Extracting the Optical Constants of Partially Absorbing TiO2 ALD Films
Abstract
:1. Introduction
2. Experimental Methods
2.1. Film Deposition
2.2. Sample Composition, Morphology, and Transmission
2.3. Spectroscopic Ellipsometry
3. Results
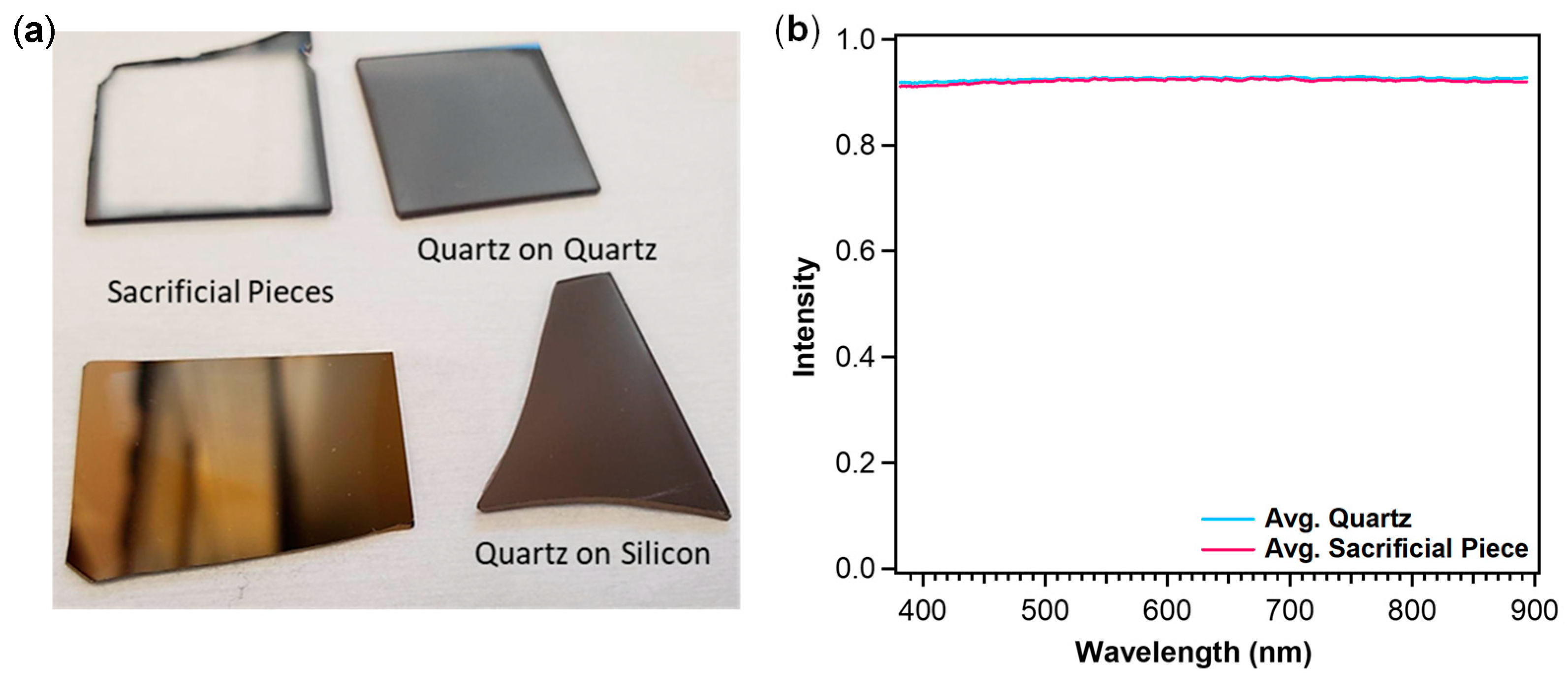
3.1. Producing ALD Films with Single-Side Deposition
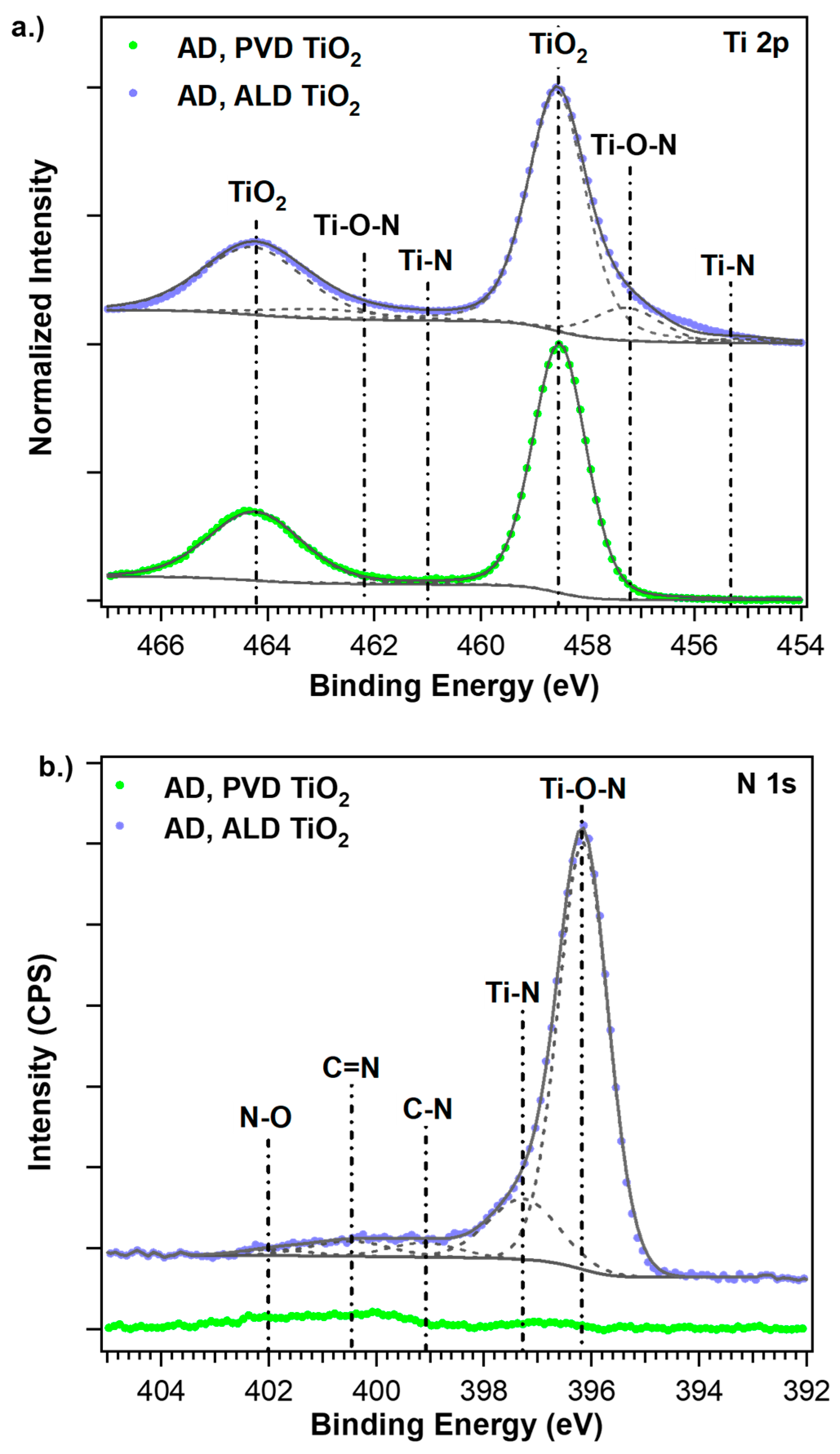
3.2. Composition and Morphology of the Films
3.3. UV–Vis Absorption of the Films
3.4. Extracting the Optical Functions of the PVD TiO2 Films
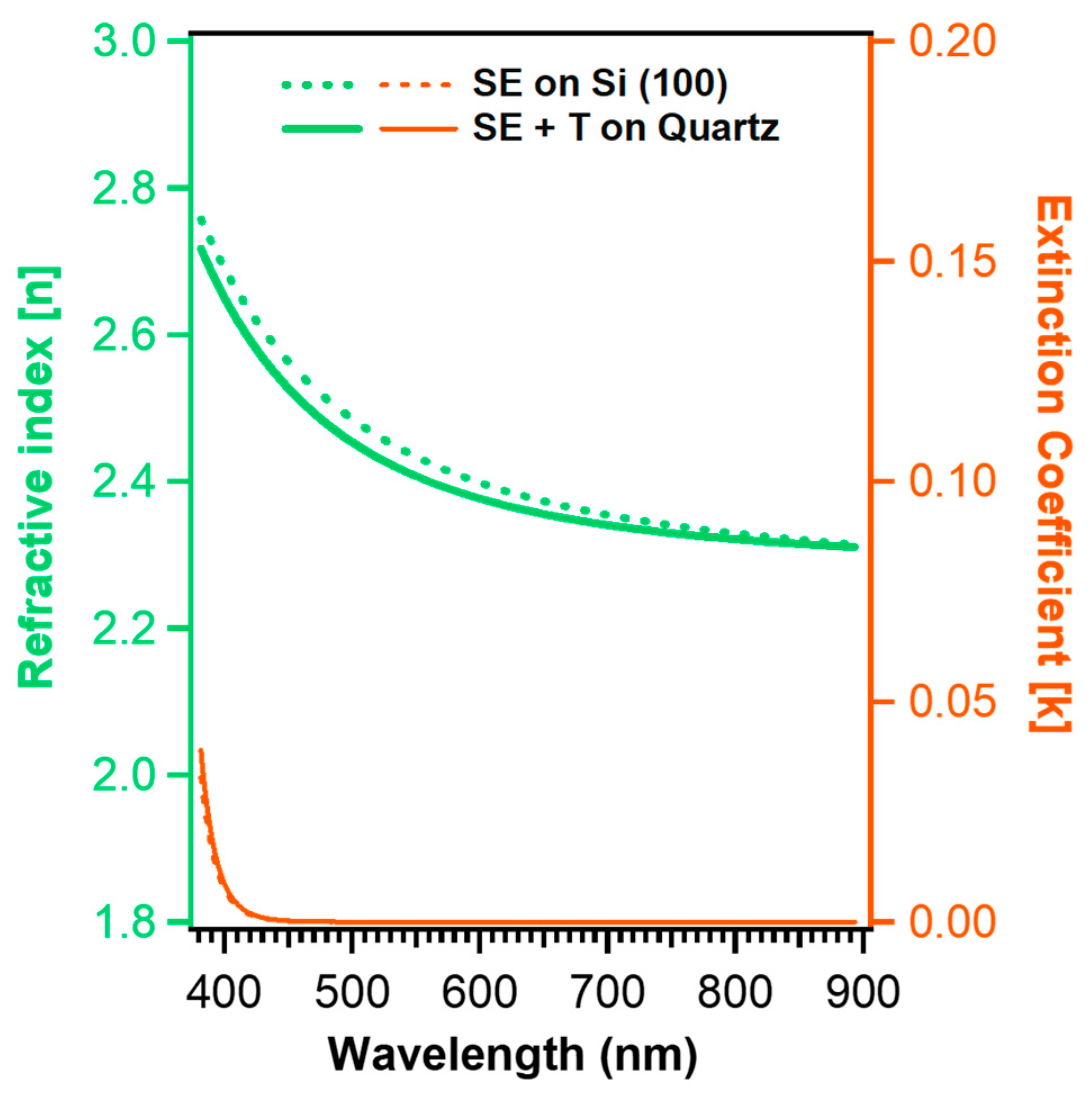
3.5. Extracting the Optical Functions of the ALD TiO2 Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Marks, T.J.; Facchetti, A. Metal Oxides for Optoelectronic Applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.L. Chemical Vapour Deposition of Coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Kaliyannan, G.V.; Palanisamy, S.V.; Priyanka, E.B.; Thangavel, S.; Sivaraj, S.; Rathanasamy, R. Investigation on Sol-Gel Based Coatings Application in Energy Sector—A Review. Mater. Today Proc. 2021, 45, 1138–1143. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mourad, A.-H.I.; Pervez, H.; Surkatti, R. Recent Developments in Multifunctional Coatings for Solar Panel Applications: A Review. Sol. Energy Mater. Sol. Cells 2019, 189, 75–102. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Detavernier, C.; Dendooven, J.; Sree, S.P.; Ludwig, K.F.; Martens, J.A. Tailoring Nanoporous Materials by Atomic Layer Deposition. Chem. Soc. Rev. 2011, 40, 5242–5253. [Google Scholar] [CrossRef]
- Ristok, S.; Flad, P.; Giessen, H. Atomic Layer Deposition of Conformal Anti-Reflective Coatings on Complex 3D Printed Micro-Optical Systems. Opt. Mater. Express 2022, 12, 2063. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Zhou, B.; Shen, J. Optical Constants of Crystallized TiO2 Coatings Prepared by Sol-Gel Process. Materials 2013, 6, 2819–2830. [Google Scholar] [CrossRef]
