Enhanced Osteogenic Differentiation Based on Combining Pulp Stem Cells with Ultralong Hydroxyapatite Nanowires and Cellulose Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the HAPNW/CF Biopaper
2.2. In Vitro Cellular Researches
2.2.1. Isolation and Culture of hDPSCs
2.2.2. Identification, Osteogenic and Adipogenic Differentiation of hDPSCs
2.2.3. Determination of Cell Adhesion Activity
2.2.4. Cell Proliferation by CCK-8 Assay
2.2.5. ALP Staining and ALP Activity Assay
2.2.6. Live–Dead Staining
2.2.7. Western Blot
2.3. Statistical Analysis
3. Results
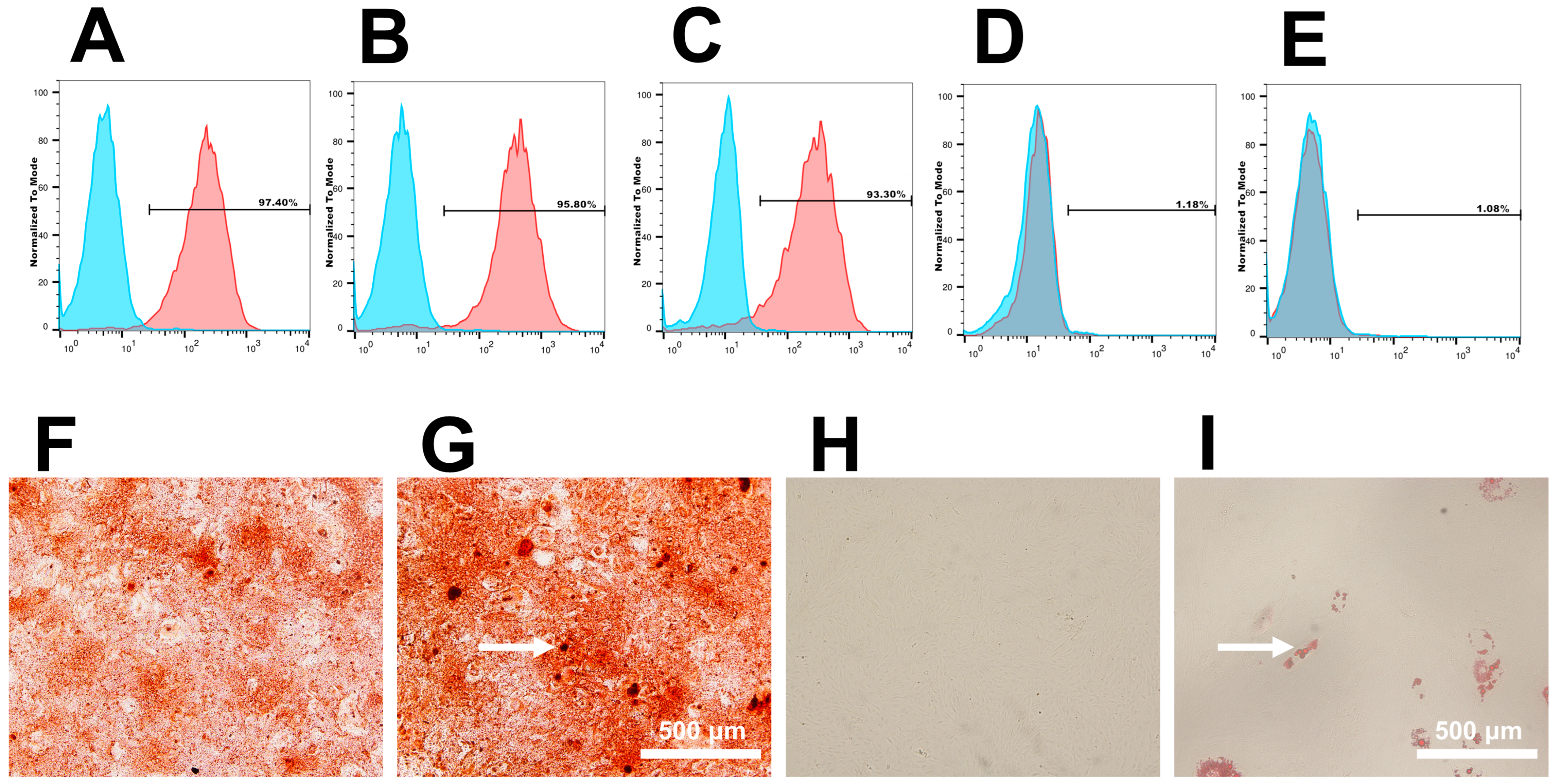
3.1. Cell Culture and Identification
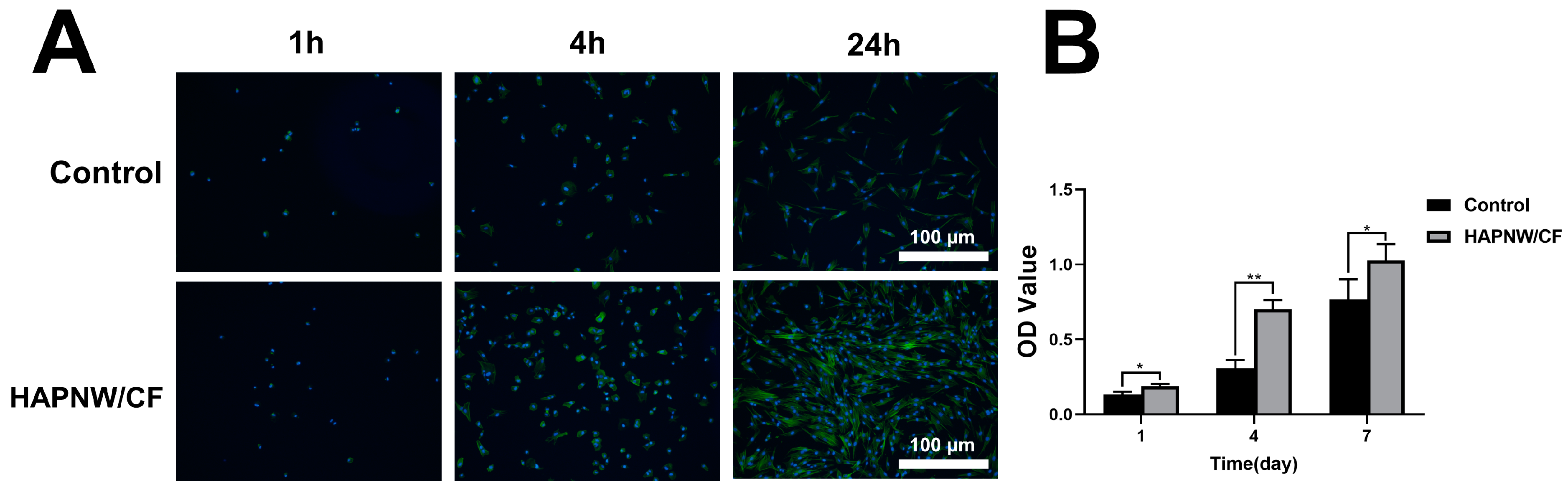
3.2. Cell Viability, Proliferation, and Adhesion Activity of hDPSCs on the HAPNW/CF Biopaper
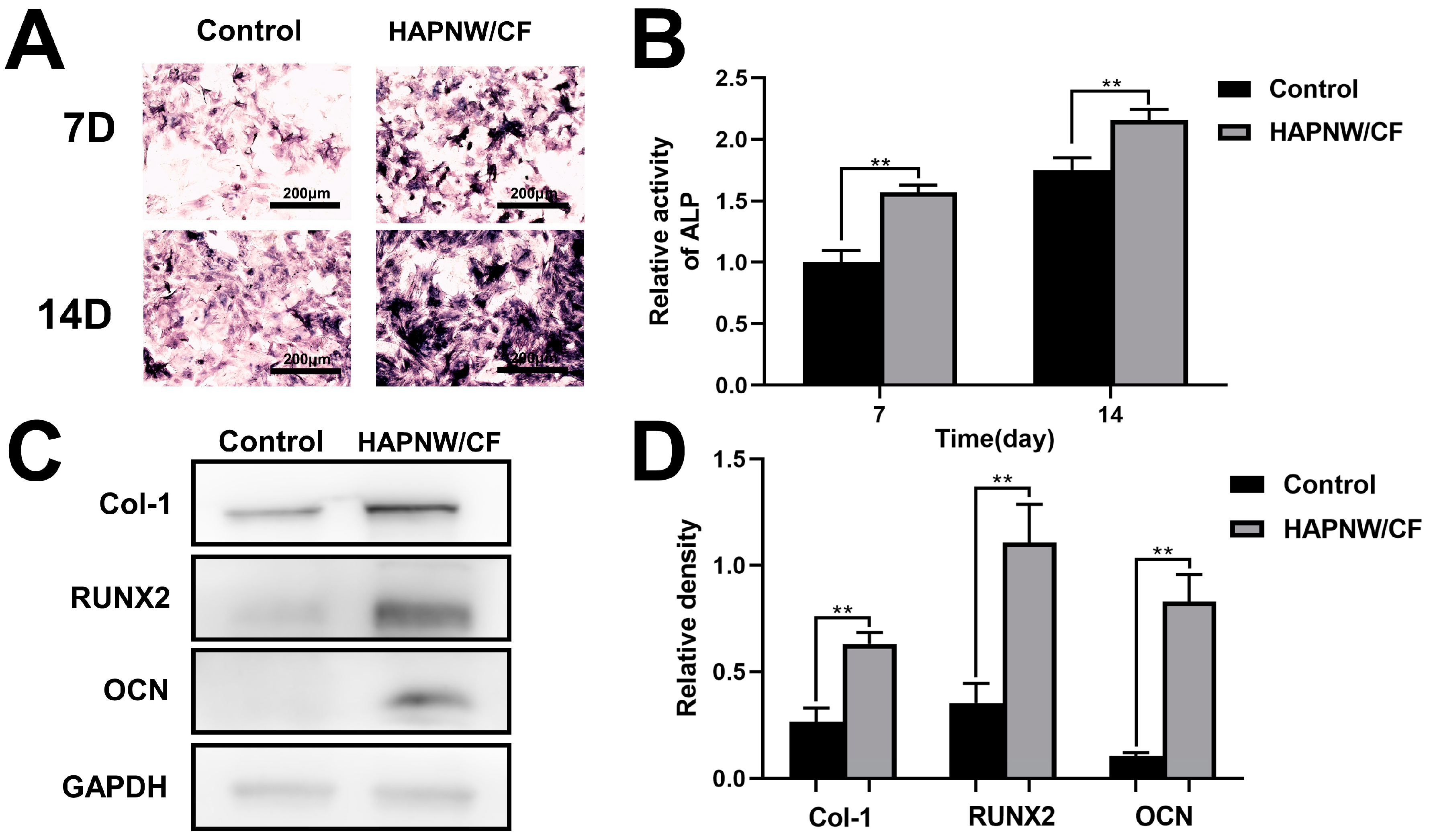
3.3. In Vitro Pro-Osteogenic and Pro-Angiogenic Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Liu, J.; Zhu, T.; Le, H.; Wang, X.; Guo, J.; Liu, G.; Ding, J. Functional Macromolecular Adhesives for Bone Fracture Healing. ACS Appl. Mater. Interfaces 2022, 14, 1–19. [Google Scholar] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar]
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv. Mater. 2022, 34, 2202044. [Google Scholar]
- Schätzlein, E.; Kicker, C.; Söhling, N.; Ritz, U.; Neijhoft, J.; Henrich, D.; Frank, J.; Marzi, I.; Blaeser, A. 3D-Printed PLA-Bioglass Scaffolds with Controllable Calcium Release and MSC Adhesion for Bone Tissue Engineering. Polymers 2022, 14, 238. [Google Scholar]
- Zhang, Y.; Wang, C.; Fu, L.; Ye, S.; Wang, M.; Zhou, Y. Fabrication and Application of Novel Porous Scaffold in Situ-Loaded Graphene Oxide and Osteogenic Peptide by Cryogenic 3D Printing for Repairing Critical-Sized Bone Defect. Molecules 2019, 24, 1669. [Google Scholar]
- Freeman, F.; Browe, D.; Nulty, J.; Von Euw, S.; Grayson, W.; Kelly, D. Biofabrication of Multiscale Bone Extracellular Matrix Scaffolds for Bone Tissue Engineering. Eur. Cells Mater. 2019, 38, 168–187. [Google Scholar]
