The Influence of Salt Erosion on the Mechanical Performances of Ultra-High-Performance Concrete with Secondary Aluminum Dross
Abstract
:1. Introduction
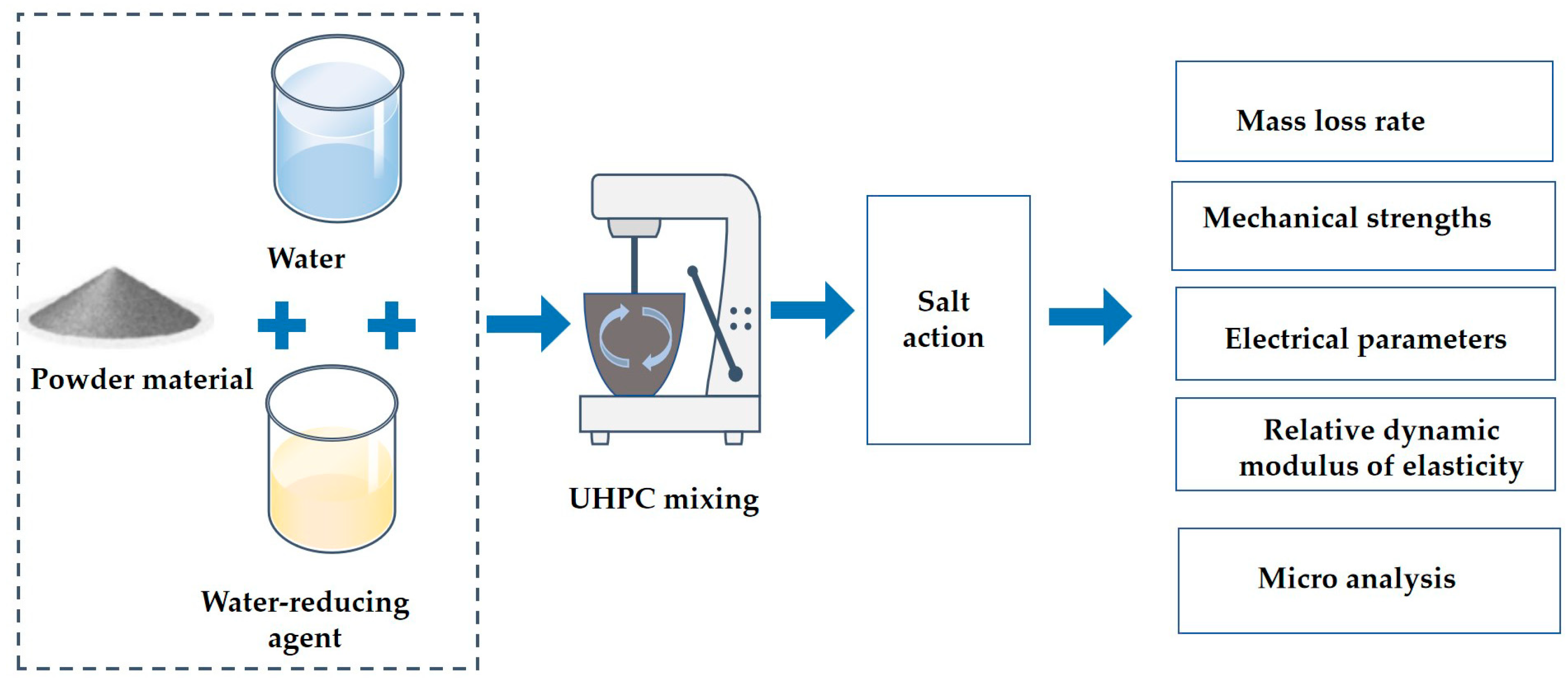
2. Experimental Section
2.1. Raw Materials
2.2. The Preparing of Specimens
2.3. The NaCl F-C Action
2.4. The NaCl and Na2SO4 D-A Actions
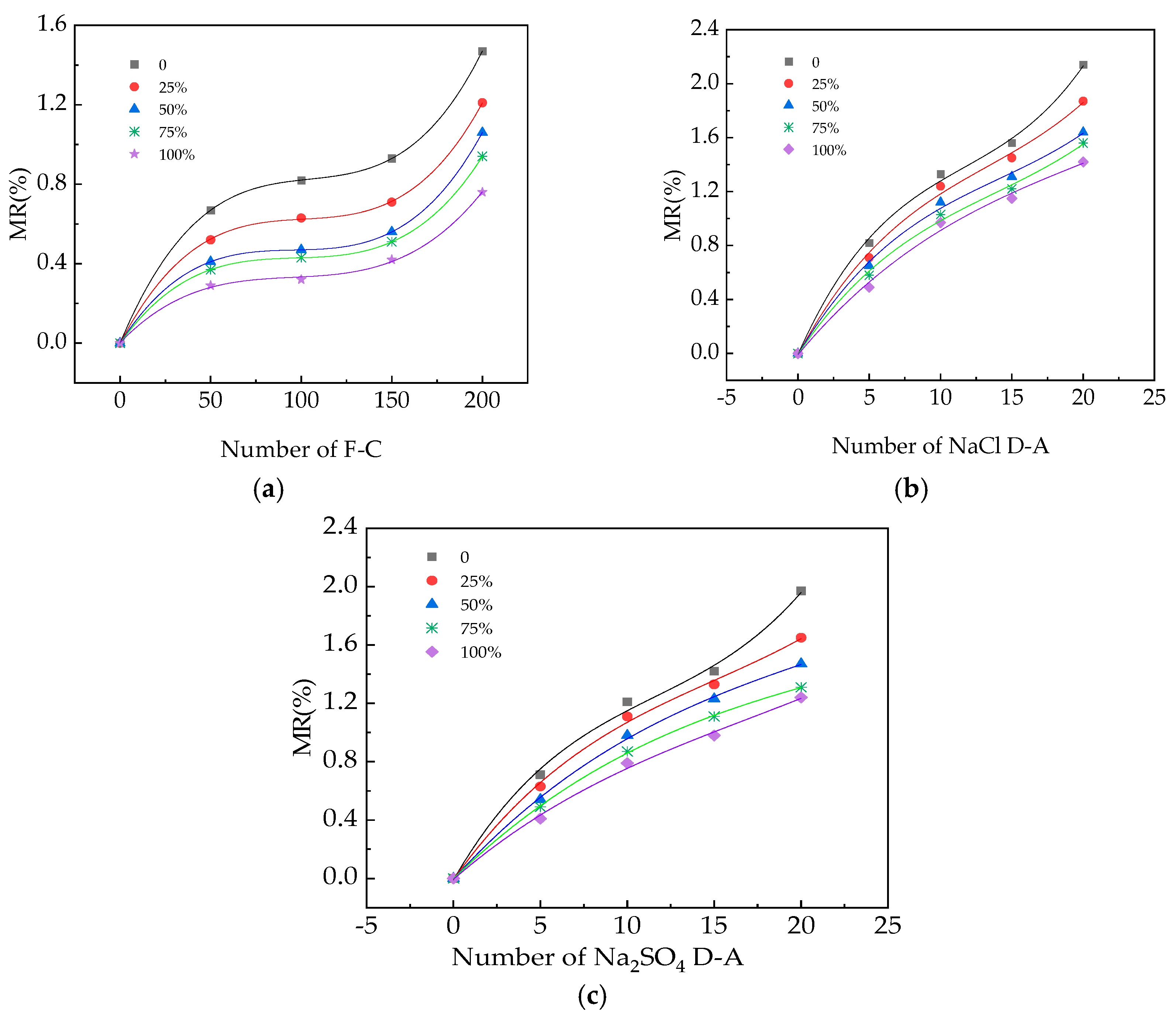
2.5. The Measurement of MR
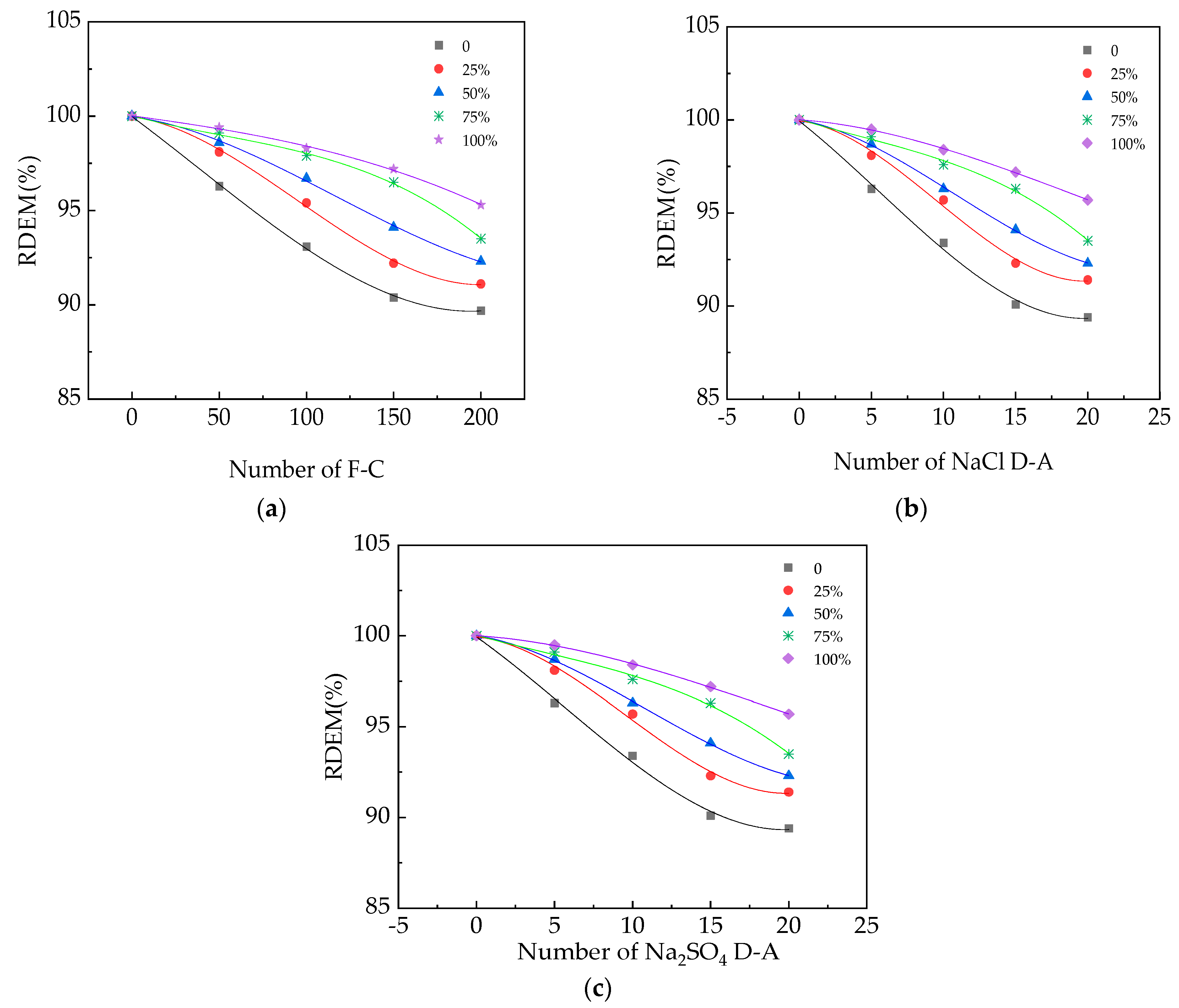
2.6. The Measurement of the Relative Dynamic Modulus of Elasticity
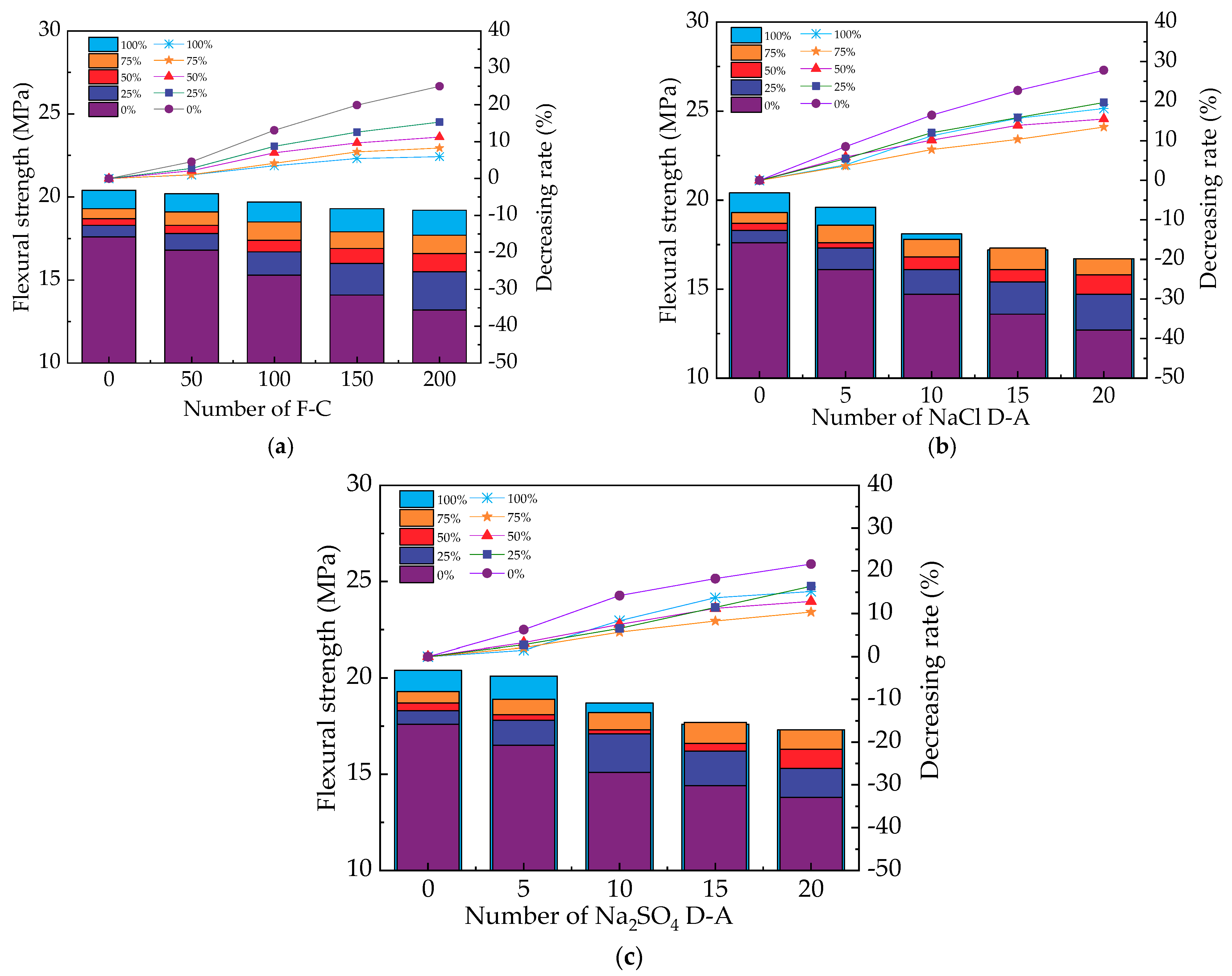
2.7. The Measurement of Mechanical Strengths
2.8. The Chloride Ion Migration Coefficient
2.9. The Carbonation Depth
2.10. The SEM Experiment
3. Results and Discussions
3.1. The MR of UHPC
3.2. The RDME of UHPC
3.3. The Mechanical Strengths of UHPC
3.4. The CMC of UHPC
3.5. The Carbonation Depth of UHPC
3.6. The SEM Photos of UHPC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabrera Luna, K.; Maldonado Bandala, E.E.; Nieves Mendoza, D. Supersulfated binders based on volcanic raw material: Optimization, microstructure and reaction products. Constr. Build. Mater. 2018, 176, 145–155. [Google Scholar] [CrossRef]
- Han, F.; Song, S.; Liu, J.; Huang, S. Properties of steam-cured precast concrete containing iron tailing powder. Powder Technol. 2019, 345, 292–299. [Google Scholar] [CrossRef]
- Ince, C.; Derogar, S.; Gürkaya, K.; James Ball, R. Properties, durability and cost efficiency of cement and hydrated lime mortars reusing copper mine tailings of Lefke-Xeros in Cyprus. Constr. Build. Mater. 2021, 268, 121070. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Plaza, M.G.; Gonazlez, A.S.; Rubiera, F.; Pevida, C. Water vapour adsorption by a coffee-based microporous carbon: Effect on CO2 capture. J. Chem. Technol. Biotechnol. 2015, 90, 1592–1600. [Google Scholar] [CrossRef]