- Shamma, K.; Aldwayyan, A.; Albrithen, H.; Alodhayb, A. Exploiting the Properties of TiO2 Thin Films as a Sensing Layer on (MEMS)-Based Sensors for Radiation Dosimetry Applications. AIP Adv. 2021, 11, 025209. [Google Scholar] [CrossRef]
- Hilfiker, J.N.; Singh, N.; Tiwald, T.; Convey, D.; Smith, S.M.; Baker, J.H.; Tompkins, H.G. Survey of Methods to Characterize Thin Absorbing Films with Spectroscopic Ellipsometry. Thin Solid Films 2008, 516, 7979–7989. [Google Scholar] [CrossRef]
- Rafieian, D.; Ogieglo, W.; Savenije, T.; Lammertink, R.G.H. Controlled Formation of Anatase and Rutile TiO2 Thin Films by Reactive Magnetron Sputtering. AIP Adv. 2015, 5, 097168. [Google Scholar] [CrossRef]
- Zeiss, C. Application of the Tauc-Lorentz Formulation to the Interband Absorption of Optical Coating Materials. Appl. Opt. 2001, 41, 3137–3141. [Google Scholar]
- Wang, L.; Liu, H.; Li, S.; Jiang, C.; Ji, Y.; Chen, D. Study on the Composite Dispersion Model of Optical Constants of Metal-Oxide Films in the Range from Ultraviolet to near Infrared. Optik 2018, 168, 892–900. [Google Scholar] [CrossRef]
- Hilfiker, J.N.; Linford, M.R. Fitting the Spectroscopic Ellipsometry Data from a Rather Thick (Organic?) Film on Fused Silica. Vac. Technol. Coat. 2021, 22, 34–38. [Google Scholar]
- Hilfiker, J.N.; Linford, M.R. Combining Spectroscopic Ellipsometry and Transmission Spectrophotometric Data for the Analysis of Thin Metal Films. Vac. Technol. Coat. 2022, 23, 33–37. [Google Scholar]
- Hackley, J.C.; Gougousi, T.; Demaree, J.D. Nucleation of HfO2 Atomic Layer Deposition Films on Chemical Oxide and H-Terminated Si. J. Appl. Phys. 2007, 102, 034101. [Google Scholar] [CrossRef]
- Ylilammi, M. Monolayer Thickness in Atomic Layer Deposition. Thin Solid Films 1996, 279, 124–130. [Google Scholar] [CrossRef]
