Focused Review on Graphitic Carbon Nitride (g-C3N4) in Corrosion and Erosion Applications
Abstract
:1. Introduction
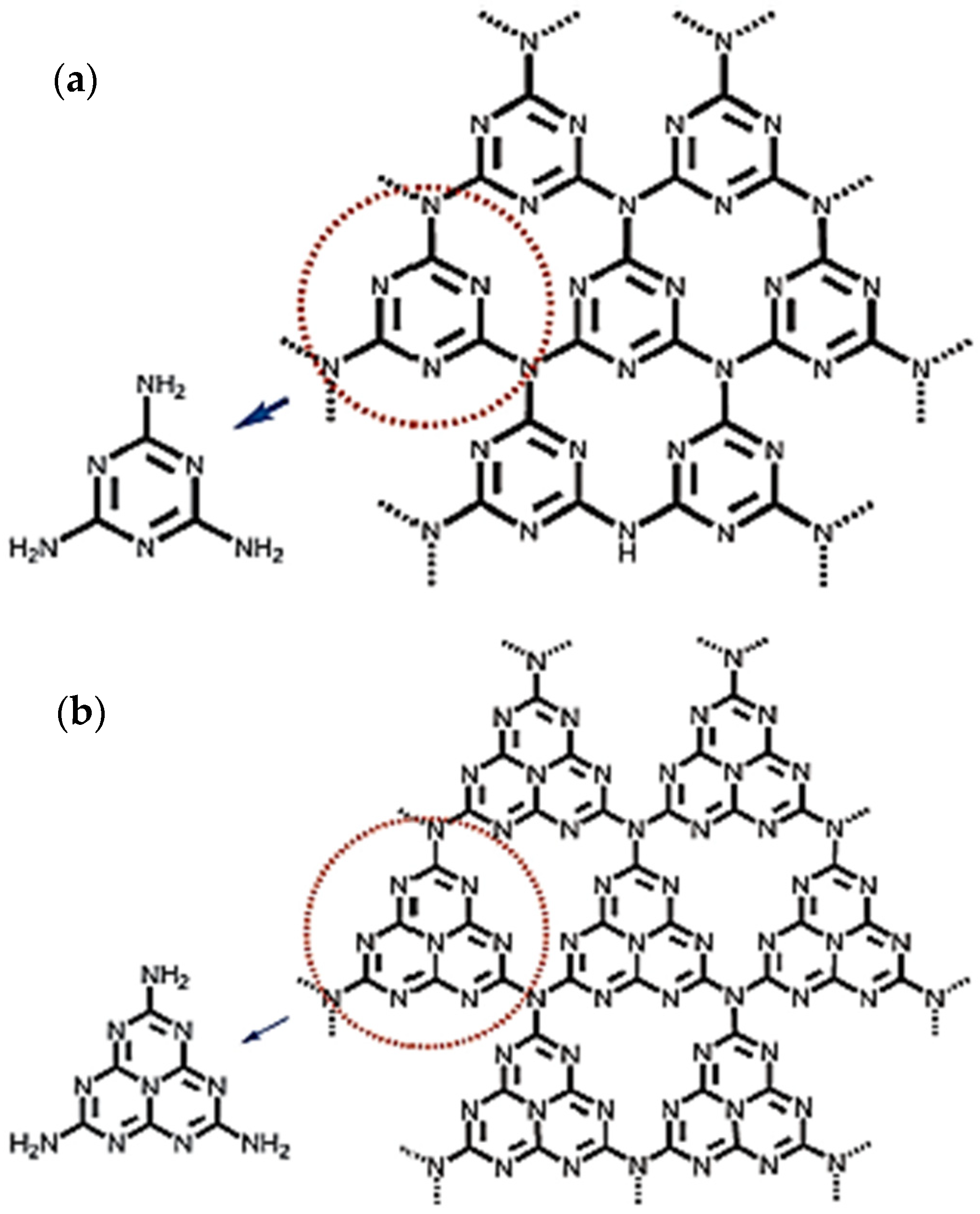
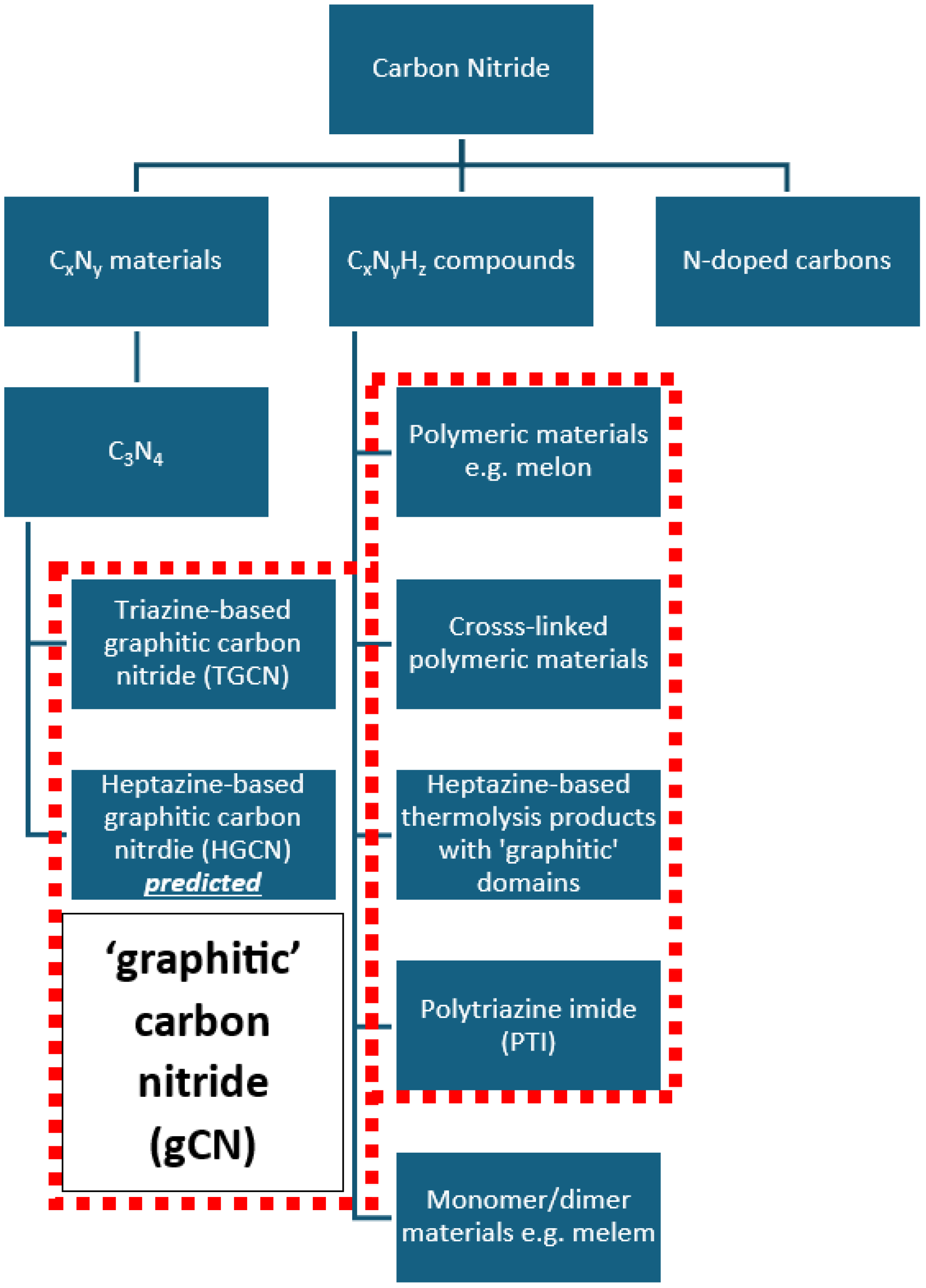
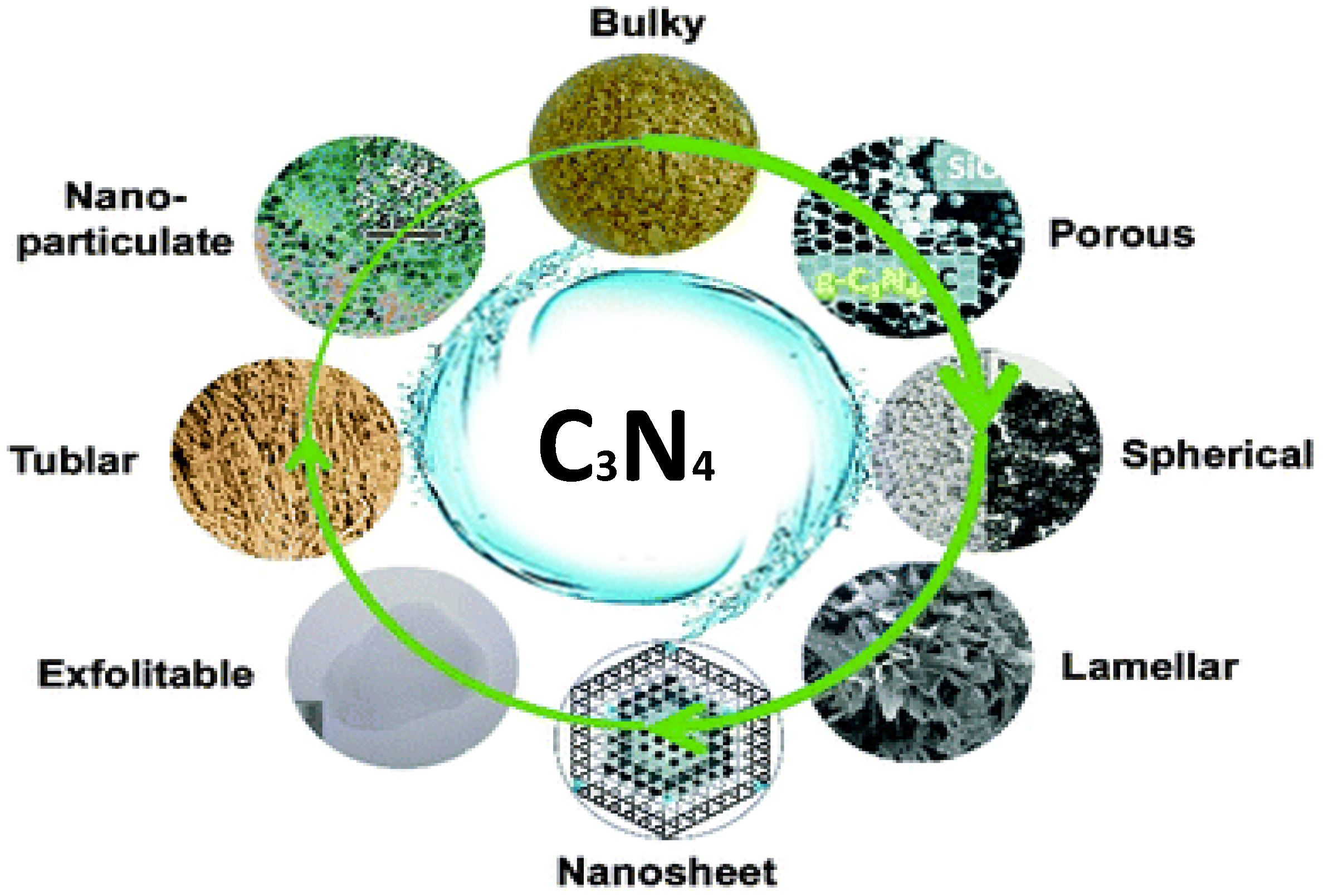
2. History, Structure, and Unique Properties of Carbon Nitride Materials
3. Standalone g-CNs Coatings for Enhanced Wear and Corrosion Resistance, Their Fundamentals, Applications, and Mechanisms
4. G-C3N4 Metallic Nanocomposite Coatings: Preparation, Importance, and Mechanism
5. G-C3N4 Organic Nanocomposite Coatings: Preparation, Importance, and Mechanism
6. Conclusions, Recommendations, and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schmitt, G.; Schütze, M.; Hays, G.F.; Burns, W.; Han, E.H.; Pourbaix, A.; Jacobson, G. Global needs for knowledge dissemination, research, and development in materials deterioration and corrosion control. World Corros. Organ. 2009, 38, 14. [Google Scholar]
- Gerhardus Koch, Jeff Varney, Neil Thompson, Oliver Moghissi, Melissa Gould, and Joe Payer, Nace International. 2016. Available online: http://impact.nace.org/documents/Nace-International-Report.pdf (accessed on 15 June 2024).
- Al Hashem, A. Corrosion in the Gulf Cooperation Council (GCC) States: Statistics and Figures; Corrosion UAE: Abu Dhabi, United Arab Emirates, 2011. [Google Scholar]
- Anti-Corrosion Coating Market by Type (Epoxy, Polyurethane, Acrylic, Alkyd, Zinc), Technology (Solvent Borne, Waterborne, Powder-Based), End-Use Industry (Marine, Oil & Gas, Industrial, Infrastructure, Power Generation), & Region—Global Forecast to 2028. MarketsandMarkets. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/anti-corrosion-coating-market-155215822.html (accessed on 10 December 2024).