- Meor Yusoff, M.S.; Muslimin, M.; Paulus, W. Effect of fractional precipitation on quality of nanostructured alumina produced from black aluminium dross waste. Adv. Mater. Res. 2011, 173, 24–28. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Lou, B.J.; Shen, H.L.; Liu, B.; Liu, J.; Zhang, S.G. Recycling secondary aluminum dross to make building materials: A review. Constr. Build. Mater. 2023, 409, 133989. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, K.; Li, H.; Zhang, X.Q.; Jiang, Y.L. Novel SCMs produced by the calcination of secondary aluminium dross with dolomite and their potential usage in cemented paste backfill. Constr. Build. Mater. 2023, 365, 130119. [Google Scholar] [CrossRef]
- Satish, R.; Neeraja, D. Mechanical and durability aspects of concrete incorporating secondary aluminium slag. Resour. Effic. Technol. 2016, 2, 225–232. [Google Scholar]
- Yoshimura, H.N.; Abreu, A.P.; Molisani, A.L.; Camargo, A.C.; Portela, J.C.S.; Narita, N.E. Evaluation of aluminum dross waste as raw material for refractories. Ceram. Int. 2008, 34, 581–591. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Ghanaym, E.E.; Sadek, H.E.H.; El Defrawy, S.A.; Ali, O.A.M. Synthesis, characterization and sinterability of pure and Ni-doped nano layered double hydroxides from aluminum dross. Ceram. Int. 2019, 45, 17598–17610. [Google Scholar] [CrossRef]
- Rotana, H.; Claudia, P.O. On utilization and mechanisms of waste aluminium in mitigating alkali-silica reaction (ASR) in concrete. J. Clean. Prod. 2019, 212, 864–879. [Google Scholar]
- Socrates, P.M.P.; Juan, M.G.C. Influence of the secondary aluminum chip on the physical and mechanical properties of concrete. Innov. Infrastruct. Solut. 2023, 8, 45. [Google Scholar]
- Kočí, V.; Vejmelková, E.; Koňáková, D.; Pommer, V.; Grzeszczyk, S.; Matuszek-Chmurowska, A.; Mordak, A.; Černý, R. Basic physical, mechanical, thermal and hygric properties of reactive powder concrete with basalt and polypropylene fibers after high-temperature exposure. Constr. Build. Mater. 2023, 374, 130922. [Google Scholar] [CrossRef]
