconate thermal barrier coatings. J. Am. Ceram. Soc. 2020, 103, 2048–2061. [Google Scholar] [CrossRef]
- Vaßen, R.; Traeger, F.; Stöver, D. New Thermal Barrier Coatings Based on Pyrochlore/YSZ Double-Layer Systems. Int. J. Appl. Ceram. Technol. 2004, 1, 351–361. [Google Scholar] [CrossRef]
- Bakan, E.; Mack, D.E.; Mauer, G.; Vaßen, R. Gadolinium Zirconate/YSZ Thermal Barrier Coatings: Plasma Spraying, Microstructure, and Thermal Cycling Behavior. J. Am. Ceram. Soc. 2014, 97, 4045–4051. [Google Scholar] [CrossRef]
- Viswanathan, V.; Dwivedi, G.; Sampath, S. Multilayer, Multimaterial Thermal Barrier Coating Systems: Design, Synthesis, and Performance Assessment. J. Am. Ceram. Soc. 2015, 98, 1769–1777. [Google Scholar] [CrossRef]
- Mahade, S.; Curry, N.; Björklund, S.; Markocsan, N.; Nylén, P. Thermal conductivity and thermal cyclic fatigue of multilayered Gd2Zr2O7/YSZ thermal barrier coatings processed by suspension plasma spray. Surf. Coat. Technol. 2015, 283, 329–336. [Google Scholar] [CrossRef]
- Mahade, S.; Curry, N.; Björklund, S.; Markocsan, N.; Nylén, P.; Vaßen, R. Functional performance of Gd2Zr2O7/YSZ multi-layered thermal barrier coatings deposited by suspension plasma spray. Surf. Coat. Technol. 2017, 318, 208–216. [Google Scholar] [CrossRef]
- Oerlikon Metco. Material Product Data Sheet: 8% Yttria Stabilized Zirconia Agglomerated and HOSP Powders. Available online: https://mymetco-europe.oerlikon.com/en-us/product/metco204ns?isRegionSelection (accessed on 1 December 2024).
- Guignard, A.; Mauer, G.; Vaßen, R.; Stöver, D. Deposition and Characteristics of Submicrometer-Structured Thermal Barrier Coatings by Suspension Plasma Spraying. J. Therm. Spray. Technol. 2012, 21, 416–424. [Google Scholar] [CrossRef]
- Vaßen, R.; Mack, D.E.; Tandler, M.; Sohn, Y.J.; Sebold, D.; Guillon, O. Unique performance of thermal barrier coatings made of yttria-stabilized zirconia at extreme temperatures (>1500° C). J. Am. Ceram. Soc. 2021, 104, 463–471. [Google Scholar] [CrossRef]
- Zhou, D.; Guillon, O.; Vaßen, R. Development of YSZ Thermal Barrier Coatings Using Axial Suspension Plasma Spraying. Coatings 2017, 7, 120. [Google Scholar] [CrossRef]
- Ganvir, A.; Calinas, R.F.; Markocsan, N.; Curry, N.; Joshi, S. Experimental visualization of microstructure evolution during suspension plasma spraying of thermal barrier coatings. J. Eur. Ceram. Soc. 2019, 39, 470–481. [Google Scholar] [CrossRef]
- Vaßen, R.; Kaßner, H.; Mauer, G.; Stöver, D. Suspension Plasma Spraying: Process Characteristics and Applications. J. Therm. Spray. Technol. 2010, 19, 219–225. [Google Scholar] [CrossRef]
- Curry, N.; VanEvery, K.; Snyder, T.; Susnjar, J.; Bjorklund, S. Performance Testing of Suspension Plasma Sprayed Thermal Barrier Coatings Produced with Varied Suspension Parameters. Coatings 2015, 5, 338–356. [Google Scholar] [CrossRef]
- Bellippady, M.; Florent, M.; Björklund, S.; Li, X.H.; Robert, F.; Kjellman, B.; Joshi, S.; Markocsan, N. Characteristics and performance of suspension plasma sprayed thermal barrier coatings on additively manufactured superalloy substrates. Surf. Coat. Technol. 2023, 472, 129926. [Google Scholar] [CrossRef]
- Renusch, D.; Schorr, M.; Schütze, M. The role that bond coat depletion of aluminum has on the lifetime of APS-TBC under oxidizing conditions. Mater. Corros. 2008, 59, 547–555. [Google Scholar] [CrossRef]
- Elsaß, M.; Frommherz, M.; Scholz, A.; Oechsner, M. Interdiffusion in MCrAlY coated nickel-base superalloys. Surf. Coat. Technol. 2016, 307, 565–573. [Google Scholar] [CrossRef]
- Elsaß, M.; Frommherz, M.; Oechsner, M. The Influence of the Coating Deposition Process on the Interdiffusion Behavior Between Nickel-Based Superalloys and MCrAlY Bond Coats. J. Therm. Spray. Technol. 2018, 27, 379–390. [Google Scholar] [CrossRef]
- Nesbitt, J.A.; Heckel, R.W. Modeling degradation and failure of Ni-Cr-Al overlay coatings. Thin Solid. Film. 1984, 119, 281–290. [Google Scholar] [CrossRef]
