Effects of Bias Voltages on the Tribological Behaviors of DLC Coatings
Abstract
1. Introduction
2. Experiment Details
2.1. Preparation of DLC Coatings
2.2. Characterization and Analysis Methods
3. Results and Discussion
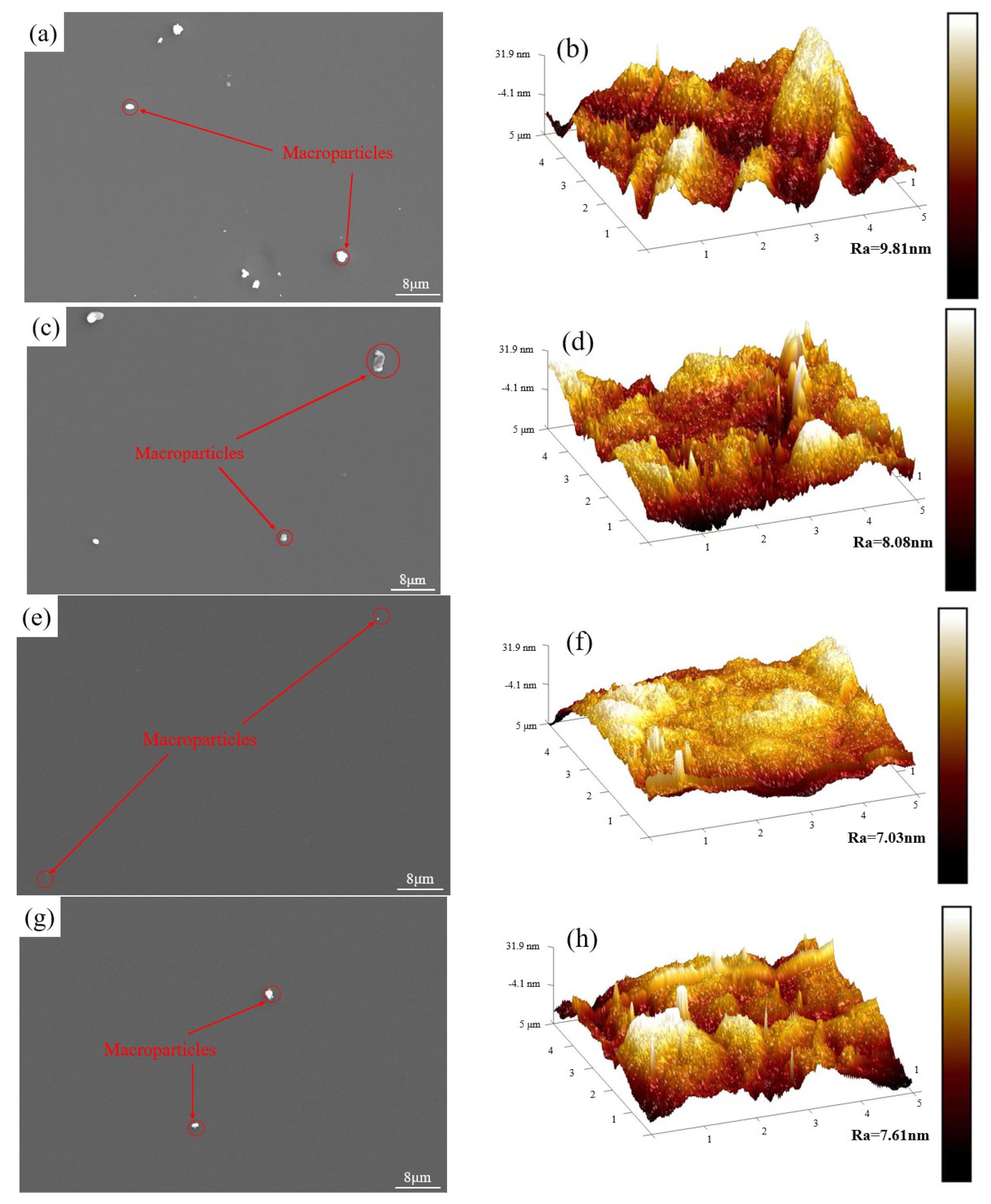
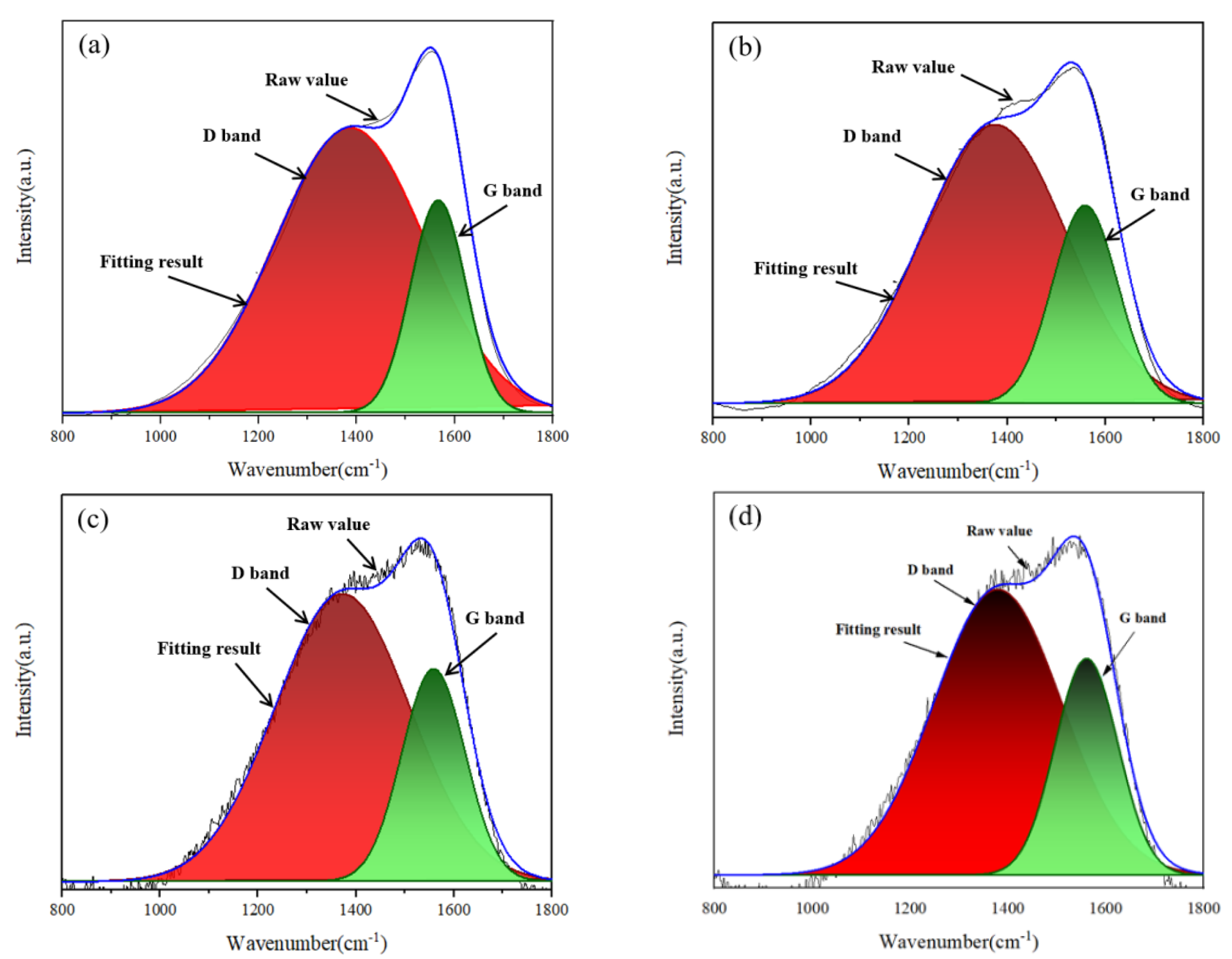
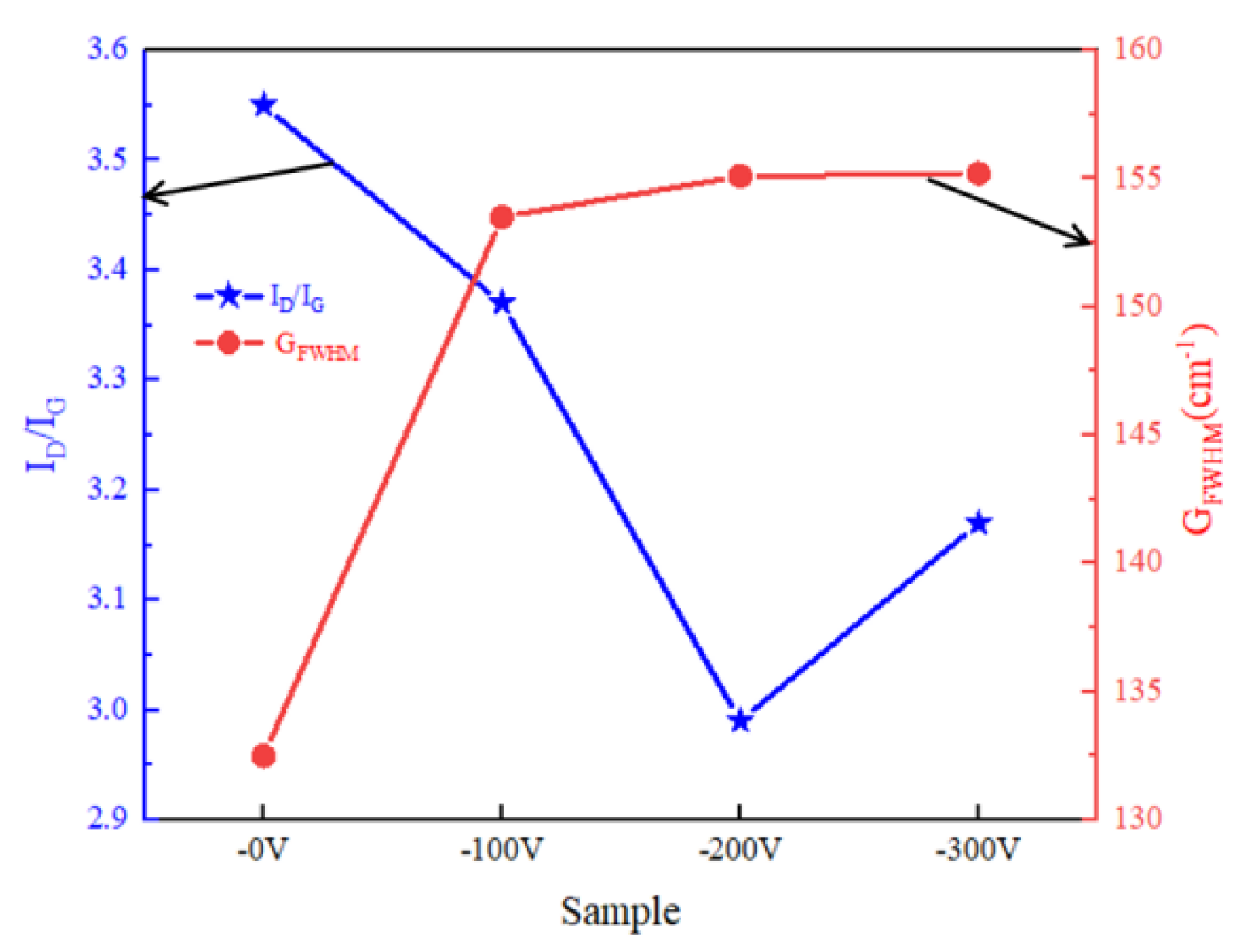
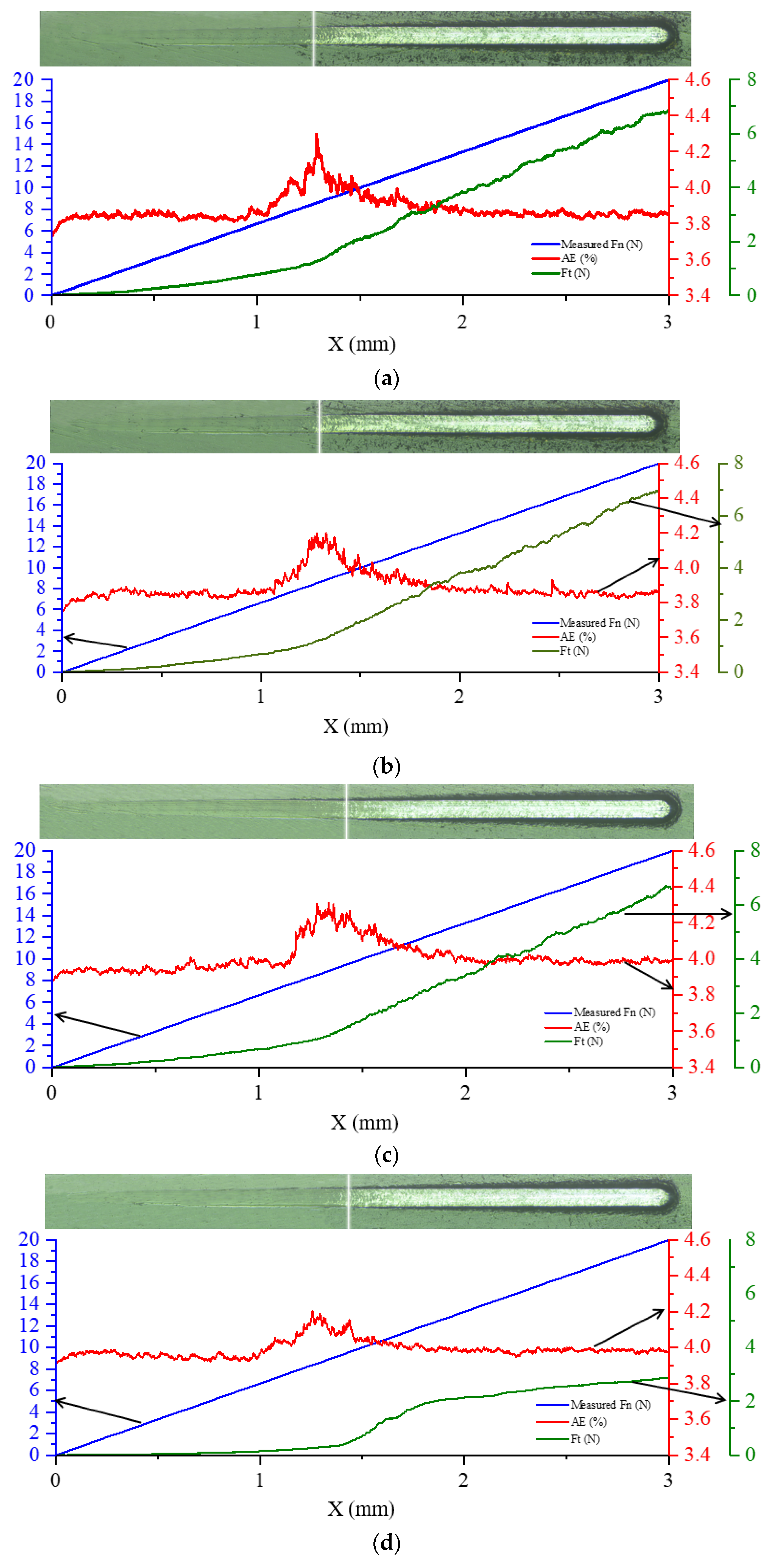
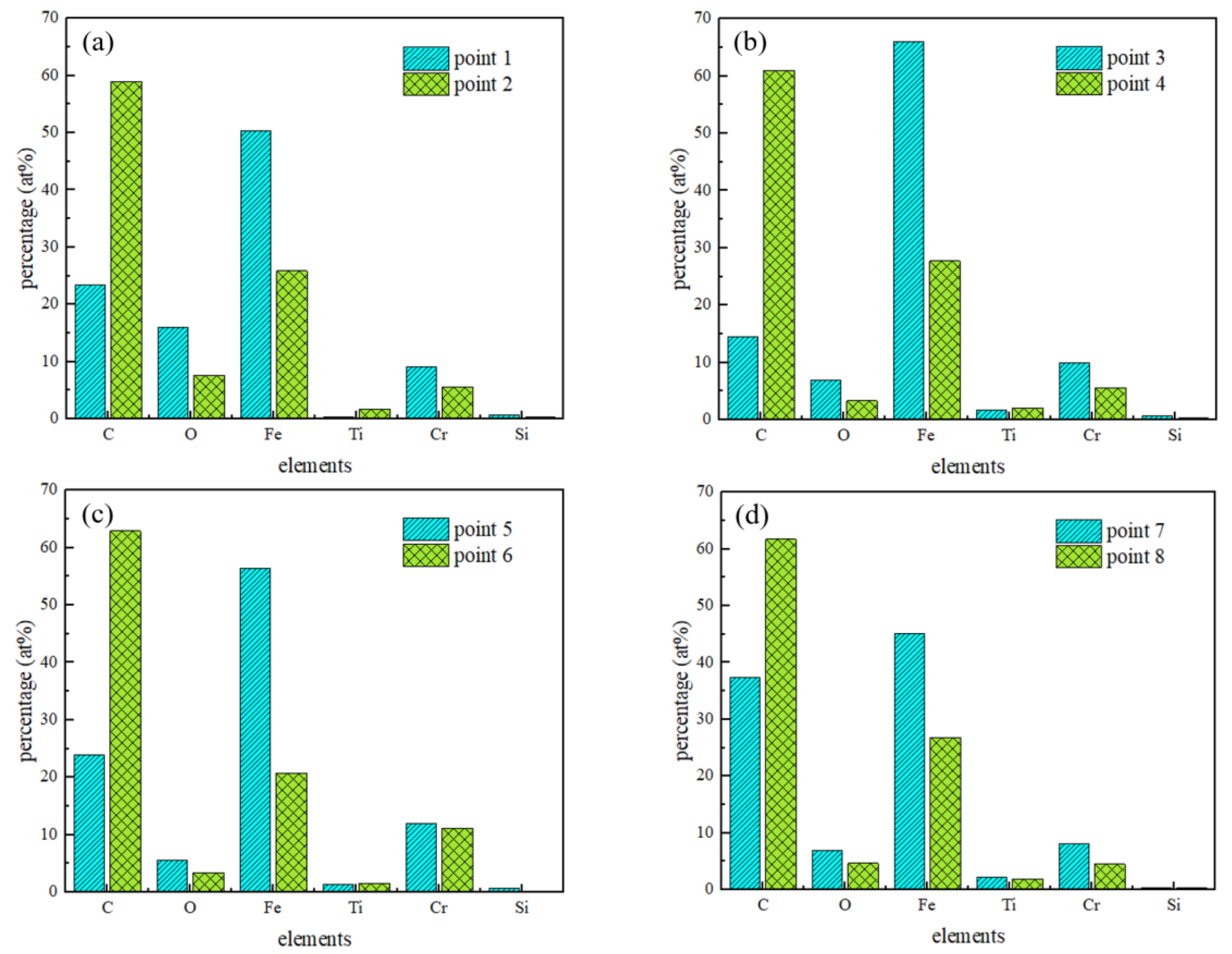
3.1. Morphological and Structure of DLC Coatings
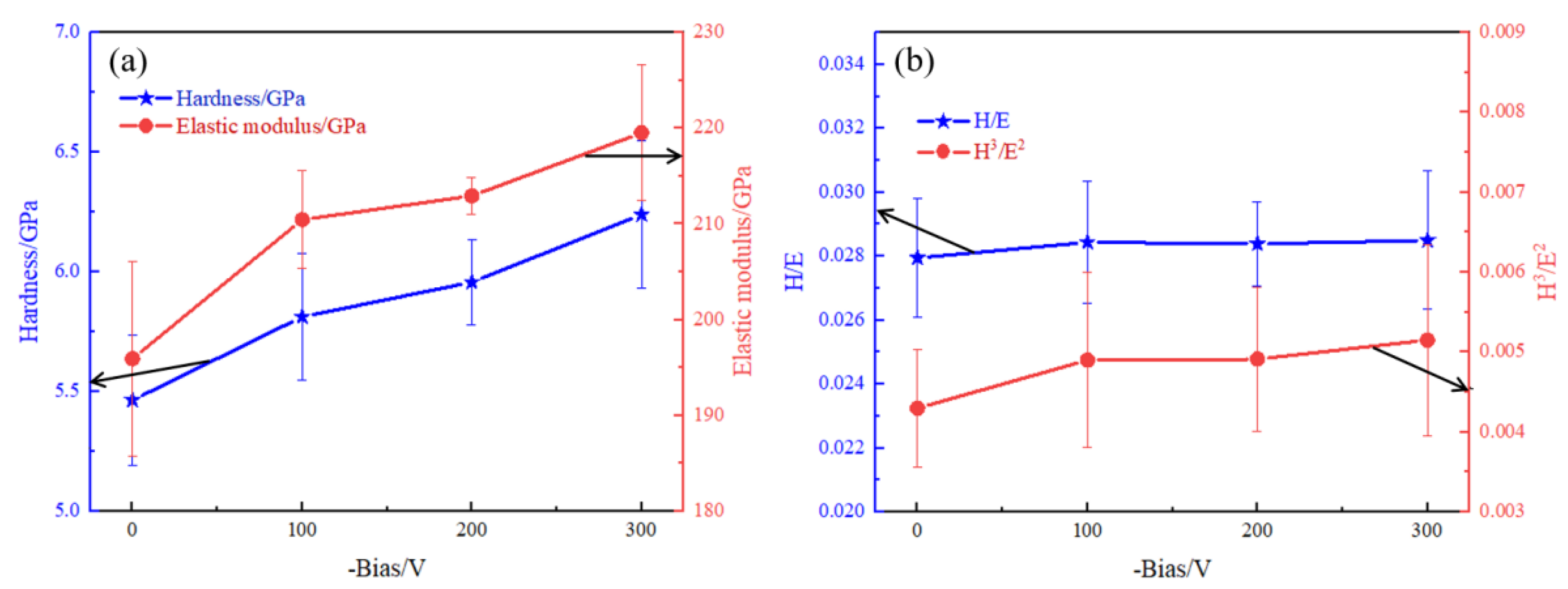
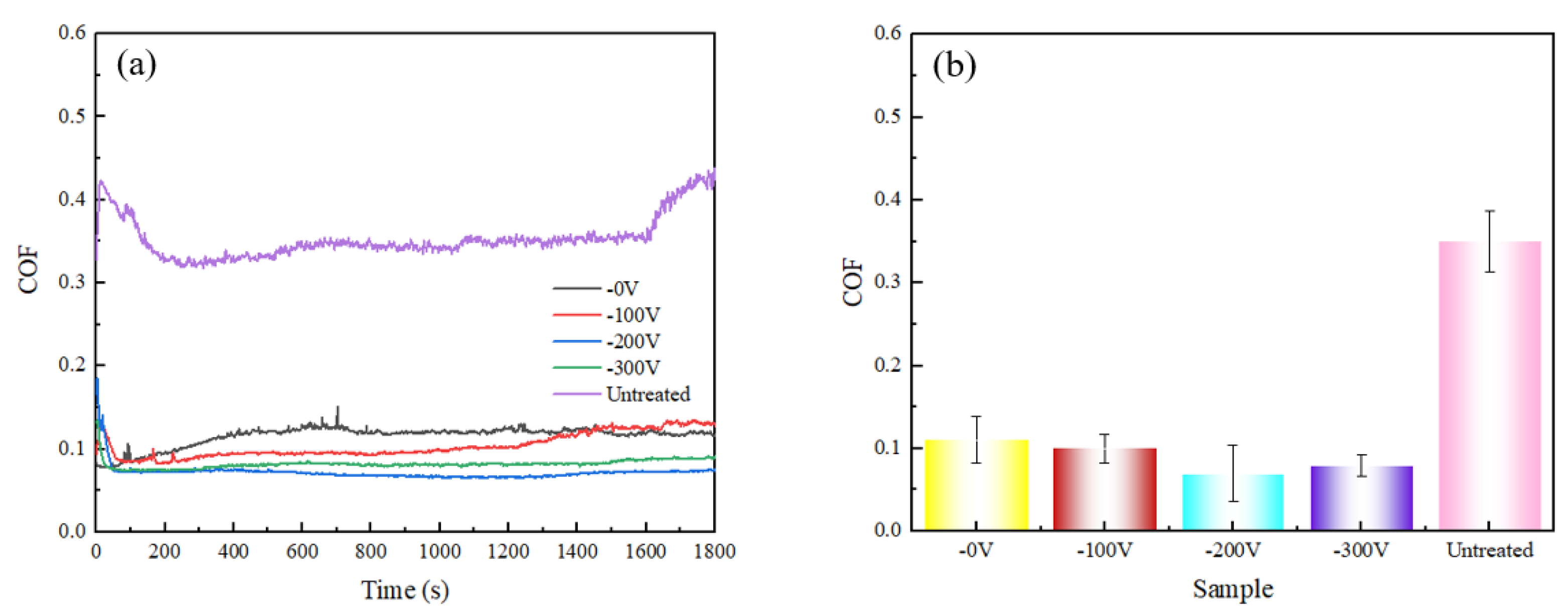
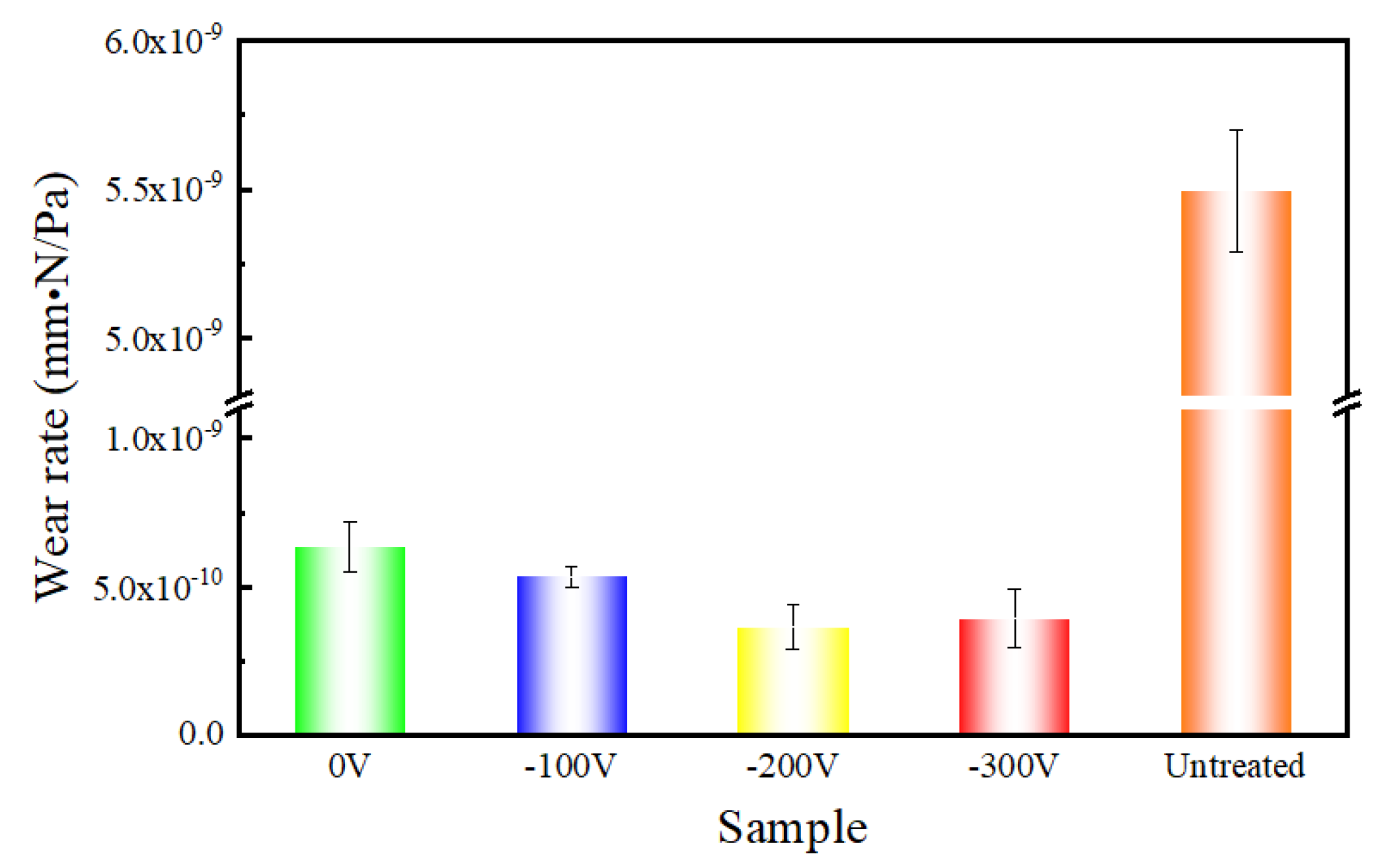
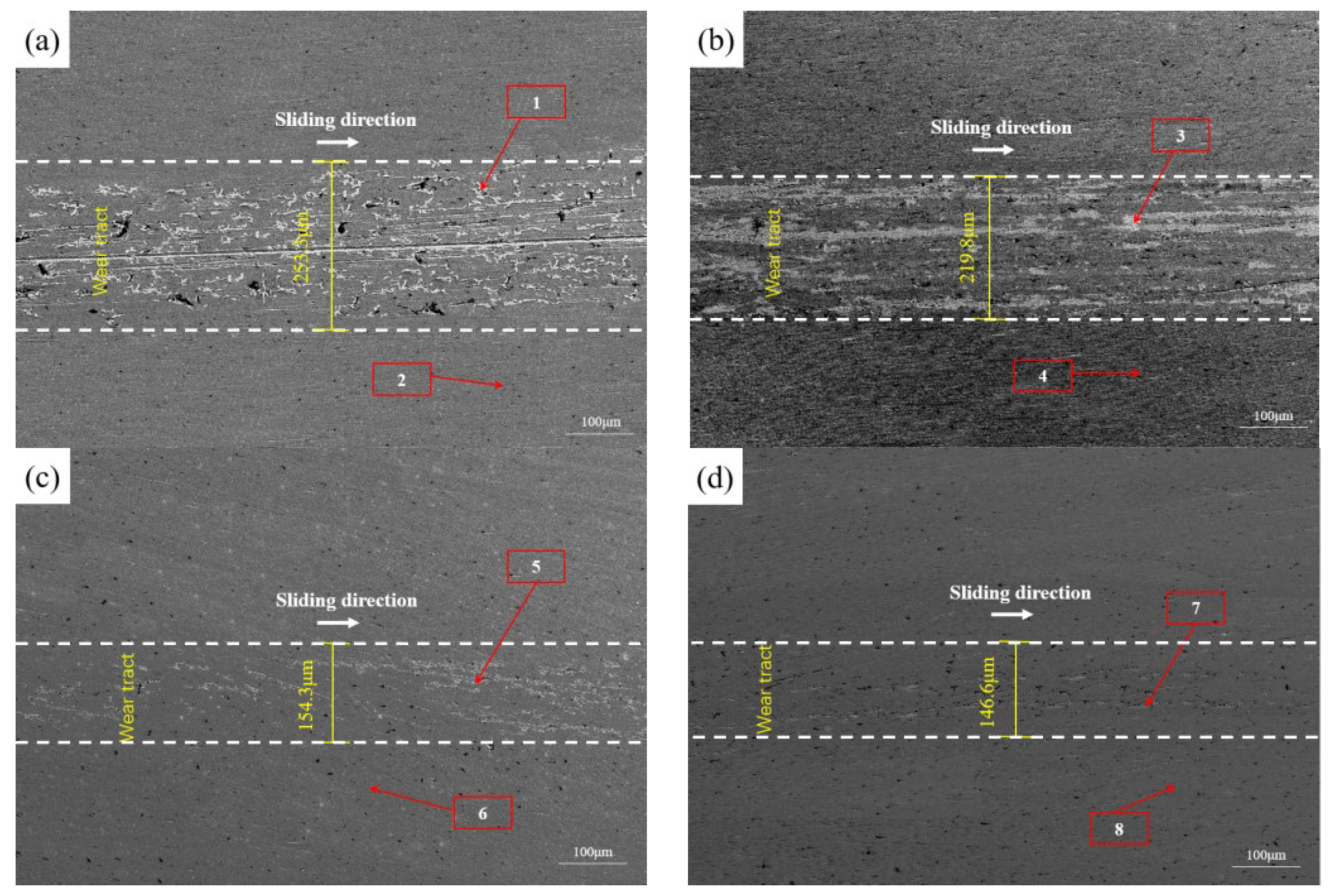
3.2. Mechanical Properties of DLC Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, A.; Cheng, X.; Zhang, S.Q.; Wang, H.M. Effects of heat treatment on microstructure and tensile properties of laser melting deposited AISI 431 martensitic stainless steel. Mater. Sci. Eng. A Struct. 2016, 666, 27–33. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelík, V.; De Hosson, J.T.M. The effect of cladding speed on phase constitution and properties of AISI 431 stainless steel laser deposited coatings. Surf. Coat. Technol. 2011, 205, 5235–5239. [Google Scholar] [CrossRef]