- Hong, X.; Wang, H.; Shi, F. Influence of NaCl freeze thaw cycles and cyclic loading on the mechanical performance and permeability of sulphoaluminate cement reactive powder concrete. Coatings 2020, 10, 1227. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Silva, R.V.; Bravo, M.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; Brito, J.D. Effect of incorporating municipal solid waste incinerated bottom ash in alkali-activated fly ash concrete subjected to accelerated CO2 curing. J. Clean. Prod. 2022, 370, 133533. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Li, L.F.; Ling, T.C. Response surface methodology for the optimization of CO2 uptake using waste concrete powder. Constr. Build. Mater. 2022, 340, 127758. [Google Scholar] [CrossRef]
- Boulahbal, A.I.; Santamaría, L.; Azizi, A.; Boutahala, M.; Korili, S.A.; Gil, A. Synthesis of Cu-Al layered double hydroxides from aluminum saline slags. Miner. Eng. 2023, 204, 108413. [Google Scholar] [CrossRef]
- Bai, L.; Liu, H.; Wang, H. The Influence of CO2-Cured Boiler Cinder on the Mechanical Strength of RPC Exposed to NaCl Erosion. Coatings 2023, 13, 1021. [Google Scholar] [CrossRef]
- Alderete, N.M.; Joseph, A.M.; Van den Heede, P.; Matthys, S.; De Belie, N. Effective and sustainable use of municipal solid waste incineration bottom ash in concrete regarding strength and durability. Resour. Conserv. Recycl. 2021, 167, 105356. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Wang, H. The Corrosion Resistance of Reinforced Reactive Powder Concrete with Secondary Aluminum Ash Exposed to NaCl Action. Materials 2023, 16, 5615. [Google Scholar] [CrossRef] [PubMed]
- Gursel, A.P.; Maryman, H.; Ostertag, C. A life-cycle approach to environmental, mechanical, and durability properties of “green” concrete mixes with rice husk ash. J. Clean. Prod. 2016, 112, 823–836. [Google Scholar] [CrossRef]
