Preparation of NaCl Particles Added Polyvinylidene Fluoride Microporous Filter and a Simple Filtration Device
Abstract
:1. Introduction
2. Experimental
2.1. Materials
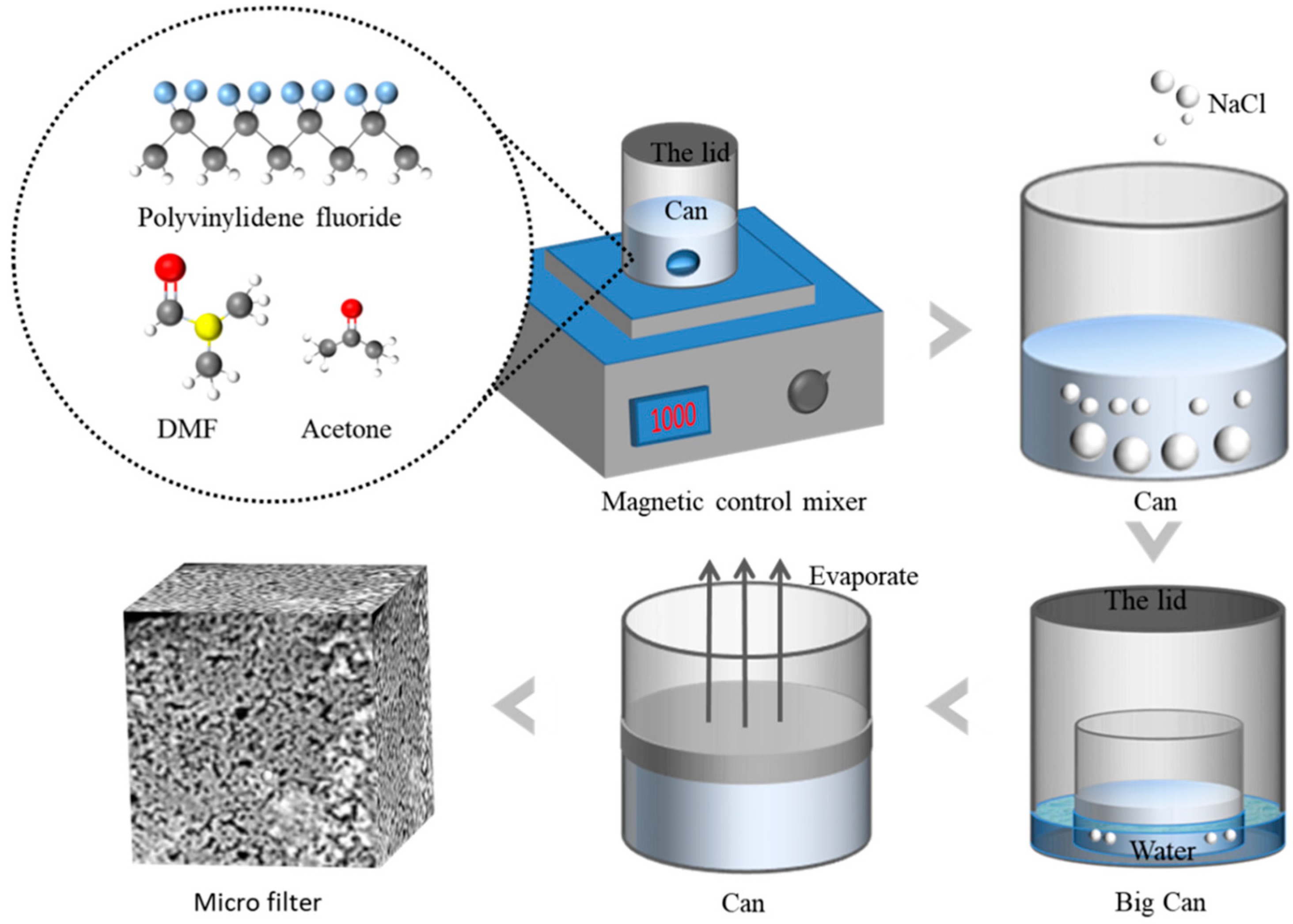
2.2. Preparation of PVDF-MPF by NIPS and VIPS Methods