- Zhao, D.; Wang, X.; Cheng, B.; Yin, M.; Hou, Z.; Li, X.; Liu, K.; Tie, C.; Yin, M. Degradation-Kinetics-Controllable and Tissue-Regeneration-Matchable Photocross-Linked Alginate Hydrogels for Bone Repair. ACS Appl. Mater. Interfaces 2022, 14, 21886–22190. [Google Scholar]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar]
- Cao, D.; Martinez, J.G.; Anada, R.; Hara, E.S.; Kamioka, H.; Jager, E.W.H. Electrochemical Control of Bone Microstructure on Electroactive Surfaces for Modulation of Stem Cells and Bone Tissue Engineering. Sci. Technol. Adv. Mater. 2023, 24, 2183710. [Google Scholar]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar]
- Golchin, A.; Chatziparasidou, A.; Ranjbarvan, P.; Niknam, Z.; Ardeshirylajimi, A. Embryonic Stem Cells in Clinical Trials: Current Overview of Developments and Challenges. In Cell Biology and Translational Medicine, Volume 11; Advances in Experimental Medicine and Biology; Turksen, K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1312, pp. 19–37. [Google Scholar]
- Guan, S.; Wang, Y.; Xie, F.; Wang, S.; Xu, W.; Xu, J.; Sun, C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules 2022, 27, 8326. [Google Scholar]
- Zhang, L.; Ma, X.-J.-N.; Fei, Y.-Y.; Han, H.-T.; Xu, J.; Cheng, L.; Li, X. Stem Cell Therapy in Liver Regeneration: Focus on Mesenchymal Stem Cells and Induced Pluripotent Stem Cells. Pharmacol. Ther. 2022, 232, 108004. [Google Scholar] [PubMed]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 11592. [Google Scholar] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of Isolation Methodology on Stem Cell Properties and Multilineage Differentiation Potential of Human Dental Pulp Stem Cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [PubMed]
- Kim, B.-C.; Bae, H.; Kwon, I.-K.; Lee, E.-J.; Park, J.-H.; Khademhosseini, A.; Hwang, Y.-S. Osteoblastic/Cementoblastic and Neural Differentiation of Dental Stem Cells and Their Applications to Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2012, 18, 235–244. [Google Scholar] [PubMed]
- Lee, J.-H.; Lee, D.-S.; Choung, H.-W.; Shon, W.-J.; Seo, B.-M.; Lee, E.-H.; Cho, J.-Y.; Park, J.-C. Odontogenic Differentiation of Human Dental Pulp Stem Cells Induced by Preameloblast-Derived Factors. Biomaterials 2011, 32, 9696–9706. [Google Scholar] [PubMed]