- Tarangini, D.; Sravana, P.; Srinivasa Rao, P. Effect of nano silica on frost resistance of pervious concrete. Mater. Today Proc. 2022, 51, 2185–2189. [Google Scholar] [CrossRef]
- Meshram, A.; Gautam, D.; Singh, K. Recycling of white aluminium dross: Production of potash alum. Trans. Indian. Inst. Met. 2020, 73, 1239–1248. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; Deventer, J.S. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross. J. Clean. Prod. 2018, 179, 93–102. [Google Scholar] [CrossRef]
- Sarikaya, Y.; Sevinç, I.; Akinç, M. The effect of calcinations temperature on some of the adsorptive properties of fine alumina powders obtained by emulsion evaporation technique. Powder Technol. 2001, 116, 109–114. [Google Scholar] [CrossRef]
- Serelis, E.; Vaitkevicius, V. Effect of waste glass powder and liquid glass on the Physico-Chemistry of Aluminum-Based Ultra-Lightweight concrete. Constr. Build. Mater. 2023, 390, 131615. [Google Scholar] [CrossRef]
- Song, Y.; Li, B.; Yang, E.-H.; Liu, Y.; Ding, T. Feasibility Study on Utilization of Municipal Solid Waste Incineration Bottom Ash as Aerating Agent for the Production of Autoclaved Aerated concrete. Cem. Concr. Compos. 2015, 56, 51–58. [Google Scholar] [CrossRef]
- Almeida, V.O.; Silvestro, L.; Gleize, P.J.; Kirchheim, A.P.; Schneider, I.A. Application of leached iron ore tailings to produce sustainable cements. Constr. Build. Mater. 2023, 377, 131095. [Google Scholar] [CrossRef]
- Miguel, F.; Brito, J.D.; Silva, R.V. Durability-related performance of recycled aggregate concrete containing alkali-activated municipal solid waste incinerator bottom ash. Const. Build. Mater. 2023, 397, 132415. [Google Scholar] [CrossRef]
- IV, G.M.; Sinnan, R.; Young, M. Evaluation of reclaimed municipal solid waste incinerator sands in concrete. J. Clean. Prod. 2019, 229, 838–849. [Google Scholar]