- Al-Kandari, H.; Abdullah, A.M.; Ahmad, Y.H.; Al-Kandari, S.; AlQaradawi, S.Y.; Mohamed, A.M. An efficient eco advanced oxidation process for phenol mineralization using a 2D/3D nanocomposite photocatalyst and visible light irradiations. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Grill, A. Tribology of diamondlike carbon and related materials: An updated review. Surf. Coat. Technol. 1997, 94, 507–513. [Google Scholar] [CrossRef]
- Liu, D.; Tu, J.; Hong, C.; Gu, C.; Mai, Y.; Chen, R. Improving mechanical properties of a-CNx films by Ti–TiN/CNx gradient multilayer. Appl. Surf. Sci. 2010, 257, 487–494. [Google Scholar] [CrossRef]
- Vepřek, S.; Weidmann, J.; Glatz, F.; Contreras, O.; Hirata, G.A.; Farías, M.H.; Cota-Araiza, L.; Dwivedi, N.; Kumar, S.; Malik, H.K.; et al. Plasma chemical vapor deposition and properties of hard C3N4 thin films. J. Vac. Sci. Technol. A 1995, 13, 2914–2919. [Google Scholar] [CrossRef]
- Cohen, M.L. Structural, electronic and optical properties of carbon nitride. Mater. Sci. Eng. A 1996, 209, 1–4. [Google Scholar] [CrossRef]
- Sjöström, H.; Hultman, L.; Sundgren, J.; Hainsworth, S.V.; Page, T.F.; Theunissen, G. Structural and mechanical properties of carbon nitride CN x (0.2 ≤ x ≤ 0.35) films. J. Vac. Sci. Technol. A Vac. Surf. Film. 1996, 14, 56–62. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, D.; Lin, X.; Dravid, V.P.; Chung, Y.-W.; Wong, M.-S.; Sproul, W.D. Analytical electron microscopy and Raman spectroscopy studies of carbon nitride thin films. J. Vac. Sci. Technol. A 1993, 11, 521–524. [Google Scholar] [CrossRef]
- Khurshudov, A.; Kato, K.; Daisuke, S. Comparison of tribological properties of carbon and carbon nitride protective coatings over magnetic media. J. Vac. Sci. Technol. A 1996, 14, 2935–2939. [Google Scholar] [CrossRef]
- Park, Y.S.; Myung, H.S.; Han, J.G.; Hong, B. Characterization of CNx thin films prepared by close field unbalanced magnetron sputtering. Thin Solid Films 2005, 475, 298–302. [Google Scholar] [CrossRef]
- Ziyang Zhou, Xiaohong Ji, Sepideh Pourhashem, Jizhou Duan, Baorong Hou, Investigating the effects of g-C3N4/Graphene oxide nanohybrids on corrosion resistance of waterborne epoxy coatings. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106568. [CrossRef]
- Broitman, E.; Hellgren, N.; Neidhardt, J.; Brunell, I.; Hultman, L. Electrical properties of carbon nitride thin films: Role of morphology and hydrogen content. J. Electron. Mater. 2002, 31, L11–L15. [Google Scholar] [CrossRef]
- Liu, D.; Tu, J.; Chen, R.; Gu, C. Microstructure, corrosion resistance and biocompatibility of titanium incorporated amorphous carbon nitride films. Surf. Coat. Technol. 2011, 206, 165–171. [Google Scholar] [CrossRef]
- Muhl, S.; Méndez, J.M. A review of the preparation of carbon nitride films. Diam. Relat. Mater. 1999, 8, 1809–1830. [Google Scholar] [CrossRef]
- Liebig, J. Uber einige Stickstoff—Verbindungen. Ann. Der Pharm. 1834, 10, 1–47. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. From triazines to heptazines: Novel Nonmetal tricyanomelaminates as precursors for graphitic carbon nitride materials. Chem. Mater. 2006, 18, 1891–1900. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed]
- Ladva, S.A.; Travis, W.; Quesada-Cabrera, R.; Rosillo-Lopez, M.; Afandi, A.; Li, Y.; Jackman, R.B.; Bear, J.C.; Parkin, I.P.; Blackman, C.; et al. Nanoscale, conformal films of graphitic carbon nitride deposited at room temperature: A method for construction of heterojunction devices. Nanoscale 2017, 9, 16586–16590. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Lei, G.; Cao, Y.; Zhao, W.; Dai, Z.; Shen, L.; Xiao, Y.; Jiang, L. Exfoliation of Graphitic Carbon Nitride for Enhanced Oxidative Desulfurization: A Facile and General Strategy. ACS Sustain. Chem. Eng. 2019, 7, 4941–4950. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Ngoc, C.T.H.; Nguyen, H.; Thi, N.H.N.; Shim, J.; Dang, N.N. Carbon Nitride Coupled Co3O4: A Pyrolysis-Based Approach for High-Performance Hybrid Energy Storage. J. Phys. Chem. Lett. 2023, 14, 9412–9423. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, A.; Antonietti, M.; Wang, X. Activation of carbon nitride solids by protonation: Morphology changes, enhanced ionic conductivity, and photoconduction experiments. J. Am. Chem. Soc. 2009, 131, 50–51. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Cacciafesta, V.; Sfondrini, M.F.; Scribante, A.; Klersy, C.; Auricchio, F. Evaluation of friction of conventional and metal-insert ceramic brackets in various bracket-archwire combinations. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.L.; Duncanson, M.G.; Nanda, R.S.; Currier, G. Relative kinetic frictional forces between sintered stainless steel brackets and orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Whitley, J.Q.; Kusy, R.P. Resistance to sliding of titanium brackets tested against stainless steel and beta-titanium archwires with second-order angulation in the dry and wet states. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Redlich, M.; Katz, A.; Rapoport, L.; Wagner, H.; Feldman, Y.; Tenne, R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS2 impregnated in electroless nickel–phosphorous film. Dent. Mater. 2008, 24, 1640–1646. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, X.; Kato, K.; Dai, Z. Friction and wear property of a-CNx coatings sliding against Si3N4 balls in water. Wear 2007, 263, 1253–1258. [Google Scholar] [CrossRef]