- Khorram, A.; Jamaloei, A.D.; Jafari, A.; Moradi, M. Nd:YAG laser surface hardening of AISI 431 stainless steel; mechanical and metallurgical investigation. Opt. Laser Technol. 2019, 119, 105617. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.S.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Deng, Q.Y.; Wang, C.M.; Zhang, T.F.; Yang, W.M.; Li, X.Y.; Huang, N.; Leng, Y.X. Regulating the uniformity of DLC films in ECR plasma with negative substrate biasing. Surf. Coat. Technol. 2019, 365, 15–23. [Google Scholar] [CrossRef]
- Mosayebi, M.J.; Hosseini, S.R. Structural and tribological properties of TiC-DLC coatings deposited by RCAE-PVD at various bias voltages. Surf. Eng. 2015, 31, 96–102. [Google Scholar] [CrossRef]
- Kowalski, S. Influence of diamond-like carbon coatings on the wear of the press joint components. Wear 2021, 486, 204076. [Google Scholar] [CrossRef]
- Kowalski, S.; Cieslikowski, B.; Barta, D.; Dizo, J.; Dittrich, A. Analysis of the Operational Wear of the Combustion Engine Piston Pin. Lubricants 2023, 11, 100. [Google Scholar] [CrossRef]
- Talibouya, B.E.C.; Sérgio, M.P.; Eduardo, D.S.C.; Andrade, M.L.H.; Wanderson, B.J.; Rosa, D.M. Study of the behavior of skewness (Rsk) and kurtosis (Rku) parameters in the dry drilling process of Al-Mg-Si alloy 6351 T6 using a Diamond-Like Carbon coated high-speed steel tool. Tribol. Int. 2024, 191, 109149. [Google Scholar]
- Kaneko, M.; Hiratsuka, M.; Alanazi, A.; Nakamori, H.; Namiki, K.; Hirakuri, K. Surface Reformation of Medical Devices with DLC Coating. Materials 2021, 14, 376. [Google Scholar] [CrossRef]
- Pei, Y.T.; Bui, X.L.; Zhou, X.B.; De Hosson, J.T.M. Tribological behavior of W-DLC coated rubber seals. Surf. Coat. Technol. 2008, 202, 1869–1875. [Google Scholar] [CrossRef]
- Borges, C.F.M.; Pfender, E.; Heberlein, J. Influence of nitrided and carbonitrided interlayers on enhanced nucleation of diamond on stainless steel 304. Diam. Relat. Mater. 2001, 10, 1983–1990. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Luo, J.; Liao, B.; Zhang, X.; Zhao, Y.Y.; Zeng, X.M.; Chen, L.; Pang, P.; Bao, F. Tribocorrosion and tribological behavior of Ti-DLC coatings deposited by filtered cathodic vacuum arc. Diam. Relat. Mater. 2022, 125, 108985. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, H.Y.; Ma, L.; Zhang, C.H. Impact Abrasive Wear of Cr/W-DLC/DLC Multilayer Films at Various Temperatures. Metals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Li, D.S.; Kong, N.; Li, R.S.; Zhang, B.Y.; Zhang, Y.S.; Wu, Z.G.; Zhang, Q.D. The tribological performance of W-DLC in solid-liquid lubrication system addivated with Cu nanoparticles. Surf. Topogr. Metrol. Prop. 2021, 9, 045043. [Google Scholar] [CrossRef]
- Gao, M.; Kim, S.B.; Li, Y.H.; Ramaswamy, S.H.; Choi, J. Triboelectric nanogenerator with enhanced output and durability based on Si-DLC films. Nano Energy 2023, 105, 107997. [Google Scholar] [CrossRef]
- Shang, L.L.; Gou, C.X.; Li, W.S.; He, D.Q.; Wang, S.H. Effect of microstructure and mechanical properties on the tribological and electrochemical performances of Si/DLC films under HCl corrosive environment. Diam. Relat. Mater. 2021, 116, 108385. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Uzun, Y. Tribocorrosion properties of plasma nitrided, Ti-DLC coated and duplex surface treated AISI 316L stainless steel. Surf. Coat. Technol. 2022, 441, 128587. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, H.D.; Ma, Y.; Wan, Q.; Chen, H.; Liu, Y.; Chen, Y.M.; Mei, Q.S.; Yang, B. Effect of Ion Source Current on the Microstructure and Properties of Cr-DLC Coatings Prepared by Ion Beam-Assisted Arc Ion Plating. NANO 2017, 12, 1750053. [Google Scholar] [CrossRef]