- Feng, H.G.; Wang, L.; Li, G.; Jin, Q. Removal of impurities for the synthesis of high-property alumina-spinel ceramic from secondary aluminum dross. Ceram. Int. 2023, 49, 34616–34626. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.B.; Liu, Y.; Chen, M.; Wang, N. Recovery of low phosphorus iron from steel slag using secondary aluminum dross as the reductant. J. Environ. Chem. Eng. 2023, 11, 110973. [Google Scholar] [CrossRef]
- Tran, H.; Sorelli, L.; Hisseine, O.A.; Bouchard, D.; Brail, V.; Sanchez, T.; Conciatori, D.; Ouellet-Plamondon, C. Development of sustainable ultra-high performance concrete recycling aluminum production waste. Constr. Build. Mater. 2023, 371, 130212. [Google Scholar] [CrossRef]
- Aninda, S.S.; Islam, M.S. Effectiveness of waste concrete powder in fabricating compressed stabilized earth blocks: Strength, durability and thermal assessment. J. Build. Eng. 2023, 80, 107989. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Z.; Zhang, S.G.; Li, X.L.; Long, H.C.; Meng, X.X.; Melnikov, A.; Gagarin, L. The influence of particle morphology on the long-term strength of sandy soil under freeze-thaw cycles. Case. Stud. Constr. Mater. 2023, 18, e02196. [Google Scholar] [CrossRef]
- Beltrame, N.A.M.; Dias, R.L.; Witzke, F.B.; Medeiros-Junior, R.A. Effect of carbonation curing on the physical, mechanical, and microstructural properties of metakaolin-based geopolymer concrete. Constr. Build. Mater. 2023, 406, 133403. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I. Superhydrophobic soot as a mechanically robust, heat insulating and anti-corrosion protective coating for concrete surfaces. Colloids Surf. A 2023, 672, 131723. [Google Scholar] [CrossRef]







| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | Na2O | Ti2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 20.3 | 5.8 | 3.9 | 1.9 | 62.2 | 2.8 | - | - | - | 3.1 |
| FA | 90.40 | 0.33 | 0.62 | 0.35 | 0.42 | 0.21 | 7.49 | - | - | - |
| QS | 98.7 | - | 1.3 | - | - | - | - | - | - | - |