- Zhou, F.; Adachi, K.; Kato, K. Friction and wear property of a-CNx coatings sliding against ceramic and steel balls in water. Diam. Relat. Mater. 2005, 14, 1711–1720. [Google Scholar] [CrossRef]
- Zhou, F.; Kato, K.; Adachi, K. Friction and wear properties of CN x/SiC in water lubrication. Tribol. Lett. 2005, 18, 153–163. [Google Scholar] [CrossRef]
- Kato, K.; Umehara, N.; Adachi, K. Friction, wear and N2-lubrication of carbon nitride coatings: A review. Wear 2003, 254, 1062–1069. [Google Scholar] [CrossRef]
- Khurshudov, A.G.; Kato, K. Tribological properties of carbon nitride overcoat for thin-film magnetic rigid disks. Surf. Coat. Technol. 1996, 86, 664–671. [Google Scholar] [CrossRef]
- Wei, S.; Shao, T.; Ding, P. Study of CNx films on 316L stainless steel for orthodontic application. Diam. Relat. Mater. 2010, 19, 648–653. [Google Scholar] [CrossRef]
- Yang, G.; Chen, T.; Feng, B.; Weng, J.; Duan, K.; Wang, J.; Lu, X. Improved corrosion resistance and biocompatibility of biodegradable magnesium alloy by coating graphite carbon nitride (g-C3N4). J. Alloy. Compd. 2018, 770, 823–830. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Sealy, M.; Guo, Y. Surface integrity and process mechanics of laser shock peening of novel biodegradable magnesium–calcium (Mg–Ca) alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Pompa, L.; Rahman, Z.U.; Munoz, E.; Haider, W. Surface characterization and cytotoxicity response of biodegradable magnesium alloys. Mater. Sci. Eng. C 2015, 49, 761–768. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Marton, M.; Kovalčík, D.; Vojs, M.; Zdravecká, E.; Varga, M.; Michalíková, L.; Veselý, M.; Redhammer, R.; Písečný, P. Electrochemical corrosion behavior of amorphous carbon nitride thin films. Vacuum 2012, 86, 696–698. [Google Scholar] [CrossRef]
- Piedrahita, W.; Caicedo, J.; Aperador, W. Tribological and electrochemical properties of AISI D3 steel coated with hafnium carbon nitride. Tribol. Ind. 2018, 40, 488–500. [Google Scholar] [CrossRef]
- Guruz, M.; Dravid, V.; Chung, Y.; Lacerda, M.; Bhatia, C.; Yu, Y.; Lee, S. Corrosion performance of ultrathin carbon nitride overcoats synthesized by magnetron sputtering. Thin Solid Films 2001, 381, 6–9. [Google Scholar] [CrossRef]
- Li, D.J.; Niu, L.F. Influence of N atomic percentages on cell attachment for CNx coatings. Bull. Mater. Sci. 2003, 26, 371–375. [Google Scholar] [CrossRef]
- Peng, G.; Jun, X.; Wan-Yu, D.; Chuang, D. Ultra-thin silicon carbon nitride film: A promising protective coating for read/write heads in magnetic storage devices. Chin. Phys. Lett. 2009, 26, 18–22. [Google Scholar] [CrossRef]
- Li, D.-J.; Chung, Y.-W. Ultrasmooth CN/sub x/overcoats for next-generation hard disks. IEEE Trans. Magn. 2003, 39, 765–768. [Google Scholar] [CrossRef]
- Miyake, S.; Saito, T.; Wang, M.; Watanabe, S. Tribological properties of extremely thin protective carbon nitride films deposited on magnetic discs by complex treatment. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 587–595. [Google Scholar] [CrossRef]
- Januś, M.; Kyzioł, K.; Kluska, S.; Konefał-Góral, J.; Małek, A.; Jonas, S. Plasma Assisted Chemical Vapour Deposition—Technological Design Of Functional Coatings. Arch. Met. Mater. 2015, 60, 909–914. [Google Scholar] [CrossRef]
- Lanning, B.R.; Wei, R. High intensity plasma ion nitriding of orthopedic materials. Surf. Coat. Technol. 2004, 186, 314–319. [Google Scholar] [CrossRef]
- Ueda, M.; Silva, M.; Otani, C.; Reuther, H.; Yatsuzuka, M.; Lepienski, C.; Berni, L. Improvement of tribological properties of Ti6Al4V by nitrogen plasma immersion ion implantation. Surf. Coat. Technol. 2003, 169–170, 408–410. [Google Scholar] [CrossRef]
- Avelar-Batista, J.; Spain, E.; Fuentes, G.; Sola, A.; Rodriguez, R.; Housden, J. Triode plasma nitriding and PVD coating: A successful pre-treatment combination to improve the wear resistance of DLC coatings on Ti6Al4V alloy. Surf. Coat. Technol. 2006, 201, 4335–4340. [Google Scholar] [CrossRef]
- Khaled, M.; Yilbas, B.; Shirokoff, J. Electrochemical study of laser nitrided and PVD TiN coated Ti–6Al–4V alloy: The observation of selective dissolution. Surf. Coat. Technol. 2001, 148, 46–54. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Matthews, A. Tribological and electrochemical performance of PVD TiN coatings on the femoral head of Ti–6Al–4V artificial hip joints. Surf. Coat. Technol. 2003, 163–164, 597–604. [Google Scholar] [CrossRef]