- Becker, J.S.; Kim, E.; Gordon, R.G. Atomic Layer Deposition of Insulating Hafnium and Zirconium Nitrides. Chem. Mater. 2004, 16, 3497–3501. [Google Scholar] [CrossRef]
- Hausmann, D.M.; de Rouffignac, P.; Smith, A.; Gordon, R.; Monsma, D. Highly Conformal Atomic Layer Deposition of Tantalum Oxide Using Alkylamide Precursors. Thin Solid Films 2003, 443, 1–4. [Google Scholar] [CrossRef]
- Synowicki, R.A. Suppression of Backside Reflections from Transparent Substrates. Phys. Status Solidi C 2008, 5, 1085–1088. [Google Scholar] [CrossRef]
- J.A. Woollam Co., Inc. CompleteEASE Data Analysis Manual 2011; J.A. Woollam Co., Inc.: Lincoln, NE, USA, 2011. [Google Scholar]
- Stefanov, P.; Shipochka, M.; Stefchev, P.; Raicheva, Z.; Lazarova, V.; Spassov, L. XPS Characterization of TiO2 Layers Deposited on Quartz Plates. J. Phys. Conf. Ser. 2008, 100, 012039. [Google Scholar] [CrossRef]
- Driessen, J.P.A.M.; Schoonman, J.; Jensen, K.F. Infrared Spectroscopic Study of Decomposition of Ti(N(CH3)2)4. J. Electrochem. Soc. 2001, 148, G178–G184. [Google Scholar] [CrossRef]
- Song, X.; Gopireddy, D.; Takoudis, C.G. Characterization of Titanium Oxynitride Films Deposited by Low Pressure Chemical Vapor Deposition Using Amide Ti Precursor. Thin Solid Films 2008, 516, 6330–6335. [Google Scholar] [CrossRef]
- Khwansungnoen, P.; Chaiyakun, S.; Rattana, T. Room Temperature Sputtered Titanium Oxynitride Thin Films: The Influence of Oxygen Addition. Thin Solid Films 2020, 711, 138269. [Google Scholar] [CrossRef]
- Olayo, M.G.; Alvarado, E.J.; González-Torres, M.; Gómez, L.M.; Cruz, G.J. Quantifying Amines in Polymers by XPS. Polym. Bull. 2024, 81, 2319–2328. [Google Scholar] [CrossRef]
- Shard, A.G. Practical Guides for X-Ray Photoelectron Spectroscopy: Quantitative XPS. J. Vac. Sci. Technol. A 2020, 38, 041201. [Google Scholar] [CrossRef]