| SAD | 4.7 | 79.2 | 4.3 | 5.2 | 1.6 | - | - | 0.9 | - | 3.8 |
| Types | Particle Size/μm | ||||||
|---|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1 | 4 | 8 | 64 | 360 | |
| OPC | 0.13 | 0.36 | 3.1 | 14.8 | 29.2 | 92.8 | 100 |
| FA | 32.11 | 59.12 | 83.16 | 100 | 100 | 100 | 100 |
| QS | 0 | 0 | 0 | 0 | 0.035 | 24 | 100 |
| SAD | 0.04 | 0.31 | 0.55 | 1.11 | 3.95 | 25.4 | 88.13 |
| Samples | Water | OPC | SAD | FA | QS | WR |
|---|---|---|---|---|---|---|
| SAD-0% | 241.2 | 843.3 | 0 | 365.5 | 965.4 | 16.1 |
| SAD-25% | 241.2 | 843.3 | 91.4 | 274.1 | 965.4 | 16.1 |
| SAD-50% | 241.2 | 843.3 | 182.8 | 182.8 | 965.4 | 16.1 |
| SAD-75% | 241.2 | 843.3 | 274.1 | 91.4 | 965.4 | 16.1 |
| SAD-100% | 241.2 | 843.3 | 365.5 | 0 | 965.4 | 16.1 |
| Equation Types | SAD Content | a | b | c | d | R2 |
|---|---|---|---|---|---|---|
| NaCl F-C | 0% | 6.33 × 10−7 | −1.96 × 10−4 | 2.17 × 10−2 | 1.42857 × 10−4 | 0.99 |
| 25% | 5.58 × 10−7 | −1.75 × 10−4 | 1.75 × 10−2 | −1 × 10−3 | 0.99 | |
| 50% | 5.12 × 10−7 | −1.51 × 10−4 | 1.42 × 10−2 | −1.99 × 10−16 | 1.00 | |
| 75% | 4.44 × 10−7 | −1.28 × 10−4 | 1.27 × 10−2 | −5.96 × 10−16 | 1.00 | |
| 100% | 3.33 × 10−7 | −9.51 × 10−5 | 9.5 × 10−3 | 2.29 × 10−3 | 0.98 | |
| NaCl D-A | 0% | 4.40 × 10−4 | −1.54 × 10−2 | 2.38 × 10−1 | −8.57 × 10−3 | 0.99 |
| 25% | 2.60 × 10−4 | −1.04 × 10−2 | 1.97 × 10−1 | −9.57 × 10−3 | 0.98 | |
| 50% | 2.13 × 10−4 | −9.03 × 10−3 | 1.77 × 10−1 | −7.43 × 10−3 | 0.99 | |
| 75% | 1.87 × 10−4 | −7.71 × 10−3 | 1.58 × 10−1 | −7.71 × 10−3 | 0.99 | |
| 100% | 6.67 × 10−5 | −4.11 × 10−3 | 1.27 × 10−1 | −9.71 × 10−3 | 0.98 | |
| Na2SO4 D-A | 0% | 3.67 × 10−4 | −1.27 × 10−3 | 0.21 | −1.01 × 10−2 | 0.99 |
| 25% | 1.67 × 10−4 | −7.51 × 10−3 | 0.17 | −6.71 × 10−3 | 0.99 | |
| 50% | 6.00 × 10−5 | −4.06 × 10−3 | 0.13 | −3.86 × 10−3 | 0.99 | |
| 75% | 4.67 × 10−5 | −3.46 × 10−3 | 0.12 | −1.86 × 10−3 | 0.99 | |
| 100% | 6.67 × 10−5 | −3.40 × 10−3 | 0.10 | −6 × 10−3 | 0.98 |