- Nolan, D.; Huang, S.; Leskovsek, V.; Braun, S. Sliding wear of titanium nitride thin films deposited on Ti–6Al–4V alloy by PVD and plasma nitriding processes. Surf. Coat. Technol. 2005, 200, 5698–5705. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Huang, H.C. The Tribological Performance of Surface Treated Ti6A14V as Sliding Against Si3N4 Ball and 316L Stainless Steel Cylinder. J. Mater. Eng. Perform. 2016, 25, 5209–5219. [Google Scholar] [CrossRef]
- Gicquel, A.; Laidani, N.; Saillard, P. Plasma and nitrides: Application to the nitriding of titanium. Pure Appl. Chem. 1990, 62, 1743–1750. [Google Scholar] [CrossRef]
- Salehi, M.; Bell, T.; Morton, P.H. The effect of surface topography on tribo-oxidation of titanium nitrided surfaces. J. Phys. D Appl. Phys. 1992, 25, 889–895. [Google Scholar] [CrossRef]
- Kyzioł, K.; Kaczmarek, K.; Brzezinka, G.; Kyzioł, A. Structure, characterization and cytotoxicity study on plasma surface modified Ti–6Al–4V and γ-TiAl alloys. Chem. Eng. J. 2014, 240, 516–526. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Pompa, L.; Haider, W. Influence of Electropolishing and Magnetoelectropolishing on Corrosion and Biocompatibility of Titanium Implants. J. Mater. Eng. Perform. 2014, 23, 3907–3915. [Google Scholar] [CrossRef]
- Short, K.; Zhang, Z.; Finnie, K.; Collins, G.; Figueroa, C. A new duplex process for corrosion protection by PI3. Mater. Lett. 2008, 62, 3156–3158. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Chang, C.Y. Enhanced Tribological, Electrochemical, and Biocompatibility Properties of Ti6Al4V Alloy Through Gas Nitriding and CN Coating Deposition. J. Mater. Eng. Perform. 2018, 27, 5329–5340. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single layer and multilayer wear resistant coatings of (Ti,Al)N: A review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Cavaleiro, A.; Trindade, B.; Vieira, M. Influence of Ti addition on the properties of W–Ti–C/N sputtered films. Surf. Coat. Technol. 2003, 174–175, 68–75. [Google Scholar] [CrossRef]
- Balaceanu, M.; Braic, V.; Braic, M.; Vladescu, A.; Zoita, C.; Grigorescu, C.; Grigore, E.; Ripeanu, R. Characteristics of Ti–Nb, Ti–Zr and Ti–Al containing hydrogenated carbon nitride films. Solid State Sci. 2009, 11, 1773–1777. [Google Scholar] [CrossRef]
- Shtansky, D.; Sheveiko, A.; Petrzhik, M.; Kiryukhantsev-Korneev, F.; Levashov, E.; Leyland, A.; Yerokhin, A.; Matthews, A. Hard tribological Ti–B–N, Ti–Cr–B–N, Ti–Si–B–N and Ti–Al–Si–B–N coatings. Surf. Coat. Technol. 2005, 200, 208–212. [Google Scholar] [CrossRef]
- Yao, S.; Su, Y.; Kao, W.; Cheng, K. Wear behavior of DC unbalanced magnetron sputter deposited ZrCN films. Mater. Lett. 2005, 59, 3230–3233. [Google Scholar] [CrossRef]
- Braic, V.; Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.; Titorencu, I.; Jinga, V. (Zr,Ti)CN coatings as potential candidates for biomedical applications. Surf. Coat. Technol. 2011, 206, 604–609. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, T.; Takeda, M. Structural analysis of (Cr1−xSix)N coatings and tribological property in water environment. Surf. Coat. Technol. 2005, 193, 167–172. [Google Scholar] [CrossRef]
- Kim, G.; Kim, B.; Lee, S. High-speed wear behaviors of CrSiN coatings for the industrial applications of water hydraulics. Surf. Coat. Technol. 2005, 200, 1814–1818. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, F.; Wang, Q.; Zhou, Z.; Yan, J.; Li, L.K.-Y. Influence of trimethylsilane flow on the microstructure, mechanical and tribological properties of CrSiCN coatings in water lubrication. Appl. Surf. Sci. 2015, 355, 516–530. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Ding, X.; Zhou, Z.; Wang, C.; Zhang, W.; Li, L.K.-Y.; Lee, S.-T. Microstructure and water-lubricated friction and wear properties of CrN(C) coatings with different carbon contents. Appl. Surf. Sci. 2013, 268, 579–587. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Wang, C.; Li, J.; Yao, Y. An analysis on tribological performance of CrCN coatings with different carbon contents in seawater. Tribol. Int. 2015, 91, 131–139. [Google Scholar] [CrossRef]
- Tong, C.-Y.; Lee, J.-W.; Kuo, C.-C.; Huang, S.-H.; Chan, Y.-C.; Chen, H.-W.; Duh, J.-G. Effects of carbon content on the microstructure and mechanical property of cathodic arc evaporation deposited CrCN thin films. Surf. Coat. Technol. 2013, 231, 482–486. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Jang, C.S.; Yoon, S.-Y.; Shin, B.-C.; Kim, K.H. Effects of Si addition on the characteristic evolution and syntheses of nanocomposite Cr–Si–C–N coatings prepared by a hybrid coating system. Surf. Coat. Technol. 2005, 200, 1635–1639. [Google Scholar] [CrossRef]
- Lin, J.; Jang, J.; Park, I.-W.; Wei, R. Structure and properties of CrSiCN coatings deposited by pulsed dc magnetron sputtering for wear and erosion protection. Surf. Coat. Technol. 2016, 287, 44–54. [Google Scholar] [CrossRef]