- Song, R.W.; Chen, S.; Liu, Z.W.; Huo, C.Q.; Chen, Q. Effect of W-doping on the structure and properties of DLC films prepared by combining physical and chemical vapor deposition. Diam. Relat. Mater. 2023, 132, 109687. [Google Scholar] [CrossRef]
- Zhao, Q.P.; Kang, S.M.; Zou, F.Z.; Huo, Z.K. Structure and properties of Si and N co-doping on DLC film corrosion resistance. Ceram. Int. 2023, 49, 2121–2129. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yan, M.F.; Yang, Y.; Zhang, Y.X.; Yan, F.Y.; Li, H.T. Excellent mechanical, tribological and anti-corrosive performance of novel Ti-DLC nanocomposite thin films prepared via magnetron sputtering method. Carbon 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Dalibon, E.L.; Moreira, R.D.; Guitar, M.A.; Trava-Airoldi, V.J.; Bruhl, S.P. Influence of the substrate pre-treatment on the mechanical and corrosion response of multilayer DLC coatings. Diam. Relat. Mater. 2021, 118, 108507. [Google Scholar] [CrossRef]
- Guo, C.Q.; Pei, Z.L.; Fan, D.; Gong, J.; Sun, C. Microstructure and tribomechanical properties of (Cr, N)-DLC/DLC multilayer films deposited by a combination of filtered and direct cathodic vacuum arcs. Diam. Relat. Mater. 2015, 60, 66–74. [Google Scholar] [CrossRef]