- Sloan, A.; Smith, A. Stem Cells and the Dental Pulp: Potential Roles in Dentine Regeneration and Repair. Oral Dis. 2007, 13, 151–157. [Google Scholar] [PubMed]
- Stuepp, R.T.; Barros Delben, P.; Modolo, F.; Trentin, A.G.; Garcez, R.C.; Biz, M.T. Human Dental Pulp Stem Cells in Rat Mandibular Bone Defects. Cells Tissues Organs 2019, 207, 138–148. [Google Scholar]
- Song, F.; Sun, H.; Huang, L.; Fu, D.; Huang, C. The Role of Pannexin3-Modified Human Dental Pulp-Derived Mesenchymal Stromal Cells in Repairing Rat Cranial Critical-Sized Bone Defects. Cell Physiol. Biochem. 2017, 44, 2174–2188. [Google Scholar]
- Sun, T.-W.; Zhu, Y.-J.; Chen, F. Hydroxyapatite Nanowire/Collagen Elastic Porous Nanocomposite and Its Enhanced Performance in Bone Defect Repair. RSC Adv. 2018, 8, 26218–26229. [Google Scholar]
- Fernandes, M.H.; Alves, M.M.; Cebotarenco, M.; Ribeiro, I.A.C.; Grenho, L.; Gomes, P.S.; Carmezim, M.J.; Santos, C.F. Citrate Zinc Hydroxyapatite Nanorods with Enhanced Cytocompatibility and Osteogenesis for Bone Regeneration. Mater. Sci. Eng. C 2020, 115, 111147. [Google Scholar]
- Kashiwada, H.; Shimizu, Y.; Sano, Y.; Yamauchi, K.; Guang, H.; Kumamoto, H.; Unuma, H.; Zhu, Y.-J. In vivo Behaviors of Highly Flexible Paper Consisting of Ultralong Hydroxyapatite Nanowires. J. Biomed. Mater. Res. Part B 2021, 109, 1611–1621. [Google Scholar]
- George, S.M.; Nayak, C.; Singh, I.; Balani, K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2022, 8, 3162–3186. [Google Scholar]
- Cheng, D.; Ding, R.; Jin, X.; Lu, Y.; Bao, W.; Zhao, Y.; Chen, S.; Shen, C.; Yang, Q.; Wang, Y. Strontium Ion-Functionalized Nano-Hydroxyapatite/Chitosan Composite Microspheres Promote Osteogenesis and Angiogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 19951–19965. [Google Scholar]
- Maimaiti, B.; Zhang, N.; Yan, L.; Luo, J.; Xie, C.; Wang, Y.; Ma, C.; Ye, T. Stable ZnO-Doped Hydroxyapatite Nanocoating for Anti-Infection and Osteogenic on Titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar]