- Cai, F.; Huang, X.; Yang, Q.; Wei, R.; Nagy, D. Microstructure and tribological properties of CrN and CrSiCN coatings. Surf. Coat. Technol. 2010, 205, 182–188. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Q.; Zhou, F. Tribological Properties of Cr(Si)CN Coatings Sliding Against Different Mating Balls in Water. J. Mater. Eng. Perform. 2019, 28, 1491–1499. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Ju, H.; Xu, J. Microstructure, mechanical and tribological properties of magnetron sputtered VCN films. Surf. Eng. 2017, 33, 919–924. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, M.; Zhao, Y. Carbon doping to improve the high temperature tribological properties of VN coating. Tribol. Int. 2016, 97, 327–336. [Google Scholar] [CrossRef]
- Chen, H.; Xie, X.; Wang, Y.; Wang, Y.; Ye, Y. Understanding corrosion and tribology behaviors of VN and VCN coatings in seawater. Tungsten 2019, 1, 110–119. [Google Scholar] [CrossRef]
- Cai, Z.; Pu, J.; Wang, L.; Xue, Q. Synthesis of a new orthorhombic form of diamond in varying-C VN films: Microstructure, mechanical and tribological properties. Appl. Surf. Sci. 2019, 481, 767–776. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, Z.; Zou, Y.; Guo, S.; Zeng, X.; Yi, Z.; Yu, J.; Gui, J.; Liu, T.; Chen, H. Enhanced anti-wear property of VCN coating in seawater with the optimization of bias voltage. Ceram. Int. 2019, 46, 7939–7946. [Google Scholar] [CrossRef]
- Tillmann, W.; Bejarano, G.; Hoffmann, F. Deposition of hard and adherent TiBCN films for cutting tools applications. Phys. Status Solidi 2012, 209, 1520–1525. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, K.-L.; Lin, Z.-H.; Su, C.-Y.; Lin, C.-K. Bias effects on the tribological behavior of cathodic arc evaporated CrTiAlN coatings on AISI 304 stainless steel. Thin Solid Films 2010, 518, 3825–3829. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of bias voltage on structure and tribocorrosion properties of TiSiCN coating in artificial seawater. Mater. Charact. 2017, 127, 198–208. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of carbon contents on the structure and tribocorrosion properties of TiSiCN coatings on Ti6Al4V. Tribol. Int. 2016, 109, 285–296. [Google Scholar] [CrossRef]
- Eriksson, A.O.; Zhu, J.; Ghafoor, N.; Jensen, J.; Greczynski, G.; Johansson, M.P.; Sjölen, J.; Odén, M.; Hultman, L.; Rosén, J. Ti–Si–C–N thin films grown by reactive arc evaporation from Ti3SiC2 cathodes. J. Mater. Res. 2011, 26, 874–881. [Google Scholar] [CrossRef]
- Ma, S.; Ma, D.; Guo, Y.; Xu, B.; Wu, G.; Xu, K.; Chu, P.K. Synthesis and characterization of super hard, self-lubricating Ti–Si–C–N nanocomposite coatings. Acta Mater. 2007, 55, 6350–6355. [Google Scholar] [CrossRef]
- Ma, D.; Ma, S.; Dong, H.; Xu, K.; Bell, T. Microstructure and tribological behaviour of super-hard Ti–Si–C–N nanocomposite coatings deposited by plasma enhanced chemical vapour deposition. Thin Solid Films 2006, 496, 438–444. [Google Scholar] [CrossRef]
- He, J.; Zhang, F.; Mi, P.; Qin, Y.; Chen, K.; Yang, Y.; Zhang, J.; Yin, F. Microstructure and wear behavior of nano C-rich TiCN coatings fabricated by reactive plasma spraying with Ti-graphite powders. Surf. Coat. Technol. 2016, 305, 215–222. [Google Scholar] [CrossRef]
- Lin, Z.; Jining, H.; Dianran, Y. The study on TiCN coating prepared by reactive plasma spraying. Mater. Rev. 2006, 20, 468–470. [Google Scholar]
- Mi, P.; He, J.; Qin, Y.; Chen, K. Nanostructure reactive plasma sprayed TiCN coating. Surf. Coat. Technol. 2017, 309, 1–5. [Google Scholar] [CrossRef]
- Zheng, G.; Jiao, Q.; Li, C.; Ding, Y.; He, J.; Jiang, Y.; Zhao, H. Influence of nitridation on the microstructure and corrosion behavior of reactive plasma sprayed TiCN coatings. Surf. Coat. Technol. 2020, 396, 125954. [Google Scholar] [CrossRef]
- Vlcak, P.; Fojt, J.; Weiss, Z.; Kopeček, J.; Perina, V. The effect of nitrogen saturation on the corrosion behaviour of Ti-35Nb-7Zr-5Ta beta titanium alloy nitrided by ion implantation. Surf. Coat. Technol. 2019, 358, 144–152. [Google Scholar] [CrossRef]
- Sharma, P.; Dhawan, A.; Sharma, S. Influence of nitrogen ion implantation on corrosion behavior of Zr55Cu30Ni5Al10 amorphous alloy. J. Non-Cryst. Solids 2019, 511, 186–193. [Google Scholar] [CrossRef]
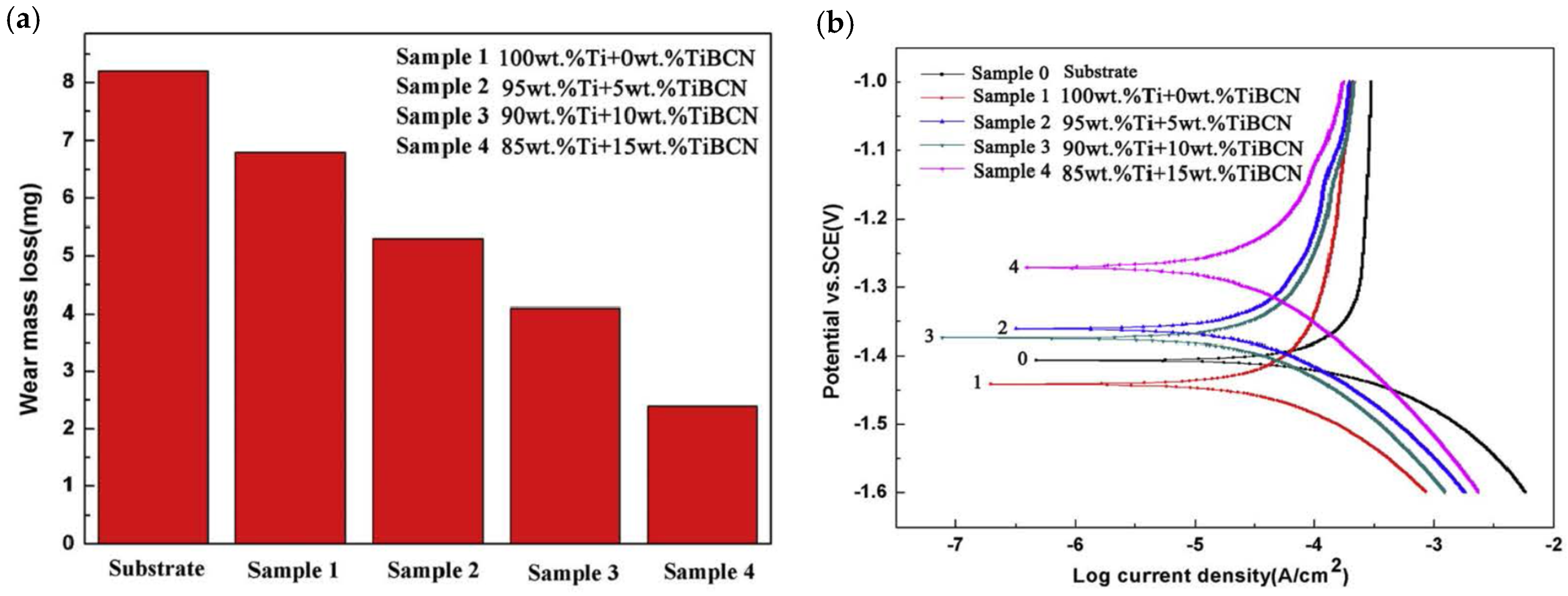
- Li, Y.; Zhang, P.; Bai, P.; Wu, L.; Liu, B.; Zhao, Z. Microstructure and properties of Ti/TiBCN coating on 7075 aluminum alloy by laser cladding. Surf. Coat. Technol. 2017, 334, 142–149. [Google Scholar] [CrossRef]
- Lu, X.-L.; Liu, X.-B.; Yu, P.-C.; Qiao, S.-J.; Zhai, Y.-J.; Wang, M.-D.; Chen, Y.; Xu, D. Synthesis and characterization of Ni60-hBN high temperature self-lubricating anti-wear composite coatings on Ti6Al4V alloy by laser cladding. Opt. Laser Technol. 2016, 78, 87–94. [Google Scholar] [CrossRef]