- Rushton, M.; Stanek, C.R.; Cleave, A.R.; Uberuaga, B.P.; Sickafus, K.E.; Grimes, R.W. Simulation of defects and defect processes in fluorite and fluorite related oxides: Implications for radiation tolerance. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 255, 151–157. [Google Scholar] [CrossRef]
- Frommherz, M.; Scholz, A.; Oechsner, M.; Bakan, E.; Vaßen, R. Gadolinium zirconate/YSZ thermal barrier coatings: Mixed-mode interfacial fracture toughness and sintering behavior. Surf. Coat. Technol. 2016, 286, 119–128. [Google Scholar] [CrossRef]


| Particle Size Distribution [µm] | Viscosity | Surface Tension | |||
|---|---|---|---|---|---|
| d90 | d50 | d10 | 10−3 [Pa×s] | [mN/m] | |
| Metco 204NS | 98.3 | 64.9 | 31.4 | - | - |
| GZO Batch 1 | 4.5 | 1.3 | 0.4 | 1.4 ± 0.08 | 21.5 |
| GZO Batch 2 | 5.3 | 1.7 | 0.3 | 1.4 ± 0.08 | 21.7 |
| ZrO2 | Y2O3 | Gd2O3 | SiO2 | TiO2 | Al2O3 | Fe2O3 | Other Oxides | Phases | |
|---|---|---|---|---|---|---|---|---|---|
| Metco 204 NS | Bal. a | 7.0–8.0 | - | <0.3 | <0.2 | <0.2 | <0.2 | <1.0 | 10% Monoclinic |
| GZO Batch 1 | Bal. b | - | 58.14 | <0.01 | <0.01 | <0.02 | 0.01 | <0.04 | 100% Cubic |
| GZO Batch 2 | Bal. c | - | 58.37 | <0.01 | <0.01 | 0.04 | 0.01 | <0.04 | 100% Cubic |
| Current [A] | Argon [nlpm] | Helium [nlpm] | Standoff [mm] | Robot Speed [mm/s] | Meander [mm] | Total Passes | |
|---|---|---|---|---|---|---|---|
| APS1 | 420 | 46 | 4 | 200 | 500 | 2 | 9 |
| APS2 a | 500 | 46 | 4 | 150 | 500 | 2 | 5 + 4 |
| APS3 a | 380 | 46 | 4 | 200 | 500 | 2 | 8 + 4 |
| Current [A] | Argon [nlpm] | Helium [nlpm] | Standoff [mm] | Robot Speed [mm/s] | Meander [mm] | |
|---|---|---|---|---|---|---|
| GZO LP | 500 | 46 | 4 | 70 | 1000 | 2 |
| GZO HP | 450 | 80 | 8 | 70 | 1000 | 2 |
| Coating Thickness [µm] | ||||
|---|---|---|---|---|
| Image in Figure 1 | System Name | Parameter Used | APS | GZO |
| (a) | A | APS1 + GZO LP | 233.3 ± 17.5 | 265.5 ± 19.1 |
| (b) | B | APS1 + GZO HP | 223.9 ± 19.2 | 263.6 ± 22.8 |
| (e) | C | APS3 + GZO LP | 223.3 ± 20.9 | 281.5 ± 11.4 |
| (f) | D | APS3 + GZO HP | 223.5 ± 20.6 | 254.0 ± 16.2 |
| Al | Cr | Co | Ni | Y | |
|---|---|---|---|---|---|
| Spectrum 1 | 7 | 22 | 41 | 30 | 0 |
| Spectrum 2 | 7 | 22 | 41 | 30 | 0 |
| Spectrum 3 | 13 | 18 | 34 | 35 | 0 |
| Spectrum 4 | 12 | 19 | 32 | 36 | 1 |
| Spectrum 5 | 10 | 21 | 37 | 31 | 1 |
| Spectrum 6 | 7 | 22 | 41 | 30 | 0 |
| Spectrum 7 | 7 | 22 | 41 | 30 | 0 |
| Mean values | 9 | 21 | 38 | 32 | 0.3 |
| Map 1 | 9 | 21 | 38 | 32 | 0 |
| Map 2 | 8 | 21 | 38 | 32 | 1 |
| Spectrum c1 | 4 | 22 | 33 | 40 | 0 |
| Spectrum c2 | 4 | 22 | 32 | 40 | 0 |
| Spectrum c3 | 4 | 22 | 33 | 40 | 0 |
| Spectrum c4 | 4 | 22 | 33 | 39 | 0 |
| Spectrum c5 | 4 | 22 | 33 | 39 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igel, J.; Razak, R.A.; Mack, D.E.; Guillon, O.; Vaßen, R. Extended Lifetime of Dual-Layer Yttria-Stabilized Zirconia APS/Gadolinium Zirconate SPS Thermal Barrier Coatings in Furnace Cycle Tests. Coatings 2024, 14, 1566. https://doi.org/10.3390/coatings14121566
Igel J, Razak RA, Mack DE, Guillon O, Vaßen R. Extended Lifetime of Dual-Layer Yttria-Stabilized Zirconia APS/Gadolinium Zirconate SPS Thermal Barrier Coatings in Furnace Cycle Tests. Coatings. 2024; 14(12):1566. https://doi.org/10.3390/coatings14121566
Chicago/Turabian StyleIgel, Jens, Raseem Ahmed Razak, Daniel Emil Mack, Olivier Guillon, and Robert Vaßen. 2024. "Extended Lifetime of Dual-Layer Yttria-Stabilized Zirconia APS/Gadolinium Zirconate SPS Thermal Barrier Coatings in Furnace Cycle Tests" Coatings 14, no. 12: 1566. https://doi.org/10.3390/coatings14121566
APA StyleIgel, J., Razak, R. A., Mack, D. E., Guillon, O., & Vaßen, R. (2024). Extended Lifetime of Dual-Layer Yttria-Stabilized Zirconia APS/Gadolinium Zirconate SPS Thermal Barrier Coatings in Furnace Cycle Tests. Coatings, 14(12), 1566. https://doi.org/10.3390/coatings14121566