- Lara, L.C.; Costa, H.; de Mello, J.D.B. Influence of layer thickness on sliding wear of multifunctional tribological coatings. Ind. Lubr. Tribol. 2015, 67, 460–467. [Google Scholar] [CrossRef]
- Radi, P.A.; Vieira, A.; Manfroi, L.; Nass, K.C.D.; Ramos, M.A.R.; Leite, P.; Martins, G.V.; Jofre, J.B.F.; Vieira, L. Tribocorrosion and corrosion behavior of stainless steel coated with DLC films in ethanol with different concentrations of water. Ceram. Int. 2019, 45, 9686–9693. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Jalilvand, M.M.; Heidarpour, A.; Jahani, A.R. Tribological behavior of AZ31/ZrO2 surface nanocomposites developed by friction stir processing. Tribol. Int. 2020, 143, 106062. [Google Scholar] [CrossRef]
- Guo, C.Q.; Li, H.Q.; Peng, Y.L.; Dai, M.J.; Lin, S.S.; Shi, Q.; Wei, C.B. Residual stress and tribological behavior of hydrogen-free Al-DLC films prepared by HiPIMS under different bias voltages. Surf. Coat. Technol. 2022, 445, 128713. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, Y.; Chen, H.; Wang, M.C. Nanoindentation-induced deformation behaviors of tetrahedral amorphous carbon film deposited by cathodic vacuum arc with different substrate bias voltages. Appl. Surf. Sci. 2022, 576, 151741. [Google Scholar] [CrossRef]
- Chu, P.K.; Li, L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Yoon, S.F.; Yang, H.; Rusli, A.; Ahn, J.; Zhang, Q. The effects of self-generated DC bias on the characteristics of diamond-like carbon films prepared using ECR-CVD. Diam. Relat. Mater. 1998, 7, 70–76. [Google Scholar] [CrossRef]
- Qi, J.W.; Wang, L.P.; Yan, F.Y.; Xue, Q.J. The tribological performance of DLC-based coating under the solid-liquid lubrication system with sand-dust particles. Wear 2013, 297, 972–985. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Lempert, G.D.; Grossman, E. Substantiation of subplantation model for diamondlike film growth by atomic force microscopy. Phys. Rev. Lett. 1994, 72, 2753–2756. [Google Scholar] [CrossRef]


| Parameters | Targets and Gas | Temperature/°C | Depositing Time/min | Bias Voltage/V | ||||
|---|---|---|---|---|---|---|---|---|
| Layer | Ti | C | Ar | N2 | ||||
| Ti | on | off | on | off | 100 | 25 | 0 V/−100 V/−200 V/−300 V | |
| TiN | on | off | on | on | 100 | 25 | 0 V/−100 V/−200 V/−300 V | |