- Tenkumo, T.; Vanegas Sáenz, J.R.; Takada, Y.; Takahashi, M.; Rotan, O.; Sokolova, V.; Epple, M.; Sasaki, K. Gene Transfection of Human Mesenchymal Stem Cells with a Nano-hydroxyapatite–Collagen Scaffold Containing DNA-functionalized Calcium Phosphate Nanoparticles. Genes Cells 2016, 21, 682–695. [Google Scholar]
- Munir, M.U.; Salman, S.; Javed, I.; Bukhari, S.N.A.; Ahmad, N.; Shad, N.A.; Aziz, F. Nano-Hydroxyapatite as a Delivery System: Overview and Advancements. Artif. Cells Nanomed. Biotechnol. 2021, 49, 717–727. [Google Scholar]
- Zhu, Y.J.; Lu, B.Q. Deformable Biomaterials Based on Ultralong Hydroxyapatite Nanowires. ACS Biomater. Sci. Eng. 2019, 5, 4951–4961. [Google Scholar]
- Gao, J.; Hao, L.-S.; Ning, B.-B.; Zhu, Y.-K.; Guan, J.-B.; Ren, H.-W.; Yu, H.-P.; Zhu, Y.-J.; Duan, J.-L. Biopaper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers Promotes Skin Wound Healing by Inducing Angiogenesis. Coatings 2022, 12, 479. [Google Scholar]
- Wang, F.; Qian, H.; Kong, L.; Wang, W.; Wang, X.; Xu, Z.; Chai, Y.; Xu, J.; Kang, Q. Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2021, 17, 1821–1836. [Google Scholar]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar]
- Ji, X.; Yuan, X.; Ma, L.; Bi, B.; Zhu, H.; Lei, Z.; Liu, W.; Pu, H.; Jiang, J.; Jiang, X.; et al. Mesenchymal Stem Cell-Loaded Thermosensitive Hydroxypropyl Chitin Hydrogel Combined with a Three-Dimensional-Printed Poly(ε-Caprolactone) /Nano-Hydroxyapatite Scaffold to Repair Bone Defects via Osteogenesis, Angiogenesis and Immunomodulation. Theranostics 2020, 10, 725–740. [Google Scholar] [PubMed]
- Huang, G.-J.; Yu, H.-P.; Wang, X.-L.; Ning, B.-B.; Gao, J.; Shi, Y.-Q.; Zhu, Y.-J.; Duan, J.-L. Highly Porous and Elastic Aerogel Based on Ultralong Hydroxyapatite Nanowires for High-Performance Bone Regeneration and Neovascularization. J. Mater. Chem. B 2021, 9, 1277–1287. [Google Scholar]
- Jia, Q.; Chen, X.; Jiang, W.; Wang, W.; Guo, B.; Ni, L. The Regulatory Effects of Long Noncoding RNA-ANCR on Dental Tissue-Derived Stem Cells. Stem Cells Int. 2016, 2016, 3146805. [Google Scholar] [PubMed]
- Zeng, L.; Sun, S.; Han, D.; Liu, Y.; Liu, H.; Feng, H.; Wang, Y. Long Non-Coding RNA H19/SAHH Axis Epigenetically Regulates Odontogenic Differentiation of Human Dental Pulp Stem Cells. Cell Signal. 2018, 52, 65–73. [Google Scholar]