- I Fayomi, O.S.; Akande, I.G.; A Sode, A. Corrosion Prevention of Metals via Electroless Nickel Coating: A review. J. Phys. Conf. Ser. 2019, 1378, 022063. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z. Recent advances in electroless-plated Ni-P and its composites for erosion and corrosion applications: A review. Emergent Mater. 2018, 1, 3–24. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Zhou, C.; Xu, H.; Gao, W. Fabrication and characterization of electroless Ni–P–ZrO2 nano-composite coatings. Appl. Nanosci. 2011, 1, 19–26. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Hassan, M.K.; Rasool, K.; Mahmoud, K.A.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z.; Abdullah, A.M. Novel electroless deposited corrosion-resistant and anti-bacterial NiP-TiNi nanocomposite coatings. Surf. Coat. Technol. 2019, 4, 75–84. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Rasheed, P.A.; Al-Qahtani, N.; Abdullah, A.M.; Hamdy, F.; Sharaf, M.A.; Hassan, M.K.; Mahmoud, K.A.; Mohamed, A.M.; Jarjoura, G.; et al. Microbiologically-influenced corrosion of the electroless-deposited NiP-TiNi—Coating. Arab. J. Chem. 2021, 14, 103445. [Google Scholar] [CrossRef]
- Rezagholizadeh, M.; Ghaderi, M.; Heidary, A.; Vaghefi, S.M.M. Electroless Ni-P/Ni-B-B4C duplex composite coatings for improving the corrosion and tribological behavior of Ck45 steel. Prot. Met. Phys. Chem. Surfaces 2015, 51, 234–239. [Google Scholar] [CrossRef]
- Shahzad, K.; Fayyad, E.M.; Nawaz, M.; Fayyaz, O.; Shakoor, R.A.; Hassan, M.K.; Umer, M.A.; Baig, M.N.; Raza, A.; Abdullah, A.M. Corrosion and Heat Treatment Study of Electroless NiP-Ti Nanocomposite Coatings Deposited on HSLA Steel. Nanomaterials 2020, 10, 1932. [Google Scholar] [CrossRef]
- Liu, S.; Bian, X.; Liu, J.; Yang, C.; Zhao, X.; Fan, J.; Zhang, K.; Bai, Y.; Xu, H.; Liu, Y.; et al. Structure and properties of Ni–P–graphite (Cg)–TiO2 composite coating. Surf. Eng. 2015, 31, 420–426. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Es′Haghi, M. Corrosion resistance enhancement of electroless Ni–P coating by incorporation of ultrasonically dispersed diamond nanoparticles. Corros. Sci. 2013, 77, 185–193. [Google Scholar] [CrossRef]
- Wang, C.; Farhat, Z.; Jarjoura, G.; Hassan, M.K.; Abdullah, A.M.; Fayyad, E.M. Investigation of fracture behavior of annealed electroless Ni-P coating on pipeline steel using acoustic emission methodology. Surf. Coat. Technol. 2017, 326, 336–342. [Google Scholar] [CrossRef]
- Lee, C. Wear-corrosion behavior of ultra-thin diamond-like carbon nitride films on aluminum alloy. Diam. Relat. Mater. 2008, 17, 306–312. [Google Scholar] [CrossRef]
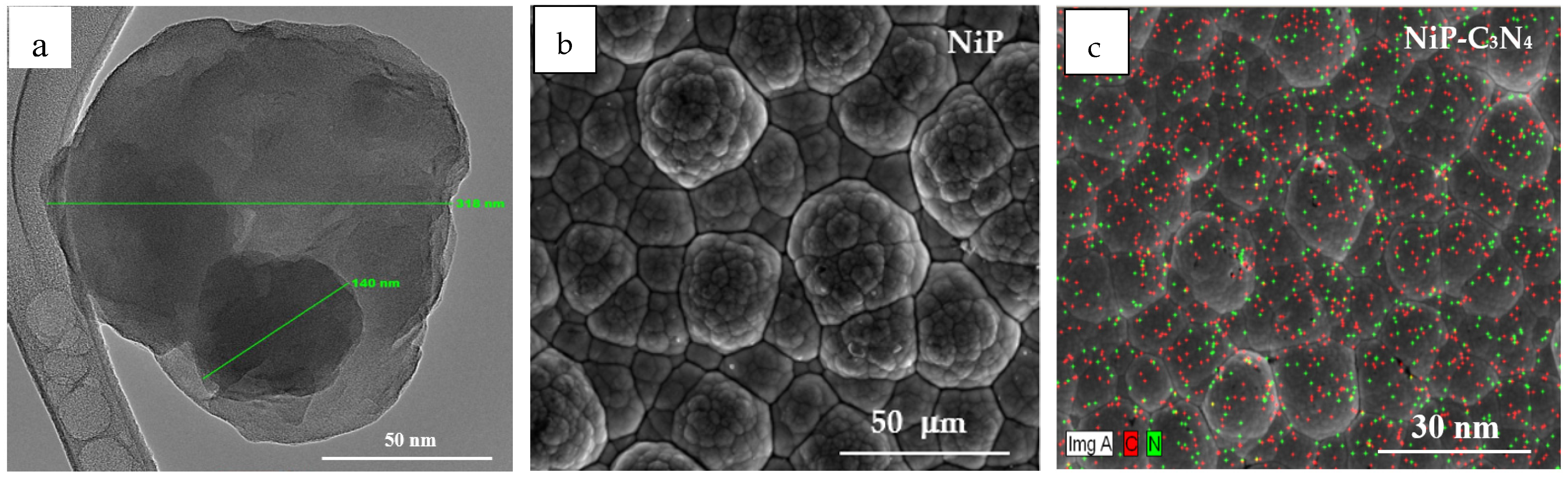
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Wang, C.; Jarjoura, G.; Farhat, Z. Synthesis, characterization, and application of novel Ni-P-carbon nitride nanocomposites. Coatings 2018, 8, 37. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z.; Hassan, M.K. Effect of electroless bath composition on the mechanical, chemical, and electrochemical properties of new NiP–C3N4 nanocomposite coatings. Surf. Coat. Technol. 2019, 362, 239–251. [Google Scholar] [CrossRef]
- Zarezadeh, A.; Shishesaz, M.R.; Ravanavard, M.; Ghobadi, M.; Zareipour, F.; Mahdavian, M. Electrochemical and Mechanical Properties of Ni/g-C3N4 Nanocomposite Coatings with Enhanced Corrosion Protective Properties: A Case Study for Modeling the Corrosion Resistance by ANN and ANFIS Models. J. Appl. Comput. Mech. 2022, 9, 590–606. [Google Scholar] [CrossRef]
- Zheng, H.; Shao, Y.; Wang, Y.; Meng, G.; Liu, B. Reinforcing the corrosion protection property of epoxy coating by using graphene oxide–poly(urea–formaldehyde) composites. Corros. Sci. 2017, 123, 267–277. [Google Scholar] [CrossRef]
- Wang, N.; Gao, H.; Zhang, J.; Li, L.; Fan, X.; Diao, X. Anticorrosive waterborne epoxy (EP) coatings based on sodium tripolyphosphate-pillared layered double hydroxides (STPP-LDHs). Prog. Org. Coat. 2019, 135, 74–81. [Google Scholar] [CrossRef]
- Wang, N.; Fu, W.; Zhang, J.; Li, X.; Fang, Q. Corrosion performance of waterborne epoxy coatings containing polyethylenimine treated mesoporous-TiO2 nanoparticles on mild steel. Prog. Org. Coat. 2015, 89, 114–122. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Alaei, M.; Moradi, M.-A.; Maklavany, D.M. Developing a new method for synthesizing amine functionalized g-C3N4 nanosheets for application as anti-corrosion nanofiller in epoxy coatings. SN Appl. Sci. 2019, 1, 108. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, Y.; Liu, W.; Yao, C.; Li, Y.; Ma, J.; Kong, Y.; Mao, H.; Li, Z.; Fu, Y. Polyaniline/g-C3N4 composites as novel media for anticorrosion coatings. J. Coat. Technol. Res. 2017, 14, 1307–1314. [Google Scholar] [CrossRef]
- Karimi, M.A.; Aghaei, V.H.; Nezhadali, A.; Ajami, N. Investigation of copper corrosion in sodium chloride solution by using a new coating of polystyrene/g-C3N4. J. Mater. Sci. Mater. Electron. 2019, 30, 6300–6310. [Google Scholar] [CrossRef]