| (Ti,N)-DLC | on | on | on | on | 100 | 25 | 0 V/−100 V/−200 V/−300 V | |
| Ti-DLC | on | on | on | off | 100 | 25 | 0 V/−100 V/−200 V/−300 V | |
| DLC | off | on | on | off | 100 | 120 | 0 V/−100 V/−200 V/−300 V | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Huang, T.; Sun, S.; Wu, S.; Yang, X.; Guo, F.; Zhang, B.; Dai, L. Effects of Bias Voltages on the Tribological Behaviors of DLC Coatings. Coatings 2024, 14, 176. https://doi.org/10.3390/coatings14020176
Zhang S, Huang T, Sun S, Wu S, Yang X, Guo F, Zhang B, Dai L. Effects of Bias Voltages on the Tribological Behaviors of DLC Coatings. Coatings. 2024; 14(2):176. https://doi.org/10.3390/coatings14020176
Chicago/Turabian StyleZhang, Shuling, Tenglong Huang, Shengdi Sun, Shuaizheng Wu, Xiangdong Yang, Feng Guo, Bo Zhang, and Longjie Dai. 2024. "Effects of Bias Voltages on the Tribological Behaviors of DLC Coatings" Coatings 14, no. 2: 176. https://doi.org/10.3390/coatings14020176
APA StyleZhang, S., Huang, T., Sun, S., Wu, S., Yang, X., Guo, F., Zhang, B., & Dai, L. (2024). Effects of Bias Voltages on the Tribological Behaviors of DLC Coatings. Coatings, 14(2), 176. https://doi.org/10.3390/coatings14020176