- Krasny, M.; Krasny, K.; Fiedor, P.; Zadurska, M.; Kamiński, A. Long-Term Outcomes of the Use of Allogeneic, Radiation-Sterilised Bone Blocks in Reconstruction of the Atrophied Alveolar Ridge in the Maxilla and Mandible. Cell Tissue Bank 2015, 16, 631–638. [Google Scholar] [PubMed]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M.; CERTiFy Study Group. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surg. 2020, 102, 179–193. [Google Scholar]
- Moshaverinia, A.; Ansari, S.; Chen, C.; Xu, X.; Akiyama, K.; Snead, M.L.; Zadeh, H.H.; Shi, S. Co-Encapsulation of Anti-BMP2 Monoclonal Antibody and Mesenchymal Stem Cells in Alginate Microspheres for Bone Tissue Engineering. Biomaterials 2013, 34, 6572–6579. [Google Scholar]
- Chen, Z.; Zhang, K.; Qiu, W.; Luo, Y.; Pan, Y.; Li, J.; Yang, Y.; Wu, B.; Fang, F. Genome-Wide Identification of Long Noncoding RNAs and Their Competing Endogenous RNA Networks Involved in the Odontogenic Differentiation of Human Dental Pulp Stem Cells. Stem Cell Res. Ther. 2020, 11, 114. [Google Scholar]
- Bao, M.; Liu, G.; Song, J.; Gao, Y. Long Non-Coding RNA MALAT1 Promotes Odontogenic Differentiation of Human Dental Pulp Stem Cells by Impairing microRNA-140-5p-Dependent Downregulation of GIT2. Cell Tissue Res. 2020, 382, 487–498. [Google Scholar]
- Agriesti, F.; Landini, F.; Tamma, M.; Pacelli, C.; Mazzoccoli, C.; Calice, G.; Ruggieri, V.; Capitanio, G.; Mori, G.; Piccoli, C.; et al. Bioenergetic Profile and Redox Tone Modulate in Vitro Osteogenesis of Human Dental Pulp Stem Cells: New Perspectives for Bone Regeneration and Repair. Stem Cell Res. Ther. 2023, 14, 215. [Google Scholar]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar]
- Li, J.; Du, H.; Ji, X.; Chen, Y.; Li, Y.; Heng, B.C.; Xu, J. ETV2 Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells through the ERK/MAPK and PI3K-Akt Signaling Pathways. Stem Cell Res. Ther. 2022, 13, 495. [Google Scholar]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as Biomimetic Tools for Stem Cell Differentiation: Applications in Dental Pulp Tissue Regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar]