- Yan, H.; Li, J.; Zhang, M.; Zhao, Y.; Feng, Y.; Zhang, Y. Enhanced corrosion resistance and adhesion of epoxy coating by two-dimensional graphite-like g-C3N4 nanosheets. J. Colloid Interface Sci. 2020, 579, 152–161. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Zhong, F.; Chen, J. Co-modification of polydopamine and KH560 on g-C3N4 nanosheets for enhancing the corrosion protection property of waterborne epoxy coating. React. Funct. Polym. 2019, 146, 104405. [Google Scholar] [CrossRef]
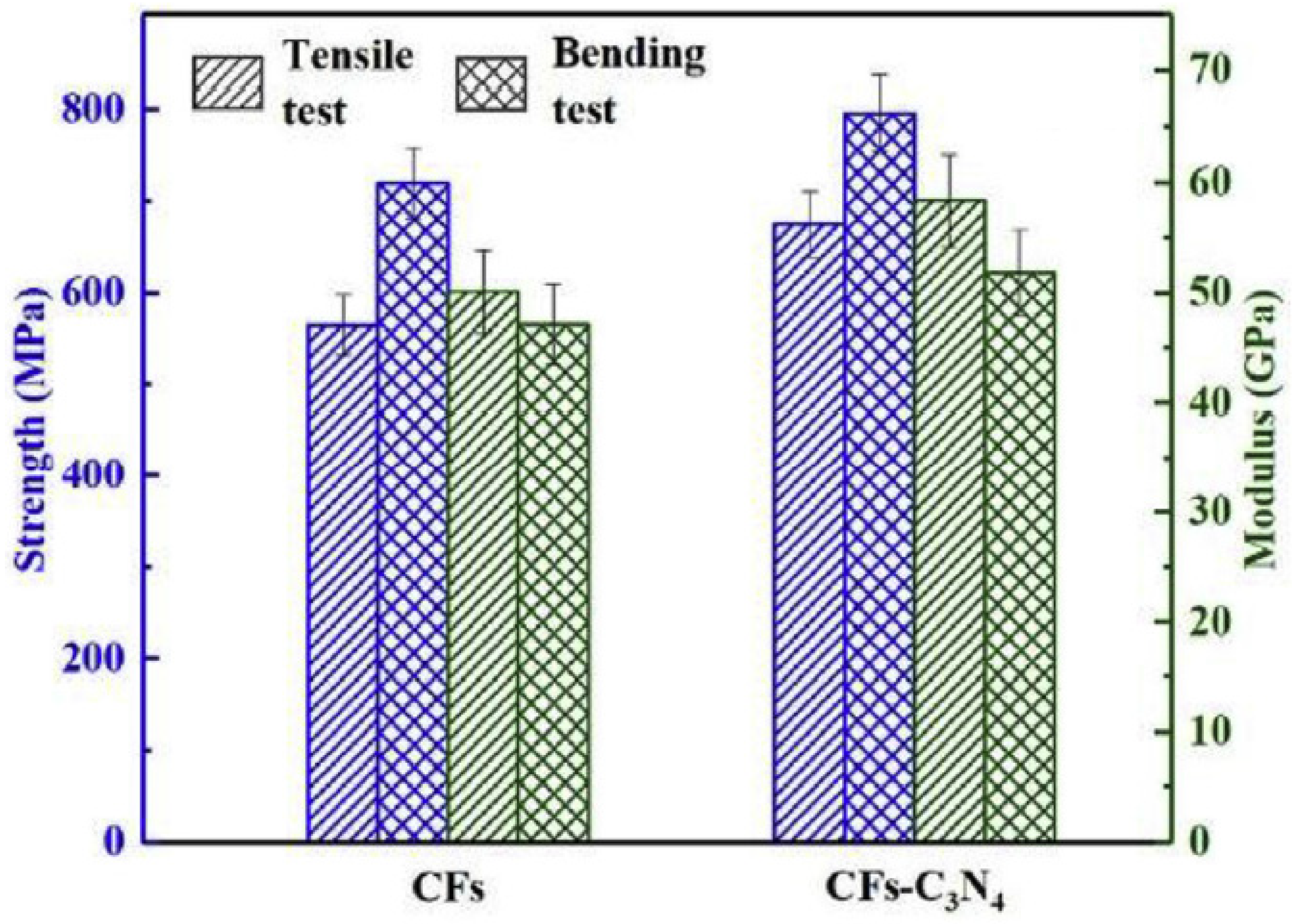
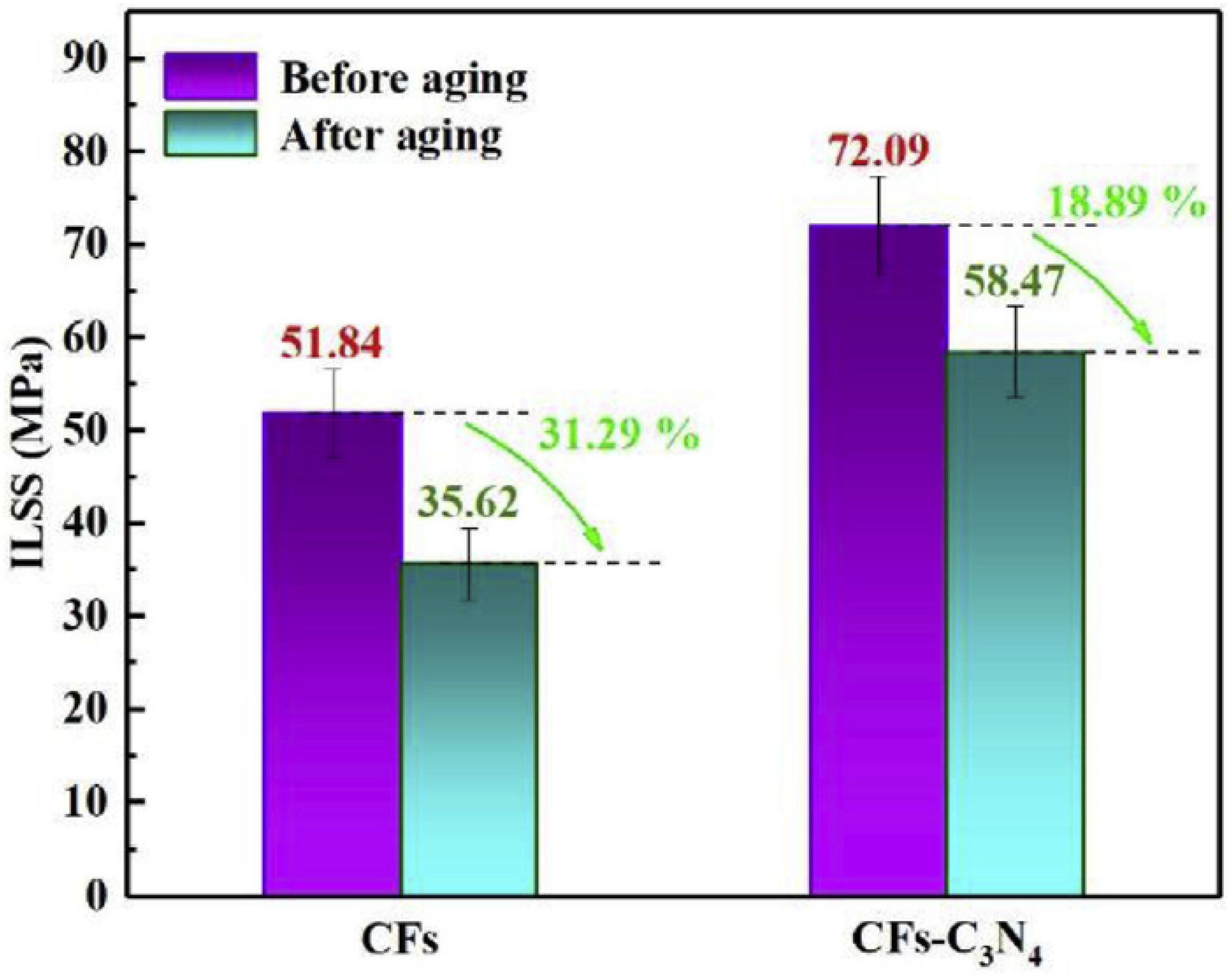
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y.; et al. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2018, 158, 259–268. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, M.Y.; Suleiman, R.K.; Khan, A.; Dafalla, H. Promising graphitic carbon nitride/MoOx nanocomposites: For surface protective performance of AA2024 alloys in marine environment. Surf. Coat. Technol. 2019, 374, 579–590. [Google Scholar] [CrossRef]
- Pourhashem, S.; Duan, J.; Guan, F.; Wang, N.; Gao, Y.; Hou, B. New effects of TiO2 nanotube/g-C3N4 hybrids on the corrosion protection performance of epoxy coatings. J. Mol. Liq. 2020, 317, 114214. [Google Scholar] [CrossRef]
- Kumar, A.M.; Khan, A.; Khan, M.Y.; Suleiman, R.K.; Jose, J.; Dafalla, H. Hierarchical graphitic carbon nitride-ZnO nanocomposite: Viable reinforcement for the improved corrosion resistant behavior of organic coatings. Mater. Chem. Phys. 2020, 251, 122987. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, N.; Zhou, Z.; Chen, C.; Wu, Y.; Zhong, F.; Lv, Y.; He, Y. Incorporating SiO2 functionalized g-C3N4 sheets to enhance anticorrosion performance of waterborne epoxy. Prog. Org. Coat. 2020, 147, 105768. [Google Scholar] [CrossRef]




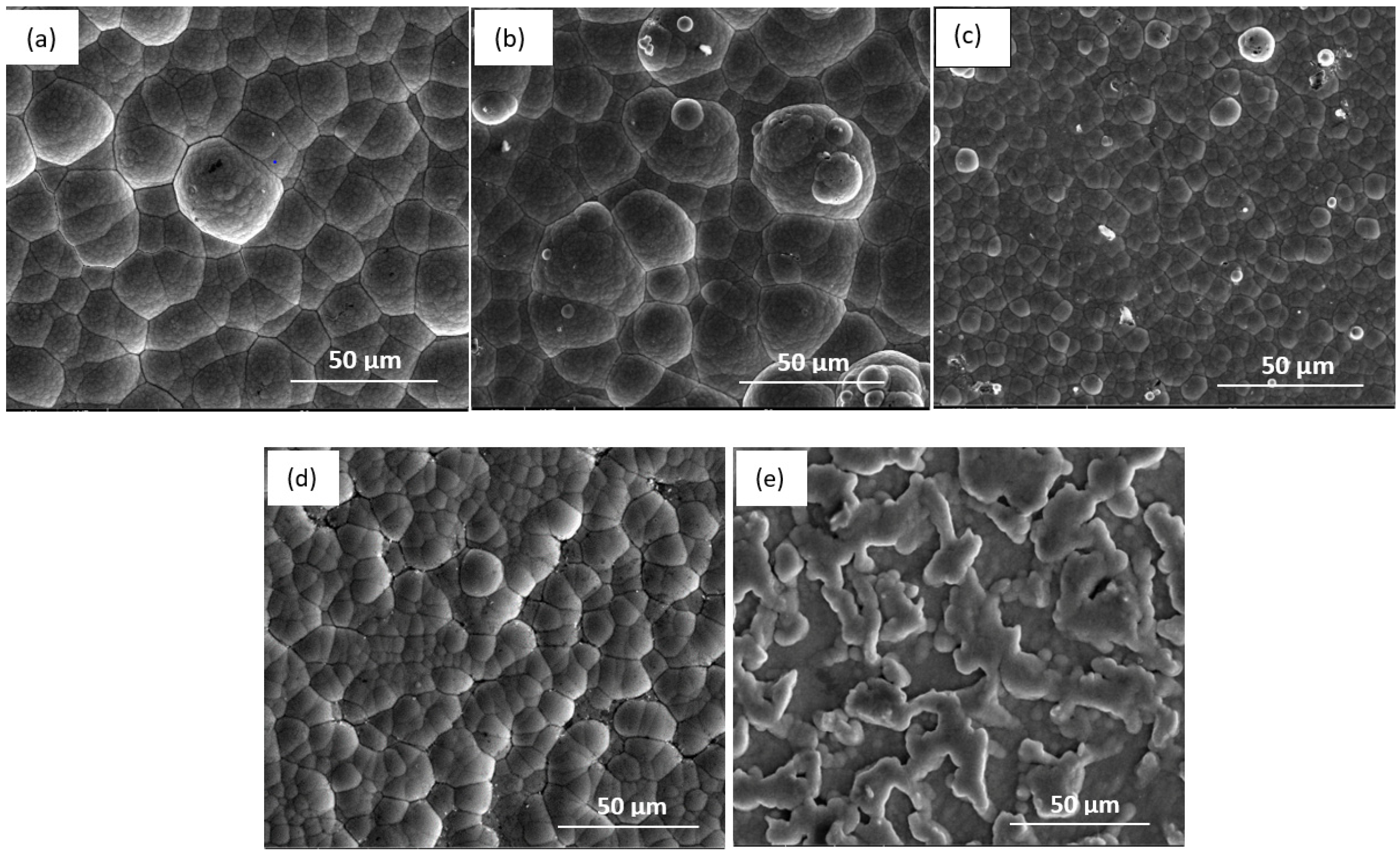


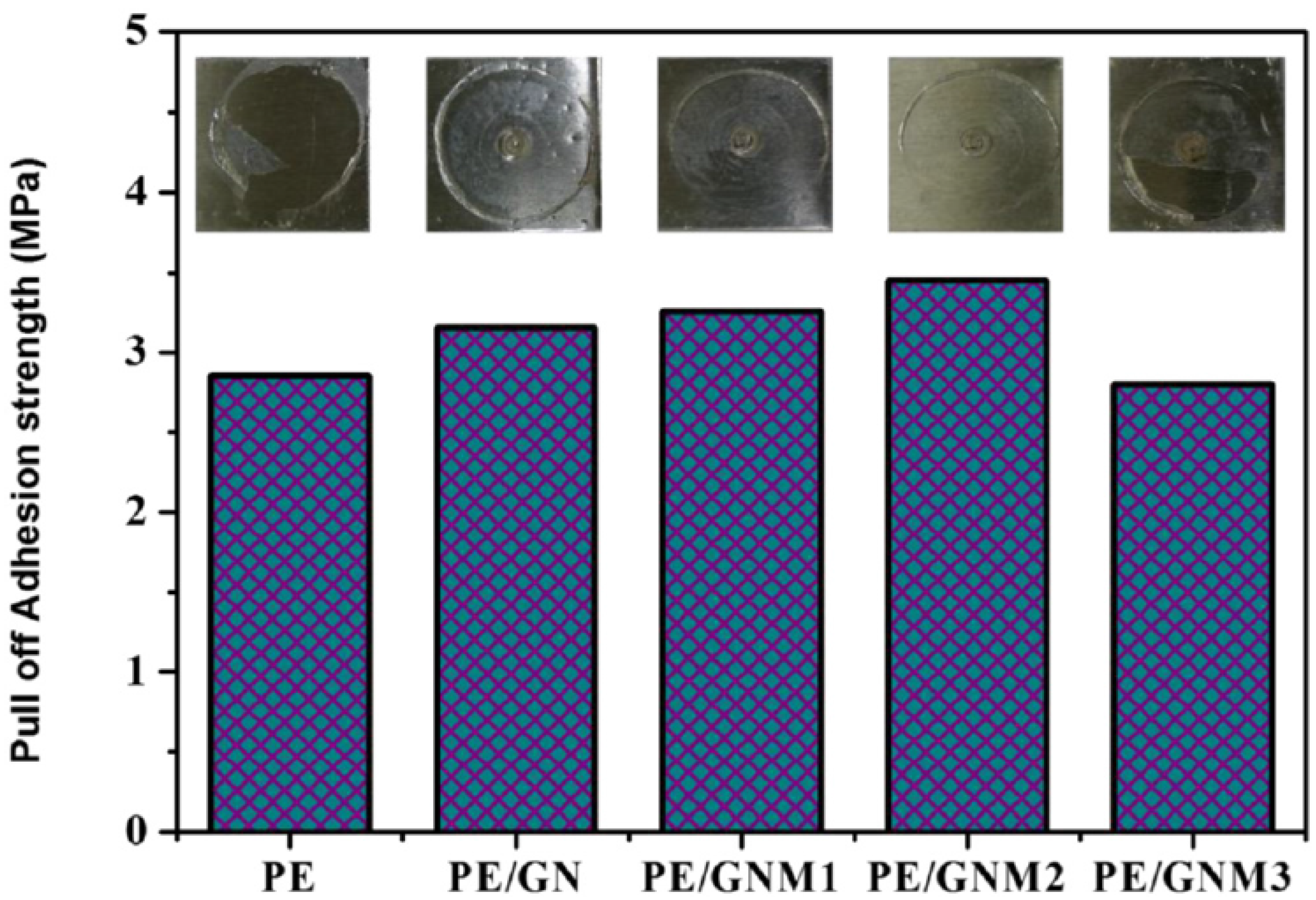


| Sample | Corrosion Spot Count |
|---|---|
| A | about 38 |
| B | about 15 |
| C | about 8 |
| D | about 6 |
| E | about 4 |
| Different Specimens | Coefficient of Friction | |
|---|---|---|
| 316L | Ti6Al4V Ball | |
| Ti6Al4V | 1.84 | 1.90 |
| NT | 1.42 | 1.78 |
| NTs | 1.38 | 1.75 |
| CN-T | 0.44 | 1.71 |
| CN-NT | 0.54 | 0.95 |
| CN-NTs | 0.41 | 0.90 |
| Specimens | Ecorr (mV) | Icorr (nA/cm2) | Eb (mV) |
|---|---|---|---|
| Ni–P | −66 | 2369 | – |
| IBD/N2 1.5 nm | 204 | 23.33 | – |
| IBD/N2 2.0 nm | 197 | 10.23 | – |
| IBD/N2 2.5 nm | 273 | 3.98 | 525 |
| IBD/N2 3.0 nm | 295 | 3.07 | 572 |
| Coating’s Type | Deposition Method/Substrate | Dopant/Concentration/Form of Coating | Corrosive Solution | Electrochemical Technique Used | Remarks and Main Results | Ref. |
|---|---|---|---|---|---|---|
| g-C3N4/Graphene oxide in epoxy coating. GO@CN nanohybrids are chemically functionalized with silane (F-GO@CN) | Solution mixing method through probe-sonication and mechanical mixing/carbon steel | 0.1 wt% F-GO@CN nanohybrids | 5 wt% NaCl | EIS and salt spray test | The epoxy coating loaded with 0.1 wt% silane-functionalized GO@CN nanohybrid has the highest chemical stability and corrosion resistance. | [15] |
| g-C3N4 prepared at different temperatures (400, 450, and 500 °C) | one-step chemical vapor deposition method (OS-CVD)/AZ31B Mg | Coating is prepared at different temperatures (400, 450, and 500 °C) | phosphate-buffered saline (PBS) solution | EIS, PDP, and immersion test | The corrosion resistance and biocompatibility of AZ31 B Mg alloy were significantly improved by g-C3N4 coating. The highest protection was at 500 °C. | [45] |
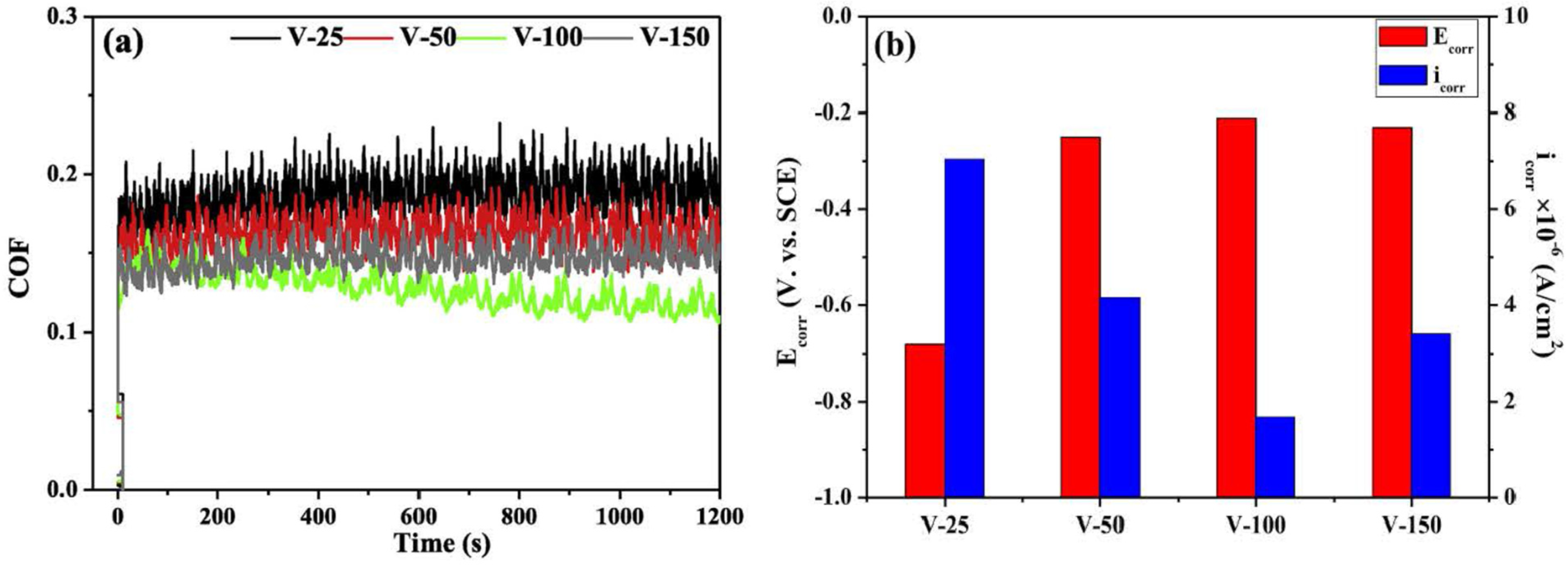
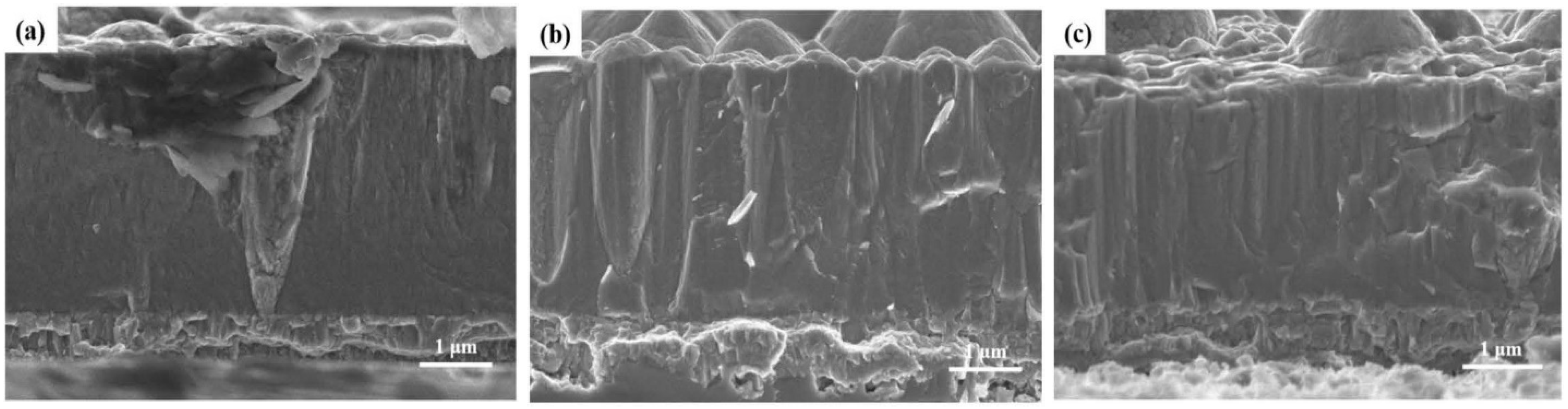
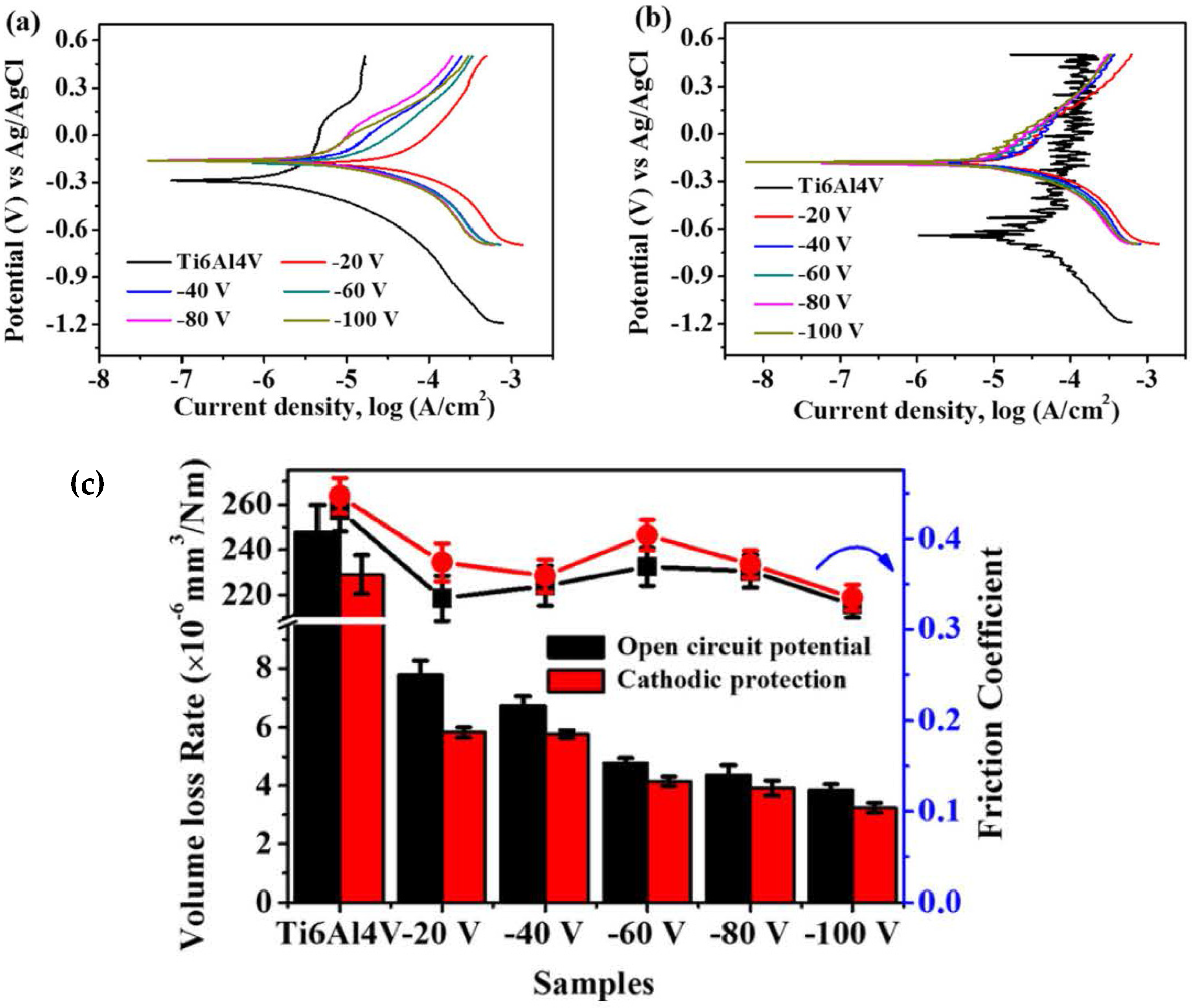
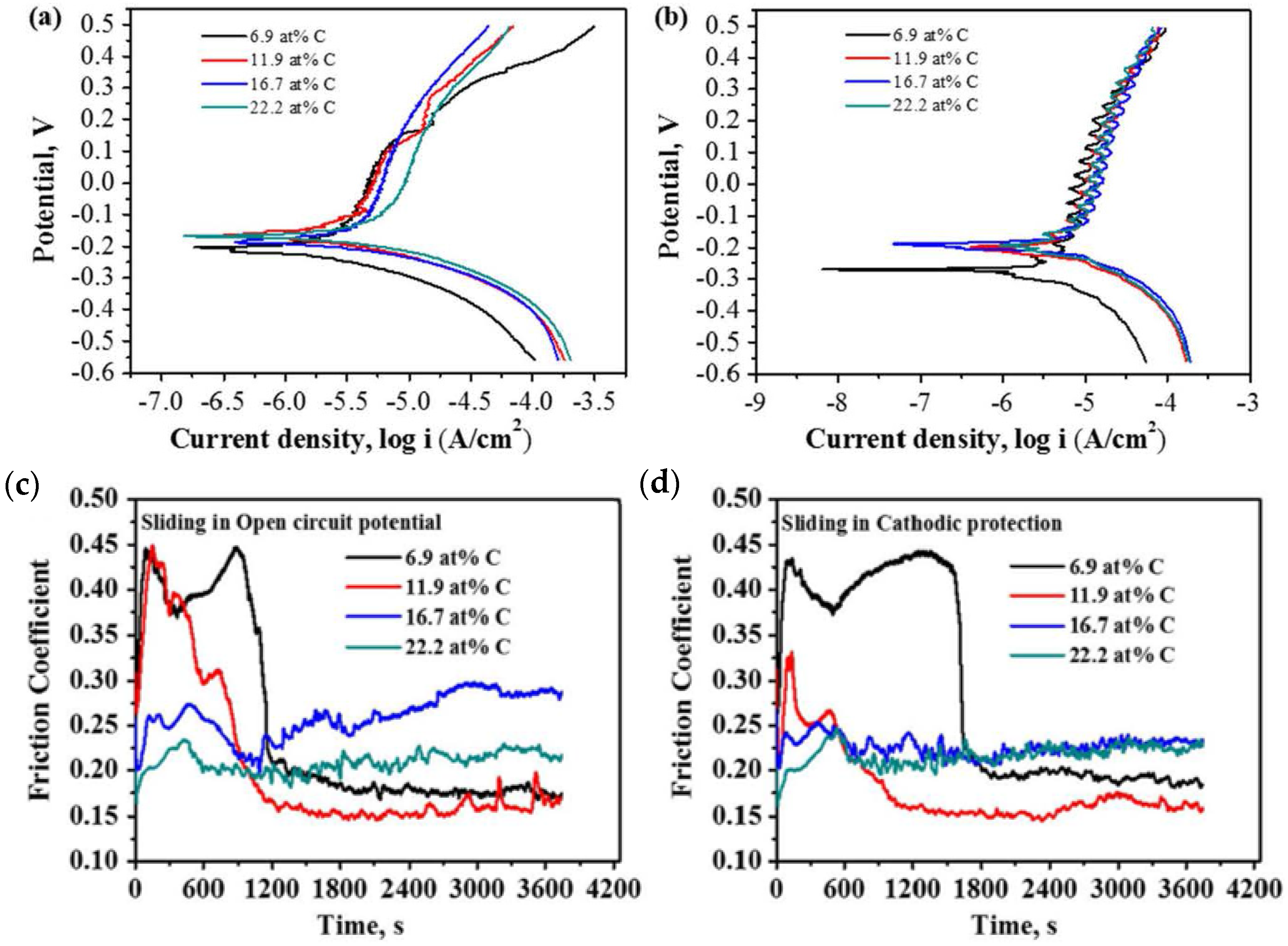
| VCN coatings | Multi-arc ion plating technology/Si and stainless steel | Coating was prepared under various bias voltages, −25 to −100 V) | Artificial seawater | PDP | The V-50, V-100, and V-150 coatings significantly reduced corrosion current density compared to the V-25 coating, demonstrating that increasing the bias voltage enhances the corrosion resistance of VCN coatings. | [91] |
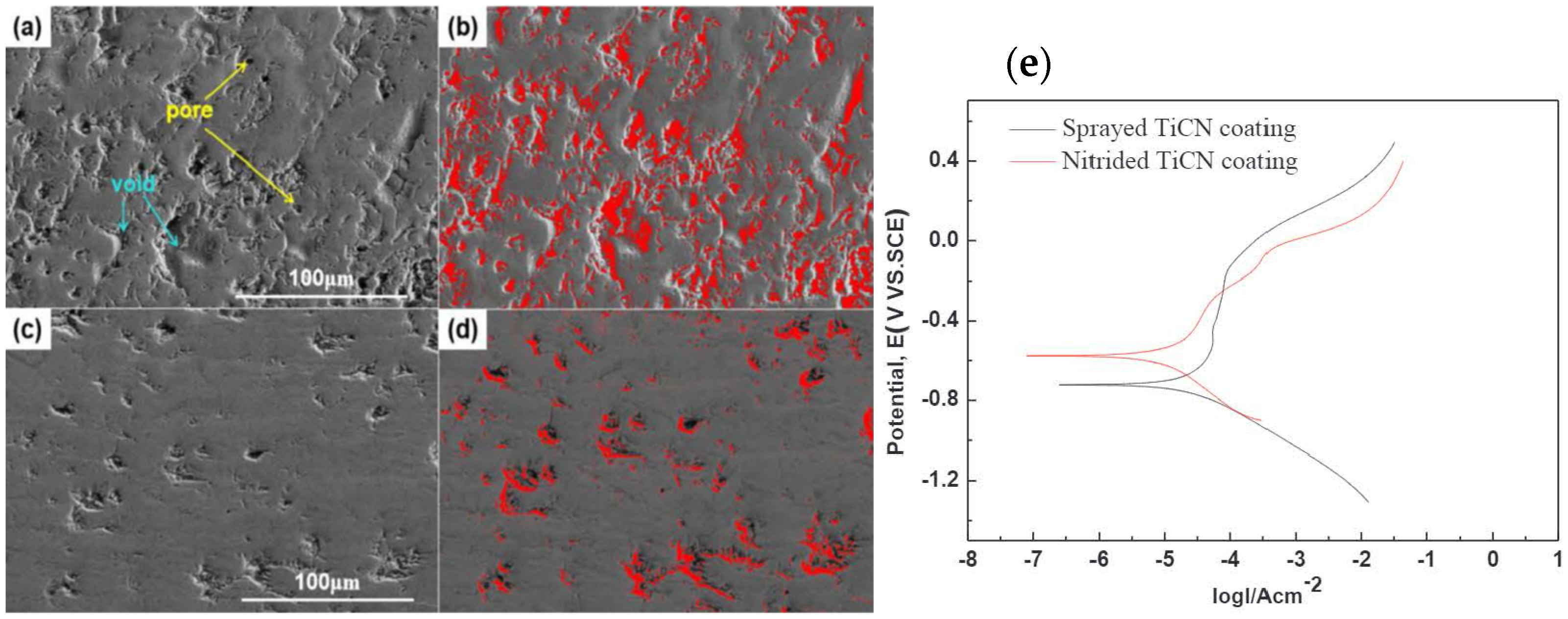
| TiCN ceramic coatings. | Reactive plasma spraying (RPS)/carbon steel | Sprayed and nitride TiCN coating | Seawater | PDP | The corrosion resistance of nitrided TiCN coatings was better than that of the sprayed TiCN coating, which was mainly due to the compact structure and the phase change resulted from nitridation. | [102] |
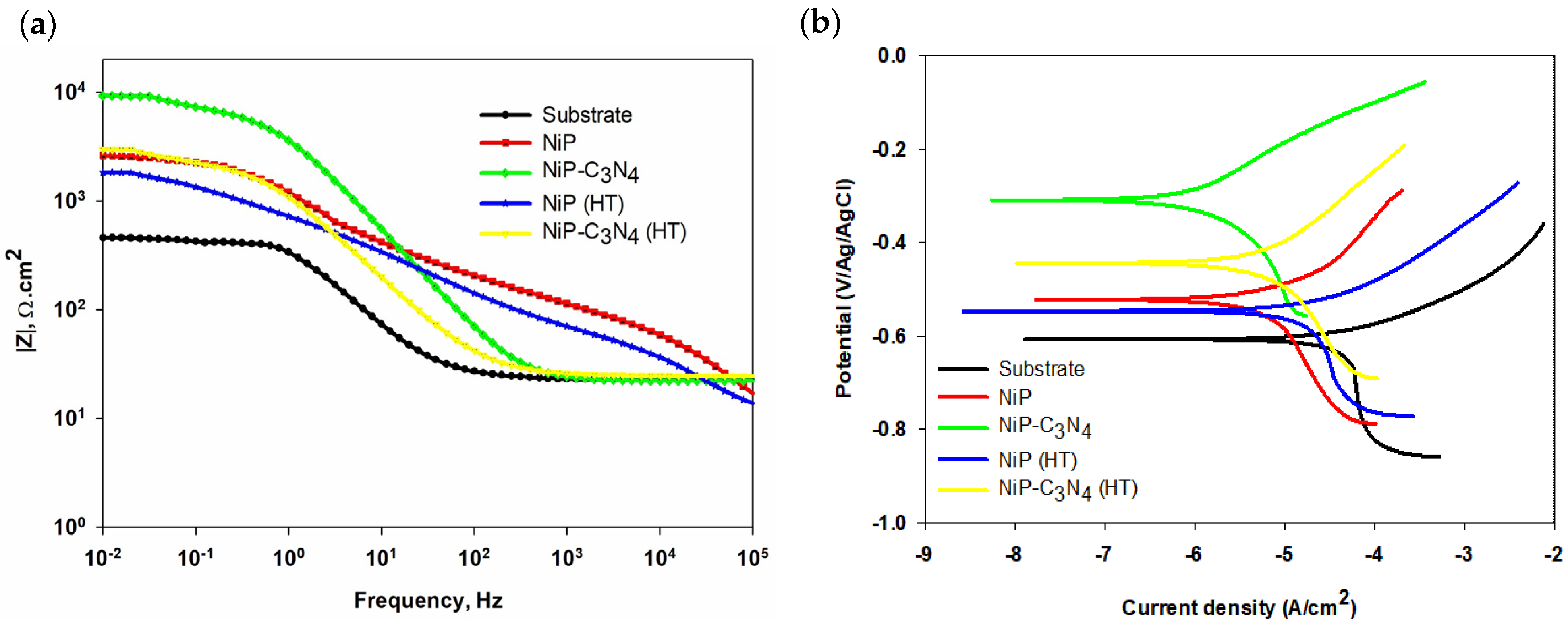
| NiP-C3N4 | Electroless deposition/API X100 carbon steel | 0.5 g of C3N4/NiP, with and without heat treatment | 3.5 wt% NaCl | EIS and PDP | The microhardness and corrosion resistance of the as-plated nanocomposite and the heat-treated nanocomposite coating were significantly enhanced compared to the Ni-P. | [118] |
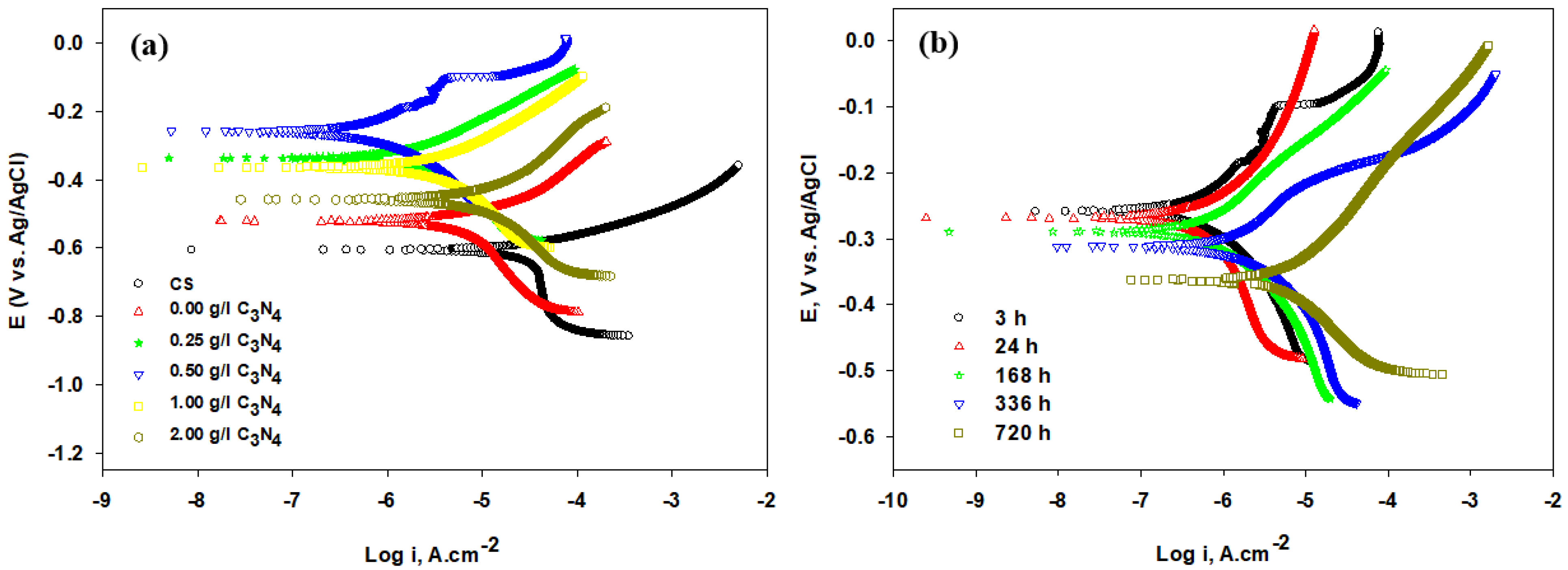