- Kuis, R.; Gougousi, T.; Basaldua, I.; Burkins, P.; Kropp, J.A.; Johnson, A.M. Engineering of Large Third-Order Nonlinearities in Atomic Layer Deposition Grown Nitrogen-Enriched TiO2. ACS Photonics 2019, 6, 2966–2973. [Google Scholar] [CrossRef]
- Gougousi, T.; Parsons, G. Postdeposition Reactivity of Sputter-Deposited High-Dielectric-Constant Films with Ambient H2O and Carbon-Containing Species. J. Appl. Phys. 2004, 95, 1391–1396. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Sammelselg, V. Morphology and Structure of TiO2 Thin Films Grown by Atomic Layer Deposition. J. Cryst. Growth 1995, 148, 268–275. [Google Scholar] [CrossRef]
- Kim, S.K.; Hoffmann-Eifert, S.; Reiners, M.; Waser, R. Relation Between Enhancement in Growth and Thickness-Dependent Crystallization in ALD TiO2 Thin Films. J. Electrochem. Soc. 2010, 158, D6. [Google Scholar] [CrossRef]
- Schöche, S.; Hofmann, T.; Korlacki, R.; Tiwald, T.E.; Schubert, M. Infrared Dielectric Anisotropy and Phonon Modes of Rutile TiO2. J. Appl. Phys. 2013, 113, 164102. [Google Scholar] [CrossRef]
- Henegar, A.J.; Gougousi, T. Stability and Surface Reactivity of Anatase TiO2 Films. ECS J. Solid State Sci. Technol. 2015, 4, P298–P304. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Yahia, I.S.; Wahab, L.A.; Fadel, M.; Afifi, M.A. Determination and Analysis of Dispersive Optical Constant of TiO2 and Ti2O3 Thin Films. Appl. Surf. Sci. 2006, 252, 8163–8170. [Google Scholar] [CrossRef]
- Ghrairi, N.; Bouaicha, M. Structural, Morphological, and Optical Properties of TiO2 Thin Films Synthesized by the Electro Phoretic Deposition Technique. Nanoscale Res. Lett. 2012, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Sreemany, M.; Sen, S. A Simple Spectrophotometric Method for Determination of the Optical Constants and Band Gap Energy of Multiple Layer TiO2 Thin Films. Mater. Chem. Phys. 2004, 83, 169–177. [Google Scholar] [CrossRef]