- Jin, Q.; Yuan, K.; Lin, W.; Niu, C.; Ma, R.; Huang, Z. Comparative Characterization of Mesenchymal Stem Cells from Human Dental Pulp and Adipose Tissue for Bone Regeneration Potential. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1577–1584. [Google Scholar]
- Petridis, X.; Diamanti, E.; Trigas, G.C.; Kalyvas, D.; Kitraki, E. Bone Regeneration in Critical-Size Calvarial Defects Using Human Dental Pulp Cells in an Extracellular Matrix-Based Scaffold. J. Cranio-Maxillofac. Surg. 2015, 43, 483–490. [Google Scholar]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human Mandible Bone Defect Repair by the Grafting of Dental Pulp Stem/Progenitor Cells and Collagen Sponge Biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar]
- Zheng, P.; Yao, Q.; Mao, F.; Liu, N.; Xu, Y.; Wei, B.; Wang, L. Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells in 3D Printed Poly-ε-Caprolactone/Hydroxyapatite Scaffolds Combined with Bone Marrow Clots. Mol. Med. Rep. 2017, 16, 5078–5084. [Google Scholar]
- Chen, Y.; Zhang, F.; Fu, Q.; Liu, Y.; Wang, Z.; Qi, N. In Vitro Proliferation and Osteogenic Differentiation of Human Dental Pulp Stem Cells in Injectable Thermo-Sensitive Chitosan/β-Glycerophosphate/Hydroxyapatite Hydrogel. J. Biomater. Appl. 2016, 31, 317–327. [Google Scholar]
- Wang, B.; Li, Y.; Wang, S.; Jia, F.; Bian, A.; Wang, K.; Xie, L.; Yan, K.; Qiao, H.; Lin, H.; et al. Electrodeposited Dopamine/Strontium-Doped Hydroxyapatite Composite Coating on Pure Zinc for Anti-Corrosion, Antimicrobial and Osteogenesis. Mater. Sci. Eng. C 2021, 129, 112387. [Google Scholar]
- Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar]
- Lin, J.H.C.; Kuo, K.H.; Ding, S.J.; Ju, C.P. Surface Reaction of Stoichiometric and Calcium-Deficient Hydroxyapatite in Simulated Body Fluid. J. Mater. Sci. Mater. Med. 2001, 12, 731–741. [Google Scholar]
- Kang, H.-J.; Makkar, P.; Padalhin, A.R.; Lee, G.-H.; Im, S.-B.; Lee, B.-T. Comparative Study on Biodegradation and Biocompatibility of Multichannel Calcium Phosphate Based Bone Substitutes. Mater. Sci. Eng. C 2020, 110, 110694. [Google Scholar]