| NiP-C3N4 | Electroless deposition/API X100 carbon steel | 0.25, 0.50, 1.0, and 2.0 g L−1 C3N4 | 3.5 wt% NaCl | EIS and PDP | An electroless bath of 0.5 g L−1 C3N4 offered a nanocomposite coating with the highest microhardness and superior corrosion resistance (96%), which decreased gradually, losing about only 2% after one week and 20% after one month of immersion time. | [119] |
| Ni/g-C3N4 | Electrodeposition/carbon steel | 0.02, 0.05, 0.1, and 0.2 C3N4 are loaded in Ni-coating | 3.5 wt% NaCl | Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP) techniques | 0.3 g/L g-C3N4 at 0.1 A/cm2 yielded Ni coatings with optimal corrosion resistance, wear performance, and hardness | [120] |
| Amine functionalized g-C3N4 (AF g-C3N4)/Epoxy coating | Spraying technique/mild steel | 0.05, 0.1, 0.3, 0.5, and 0.7 wt% of AF g-C3N4 | 3.5 wt% NaCl | EIS | The superior corrosion protection of nanocomposite coatings due to the barrier performance of AF g-C3N4. Meanwhile, an epoxy coating containing 0.5 wt% AF g-C3N4 shows the highest corrosion protection (10,650 Ω·cm2) compared to a pure epoxy sample (913 Ω cm2). | [124] |
| polystyrene/g-C3N4 (PS/g-C3N4) | One-step immersion method/Copper | 2.5, 3.0, 3.5, 4.0 and 4.5 mg mL−1 C3N4 | 3.5 wt% NaCl | EIS and PDP | The optimal concentration of g-C3N4 for achieving favorable corrosion resistance was determined to be 3.5 mg/mL. Additionally, a preparation time of 15 min was ideal for forming a uniform PS/g-C3N4 coating on copper. This coating demonstrated excellent anticorrosion performance attributed to the synergistic effect between PS and g-C3N4. Even after 216 h of immersion, the coating showed minimal corrosion. | [126] |
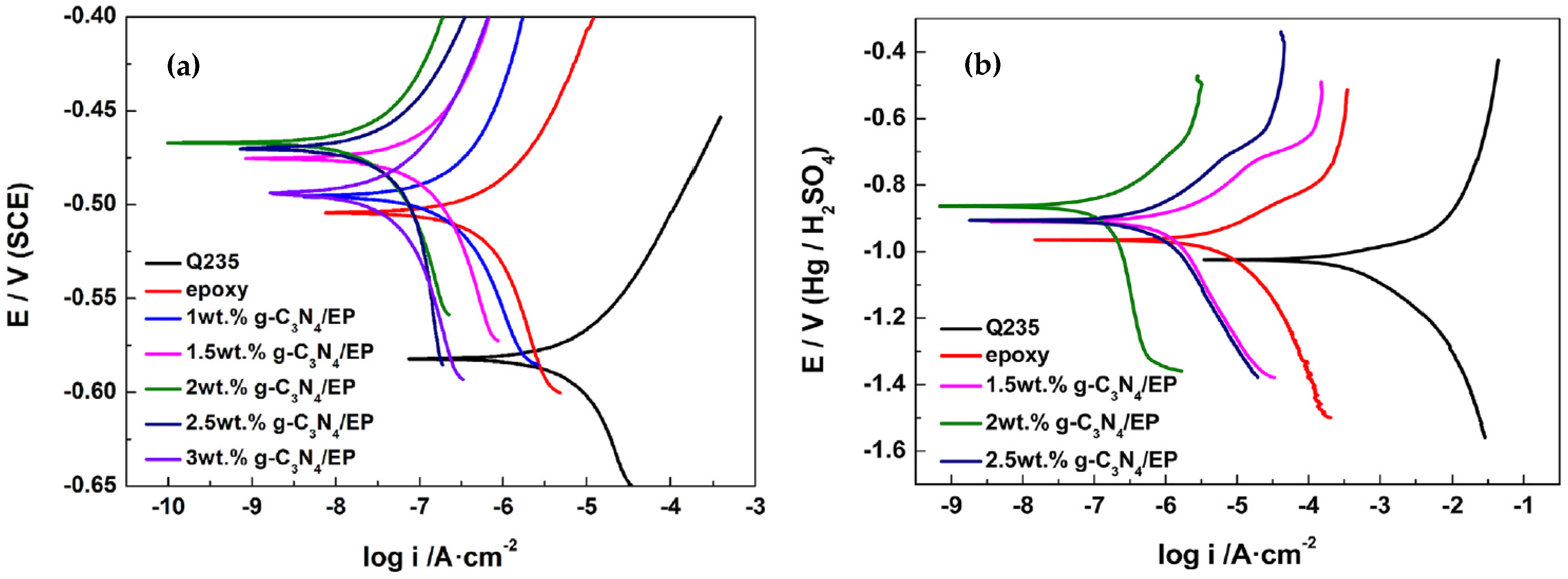
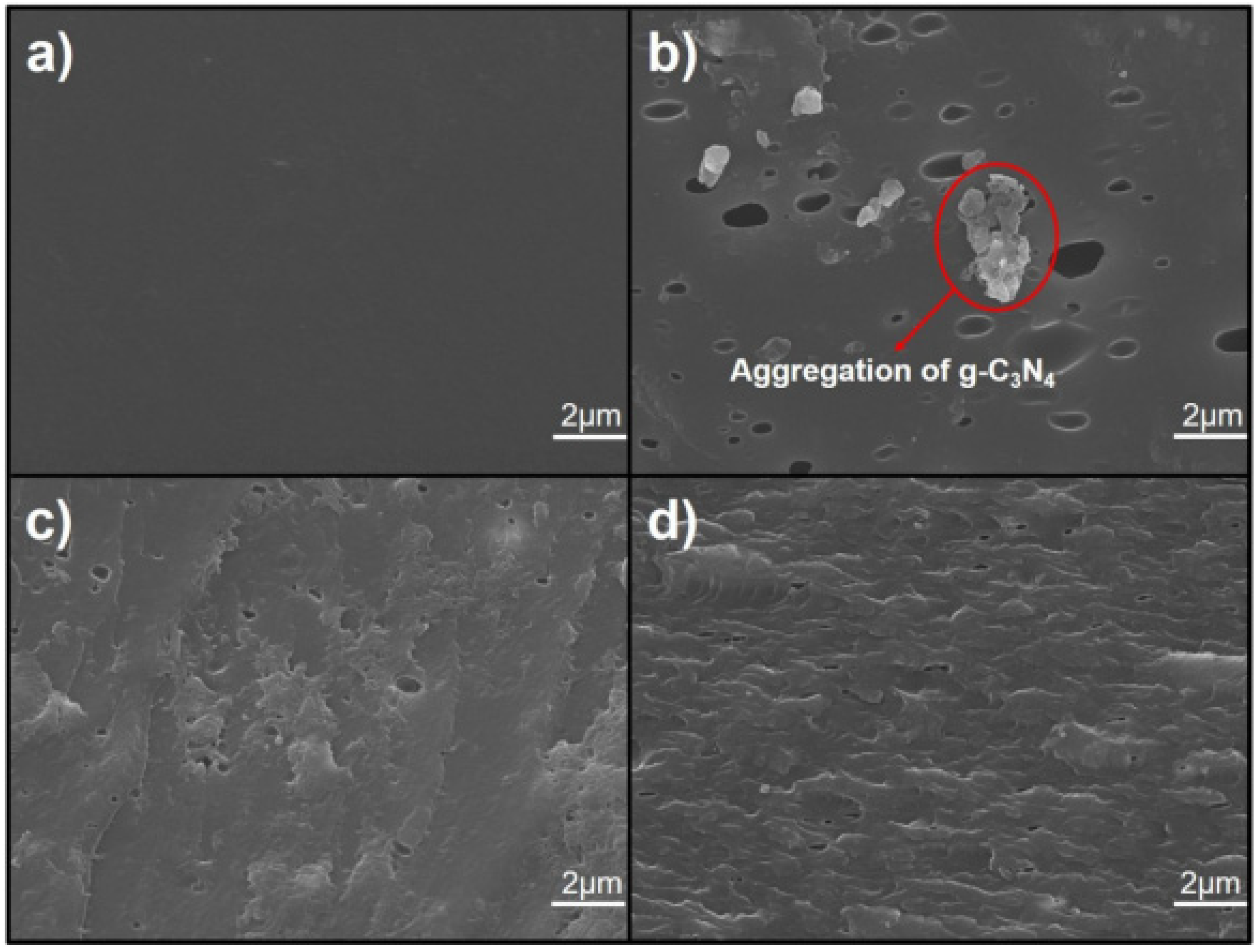
| g-C3N4/epoxy nanocomposite coatings | Spraying/Q235 steel | 1.00, 1.50, 2.00, 2.50, and 3.00 wt% C3N4 | 0.1 M H2SO4 and 3.5% NaCl solutions | EIS and PDP | Incorporating 2 wt% of g-C3N4 nanosheets into the epoxy coating results in optimal corrosion resistance. This is demonstrated by an increase in the electrochemical impedance of epoxy by two orders of magnitude in a 3.5 wt% NaCl solution and by one order of magnitude in a 0.1 M H2SO4 solution. | [127] |
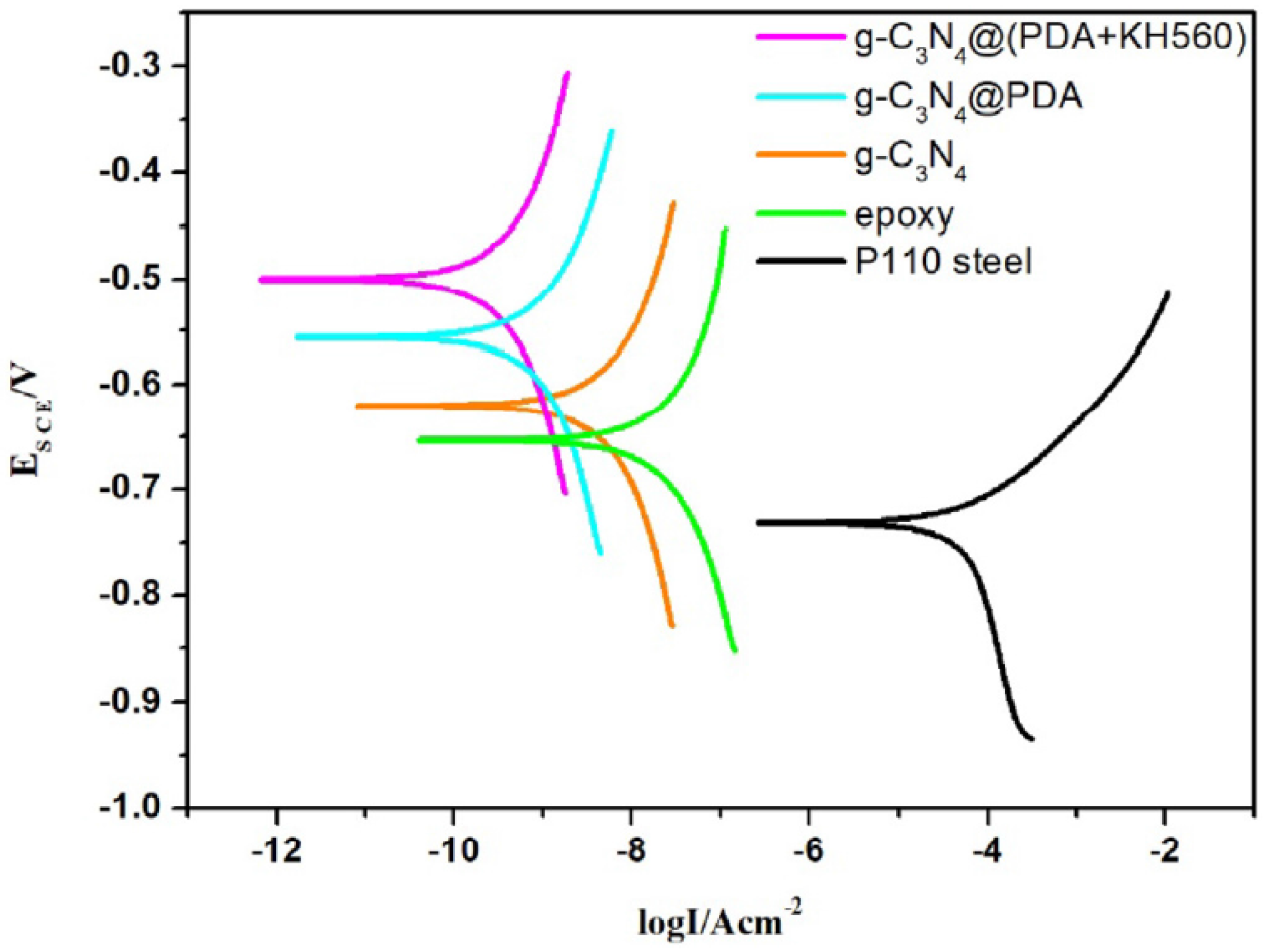
| polydopamine (DA) and silane coupling agent (KH560)/g-C3N4 /Epoxy [g C3N4@(PDA+KH560)] on Epoxy coating | Mechanical spreading/P110 steel substrates | Epoxy g-C3N4 g-C3N4@PDA g-C3N4@PDA+KH560 | NaCl | EIS, PDP, and salt spray | Polarization measurements reveal that incorporating dopamine and a silane coupling agent into the composite significantly enhances the corrosion resistance of P110 steel, achieving an almost complete protection efficiency of 99.99%. | [128] |
| g-C3N4/MoOx inside epoxy. GN-MoOx nanocomposite | Using drawdown bar coater at a constant rate/AA2024 Al alloy | 1, 3, and 5 wt% of GNMoOx | 3.5% NaCl solution | Different electrochemical techniques | Maximum protection is achieved with epoxy coatings reinforced by 3 wt% GN/MoOx. | [130] |
| TiO2 nanotube/g-C3N4)/Epoxy | Brush painting/carbon steel | 0.1, 0.3, and 0.5 wt% TiO2/g-C3N4 hybrid in epoxy | 3.5% NaCl solution | EIS | 0.3 wt% hybrids have higher corrosion resistance than that of the neat coating, and silane-functionalized nanofillers more effectively enhance the corrosion resistance. | [131] |
| g-C3N4-ZnO2 nanocomposites/Epoxy (EP) CN-ZnO/EP | Drawdown bar coater/carbon steel | 5 wt% of each GCN, ZnO, and GCN/ZnO in epoxy | 3.5% NaCl | EIS, Salt spray | Water uptake studies demonstrated that incorporating GCN/ZnO into the PE matrix enhanced its barrier properties by effectively filling micro-defects and pores. | [132] |
| g-C3N4@SiO2 nanocomposites. g-C3N4@SiO2/EP | Deposition using compressed air spray gun/carbon steel | 0.1, 0.3, and 0.5 wt% g-C3N4@SiO2 | 3.5% NaCl | EIS, PDP, and salt spray | The impedance value of the g-C3N4@SiO2 coating increased by 969% compared to the neat epoxy coating. 0.3 wt% g-C3N4@SiO2 provided the most effective shielding and achieved the highest corrosion resistance. | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayyad, E.M.; Nabhan, F.; Abdullah, A.M. Focused Review on Graphitic Carbon Nitride (g-C3N4) in Corrosion and Erosion Applications. Coatings 2024, 14, 1596. https://doi.org/10.3390/coatings14121596
Fayyad EM, Nabhan F, Abdullah AM. Focused Review on Graphitic Carbon Nitride (g-C3N4) in Corrosion and Erosion Applications. Coatings. 2024; 14(12):1596. https://doi.org/10.3390/coatings14121596
Chicago/Turabian StyleFayyad, Eman M., Fatma Nabhan, and Aboubakr M. Abdullah. 2024. "Focused Review on Graphitic Carbon Nitride (g-C3N4) in Corrosion and Erosion Applications" Coatings 14, no. 12: 1596. https://doi.org/10.3390/coatings14121596
APA StyleFayyad, E. M., Nabhan, F., & Abdullah, A. M. (2024). Focused Review on Graphitic Carbon Nitride (g-C3N4) in Corrosion and Erosion Applications. Coatings, 14(12), 1596. https://doi.org/10.3390/coatings14121596