- Demeter, A.; Samoila, F.; Tiron, V.; Stanescu, D.; Magnan, H.; Straticiuc, M.; Burducea, I.; Sirghi, L. Visible-Light Photocatalytic Activity of TiOxNy Thin Films Obtained by Reactive Multi-Pulse High Power Impulse Magnetron Sputtering. Surf. Coat. Technol. 2017, 324, 614–619. [Google Scholar] [CrossRef]
- Mosaddeq-ur-Rahman, M.; Yu, G.; Soga, T.; Jimbo, T.; Ebisu, H. Umeno Refractive Index and Degree of Inhomogeneity of Nanocrystalline TiO2 Thin Films: Effects of Substrate and Annealing Temperature. J. Appl. Phys. 2000, 88, 9. [Google Scholar] [CrossRef]
- Kasikov, A.; Aarik, J.; Mändar, H.; Moppel, M.; Pärs, M.; Uustare, T. Refractive Index Gradients in TiO2 Thin Films Grown by Atomic Layer Deposition. J. Phys. Appl. Phys. 2006, 39, 54–60. [Google Scholar] [CrossRef]
- Jolivet, A.; Labbé, C.; Frilay, C.; Debieu, O.; Marie, P.; Horcholle, B.; Lemarié, F.; Portier, X.; Grygiel, C.; Duprey, S.; et al. Structural, Optical, and Electrical Properties of TiO2 Thin Films Deposited by ALD: Impact of the Substrate, the Deposited Thickness and the Deposition Temperature. Appl. Surf. Sci. 2023, 608, 155214. [Google Scholar] [CrossRef]
- Saha, D.; Ajimsha, R.S.; Rajiv, K.; Mukherjee, C.; Gupta, M.; Misra, P.; Kukreja, L.M. Spectroscopic Ellipsometry Characterization of Amorphous and Crystalline TiO2 Thin Films Grown by Atomic Layer Deposition at Different Temperatures. Appl. Surf. Sci. 2014, 315, 116–123. [Google Scholar] [CrossRef]
- Kim, S.Y. Simultaneous Determination of Refractive Index, Extinction Coefficient, and Void Distribution of Titanium Dioxide Thin Film by Optical Methods. Appl. Opt. 1996, 35, 6703–6707. [Google Scholar] [CrossRef] [PubMed]
- Mardare, D.; Stancu, A. On the Optical Constants of TiO2 Thin Films. Ellipsometric Studies. Mater. Res. Bull. 2000, 35, 2017–2025. [Google Scholar] [CrossRef]