- Liu, Y.; Long, L.; Zhang, F.; Hu, X.; Zhang, J.; Hu, C.; Wang, Y.; Xu, J. Microneedle-Mediated Vascular Endothelial Growth Factor Delivery Promotes Angiogenesis and Functional Recovery after Stroke. J. Control. Release 2021, 338, 610–622. [Google Scholar] [PubMed]
- Abdulkadir, S.; Li, C.; Jiang, W.; Zhao, X.; Sang, P.; Wei, L.; Hu, Y.; Li, Q.; Cai, J. Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor. J. Am. Chem. Soc. 2022, 144, 270–281. [Google Scholar] [PubMed]
- Wood, W. Wound Healing: Calcium Flashes Illuminate Early Events. Curr. Biol. 2012, 22, R14–R16. [Google Scholar]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A Novel Magnesium Ion-Incorporating Dual-Crosslinked Hydrogel to Improve Bone Scaffold-Mediated Osteogenesis and Angiogenesis. Mater. Sci. Eng. C 2021, 121, 111868. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Lai, W.; Zhu, Y.; Hao, L.; Gao, J.; Yang, C.; Yuan, L.; Hao, C.; Duan, J.; Lu, Y. Enhanced Osteogenic Differentiation Based on Combining Pulp Stem Cells with Ultralong Hydroxyapatite Nanowires and Cellulose Fibers. Coatings 2024, 14, 190. https://doi.org/10.3390/coatings14020190
Liu K, Lai W, Zhu Y, Hao L, Gao J, Yang C, Yuan L, Hao C, Duan J, Lu Y. Enhanced Osteogenic Differentiation Based on Combining Pulp Stem Cells with Ultralong Hydroxyapatite Nanowires and Cellulose Fibers. Coatings. 2024; 14(2):190. https://doi.org/10.3390/coatings14020190
Chicago/Turabian StyleLiu, Kai, Wen Lai, Yuankang Zhu, Liangshi Hao, Jing Gao, Chenglong Yang, Lifeng Yuan, Changning Hao, Junli Duan, and Yongjian Lu. 2024. "Enhanced Osteogenic Differentiation Based on Combining Pulp Stem Cells with Ultralong Hydroxyapatite Nanowires and Cellulose Fibers" Coatings 14, no. 2: 190. https://doi.org/10.3390/coatings14020190
APA StyleLiu, K., Lai, W., Zhu, Y., Hao, L., Gao, J., Yang, C., Yuan, L., Hao, C., Duan, J., & Lu, Y. (2024). Enhanced Osteogenic Differentiation Based on Combining Pulp Stem Cells with Ultralong Hydroxyapatite Nanowires and Cellulose Fibers. Coatings, 14(2), 190. https://doi.org/10.3390/coatings14020190