| Equation Types | SAD Content | a | b | c | d | R2 |
|---|---|---|---|---|---|---|
| NaCl F-C | 0% | 1.00 × 10−6 | −1.14 × 10−4 | −6.86 × 10−2 | 99.98 | 0.99 |
| 25% | 1.93 × 10−6 | −5.49 × 10−4 | −1.21 × 10−2 | 99.97 | 0.99 | |
| 50% | 8.67 × 10−7 | −3.03 × 10−4 | −1.26 × 10−2 | 99.98 | 0.99 | |
| 75% | −8.67 × 10−7 | 1.34 × 10−4 | −2.47 × 10−2 | 100.02 | 0.99 | |
| 100% | −2 × 10−7 | −1.43 × 10−5 | −1.26 × 10−2 | 100.02 | 0.99 | |
| NaCl D-A | 0% | −4.67 × 10−4 | −2.69 × 10−2 | −1.04 | 99.98 | 0.99 |
| 25% | 1.73 × 10−3 | −4.43 × 10−2 | −0.33 | 99.97 | 0.99 | |
| 50% | 6.00 × 10−4 | −1.77 × 10−2 | −0.33 | 100.02 | 0.99 | |
| 75% | −1.13 × 10−3 | 2.49 × 10−3 | −0.41 | 100.03 | 0.99 | |
| 100% | 1.33 × 10−4 | −8.29 × 10−3 | −0.15 | 100.01 | 0.99 | |
| Na2SO4 D-A | 0% | 1.20 × 10−3 | −0.02 | −0.61 | 99.94 | 0.99 |
| 25% | 2 × 10−3 | −0.06 | −0.09 | 99.94 | 0.99 | |
| 50% | 1 × 10−3 | −0.03 | −0.14 | 100.02 | 0.99 | |
| 75% | −6 × 10−4 | 0.01 | −0.24 | 100.04 | 0.98 | |
| 100% | 2 × 10−4 | −0.01 | −0.06 | 100.01 | 0.99 |
| Equation Types | SAD Content | a | b | c | d | R2 |
|---|---|---|---|---|---|---|
| NaCl F-C | 0% | 1.4 × 10−7 | −3.49 × 10−5 | 1.21 × 10−2 | 1.99 | 0.96 |
| 25% | 2.67 × 10−8 | 3.06 × 10−5 | 3.12 × 10−3 | 1.94 | 0.98 | |
| 50% | 2.53 × 10−7 | −2.63 × 10−5 | 5.62 × 10−3 | 1.84 | 0.98 | |
| 75% | 3.73 × 10−7 | −5.8 × 10−5 | 7.17 × 10−3 | 1.70 | 0.97 | |
| 100% | 3.2 × 10−7 | −4.34 × 10−5 | 6.09 × 10−3 | 1.62 | 0.94 | |
| NaCl D-A | 0% | 1.63 × 10−3 | −0.06 | 0.80 | 2.06 | 0.94 |
| 25% | 1.55 × 10−3 | −0.06 | 0.74 | 1.99 | 0.97 | |
| 50% | 1.03 × 10−3 | −0.04 | 0.58 | 1.89 | 0.93 | |
| 75% | 1.29 × 10−3 | −0.05 | 0.61 | 1.75 | 0.95 | |
| 100% | 8.73 × 10−4 | −0.03 | 0.35 | 1.66 | 0.97 | |
| Na2SO4 D-A | 0% | −6.00 × 10−5 | 2.6 × 10−3 | 0.07 | −1.98 | 0.91 |
| 25% | −6.67 × 10−5 | 6.11 × 10−3 | −2.62 × 10−3 | 1.95 | 0.98 | |
| 50% | 2.27 × 10−4 | −1.63 × 10−3 | 3.59 × 10−2 | 1.84 | 0.98 | |