- Ali, S.; Magnusson, R.; Pshyk, O.; Birch, J.; Eklund, P.; Le Febvrier, A. Effect of O/N Content on the Phase, Morphology, and Optical Properties of Titanium Oxynitride Thin Films. J. Mater. Sci. 2023, 58, 10975–10985. [Google Scholar] [CrossRef]



| Sample Name | Number of Cycles | Temperature (°C) | Purge (s) |
|---|---|---|---|
| ALD 1 | 285 | 350 | 5 |
| ALD 2A, B | 350 | 350 | 5 |
| Sample Substrate | A | B | C | Model Thickness (nm) | Surface Roughness (nm) | n @ 632.8 nm | MSE | Thickness (Profilometer) (nm) |
|---|---|---|---|---|---|---|---|---|
| Silicon | 2.27 | 0.031 | 0.0058 | 91.1 ± 0.1 | 2.6 ± 0.1 | 2.38 | 7.9 | 96.0 ± 3.8 |
| Quartz | 2.28 | 0.015 | 0.0071 | 92.7 ± 0.1 | 4.0 ± 0.1 | 2.36 | 8.3 |
| Sample Name | SE + T Spot 1 (nm) | Profilometer Spot 1 (nm) | SE + T Spot 2 (nm) | Profilometer Spot 2 (nm) | SE + T Spot 3 (nm) | Profilometer Spot 3 (nm) |
|---|---|---|---|---|---|---|
| ALD 1 | 39.1 ± 0.3 | 41.8 ± 0.8 | 40.0 ± 0.1 | 41.2 ± 1.4 | N/A | N/A |
| ALD 2A | 49.9 ± 0.1 | 49.6 ± 0.9 | 49.1 ± 0.1 | 49.0 ± 0.4 | 48.2 ± 0.1 | 50.1 ± 0.6 |
| ALD 2B | 65.1 ± 0.1 | 67.7 ± 1.3 | 64.5 ± 0.1 | 62.2 ± 1.0 | 62.6 ± 0.1 | 61.6 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhary, N.K.; Gougousi, T. Extracting the Optical Constants of Partially Absorbing TiO2 ALD Films. Coatings 2024, 14, 1555. https://doi.org/10.3390/coatings14121555
Chowdhary NK, Gougousi T. Extracting the Optical Constants of Partially Absorbing TiO2 ALD Films. Coatings. 2024; 14(12):1555. https://doi.org/10.3390/coatings14121555
Chicago/Turabian StyleChowdhary, Nimarta Kaur, and Theodosia Gougousi. 2024. "Extracting the Optical Constants of Partially Absorbing TiO2 ALD Films" Coatings 14, no. 12: 1555. https://doi.org/10.3390/coatings14121555
APA StyleChowdhary, N. K., & Gougousi, T. (2024). Extracting the Optical Constants of Partially Absorbing TiO2 ALD Films. Coatings, 14(12), 1555. https://doi.org/10.3390/coatings14121555