| 75% | 3.87 × 10−4 | −5.51 × 10−3 | 0.05 | 1.71 | 0.98 | |
| 100% | 3.8 × 10−4 | −4.46 × 10−3 | 0.03 | 1.63 | 0.99 |
| Equation Types | SAD Content | a | b | c | d | R2 |
|---|---|---|---|---|---|---|
| NaCl F-C | 0% | 1.8 × 10−7 | −5.89 × 10−5 | 9.42 × 10−3 | 1.34 | 0.99 |
| 25% | 8 × 10−8 | −2.86 × 10−5 | 7.31 × 10−3 | 1.21 | 0.99 | |
| 50% | −9.33 × 10−8 | 2.49 × 10−5 | 3.16 × 10−3 | 1.15 | 0.95 | |
| 75% | −6.67 × 10−9 | 8.57 × 10−7 | 4.45 × 10−3 | 1.07 | 0.97 | |
| 100% | −8.67 × 10−8 | 2.91 × 10−5 | 1.89 × 10−3 | 1.00 | 0.98 | |
| NaCl D-A | 0% | 1.2 × 10−4 | −5.94 × 10−3 | 0.14 | 1.35 | 0.98 |
| 25% | −6.67 × 10−5 | 4 × 10−4 | 0.08 | 1.24 | 0.96 | |
| 50% | −1.8 × 10−4 | 4.77 × 10−3 | 0.04 | 1.18 | 0.91 | |
| 75% | −1 × 10−4 | −2 × 10−3 | 0.06 | 1.10 | 0.93 | |
| 100% | −1.87 × 10−4 | 4.89 × 10−3 | 0.03 | 1.02 | 0.95 | |
| Na2SO4 D-A | 0% | 6.67 × 10−6 | −2.86 × 10−5 | 0.04 | 1.33 | 0.96 |
| 25% | −2 × 10−5 | 2.86 × 10−5 | 0.05 | 1.20 | 0.97 | |
| 50% | −6 × 10−5 | 2.11 × 10−3 | 0.02 | 1.15 | 0.97 | |
| 75% | −6 × 10−4 | 5.37 × 10−3 | −6.6 × 10−3 | 1.08 | 0.99 | |
| 100% | −1.47 × 10−4 | 6.17 × 10−3 | −0.03 | 1.01 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Sun, W.; Shi, F.; Miao, L.; Wang, H. The Influence of Salt Erosion on the Mechanical Performances of Ultra-High-Performance Concrete with Secondary Aluminum Dross. Coatings 2024, 14, 189. https://doi.org/10.3390/coatings14020189
Sun H, Sun W, Shi F, Miao L, Wang H. The Influence of Salt Erosion on the Mechanical Performances of Ultra-High-Performance Concrete with Secondary Aluminum Dross. Coatings. 2024; 14(2):189. https://doi.org/10.3390/coatings14020189
Chicago/Turabian StyleSun, Houchao, Weixiang Sun, Feiting Shi, Lu Miao, and Hui Wang. 2024. "The Influence of Salt Erosion on the Mechanical Performances of Ultra-High-Performance Concrete with Secondary Aluminum Dross" Coatings 14, no. 2: 189. https://doi.org/10.3390/coatings14020189


.jpg)