2.3. Impurity Filtration Test of Two Kinds of PVDF-MPFs
2.4. Morphology of the Five Different PVDF-MPFs
2.5. Porosity of the Five Different PVDF-MPFs
3. Results and Discussions
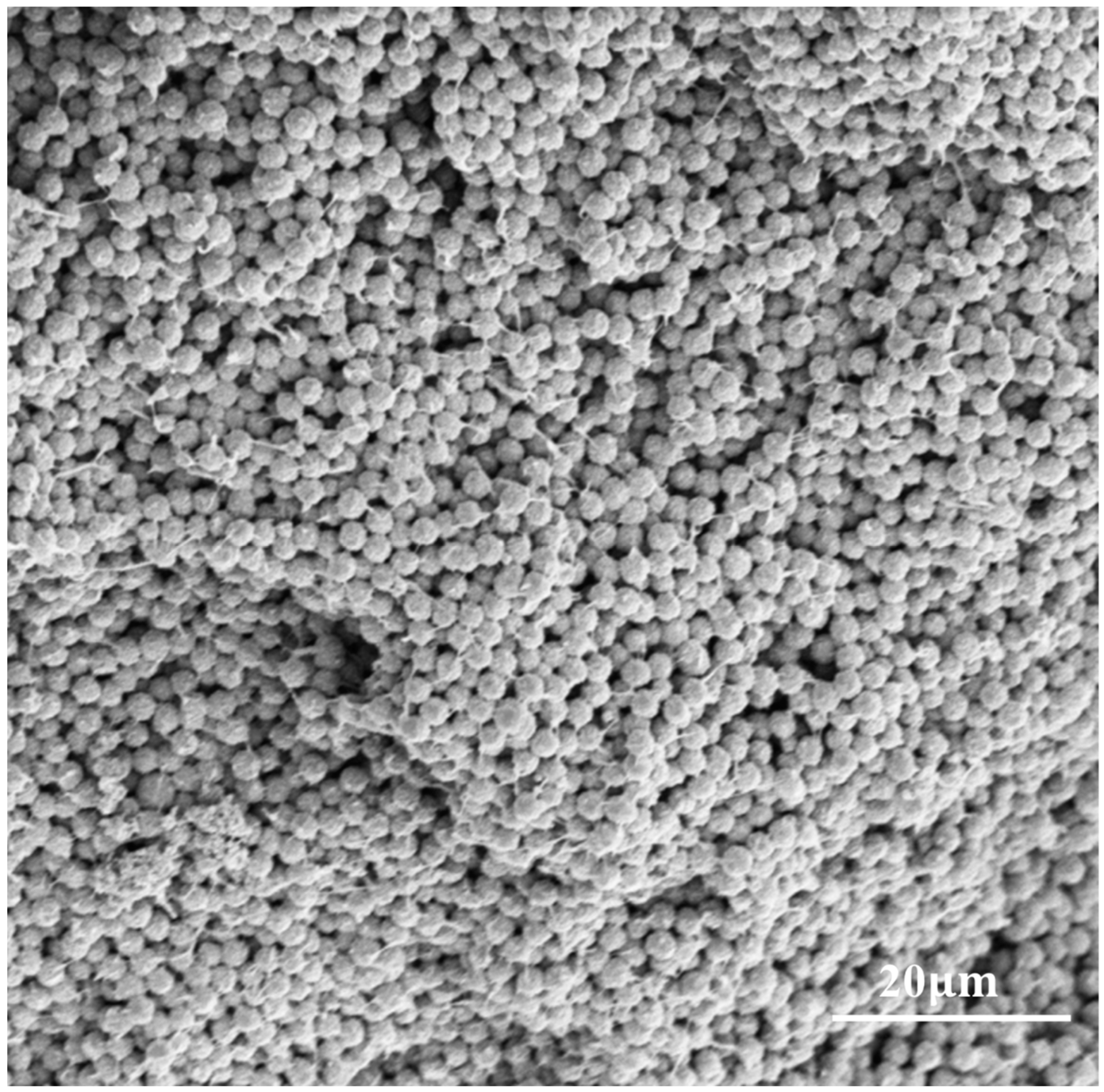
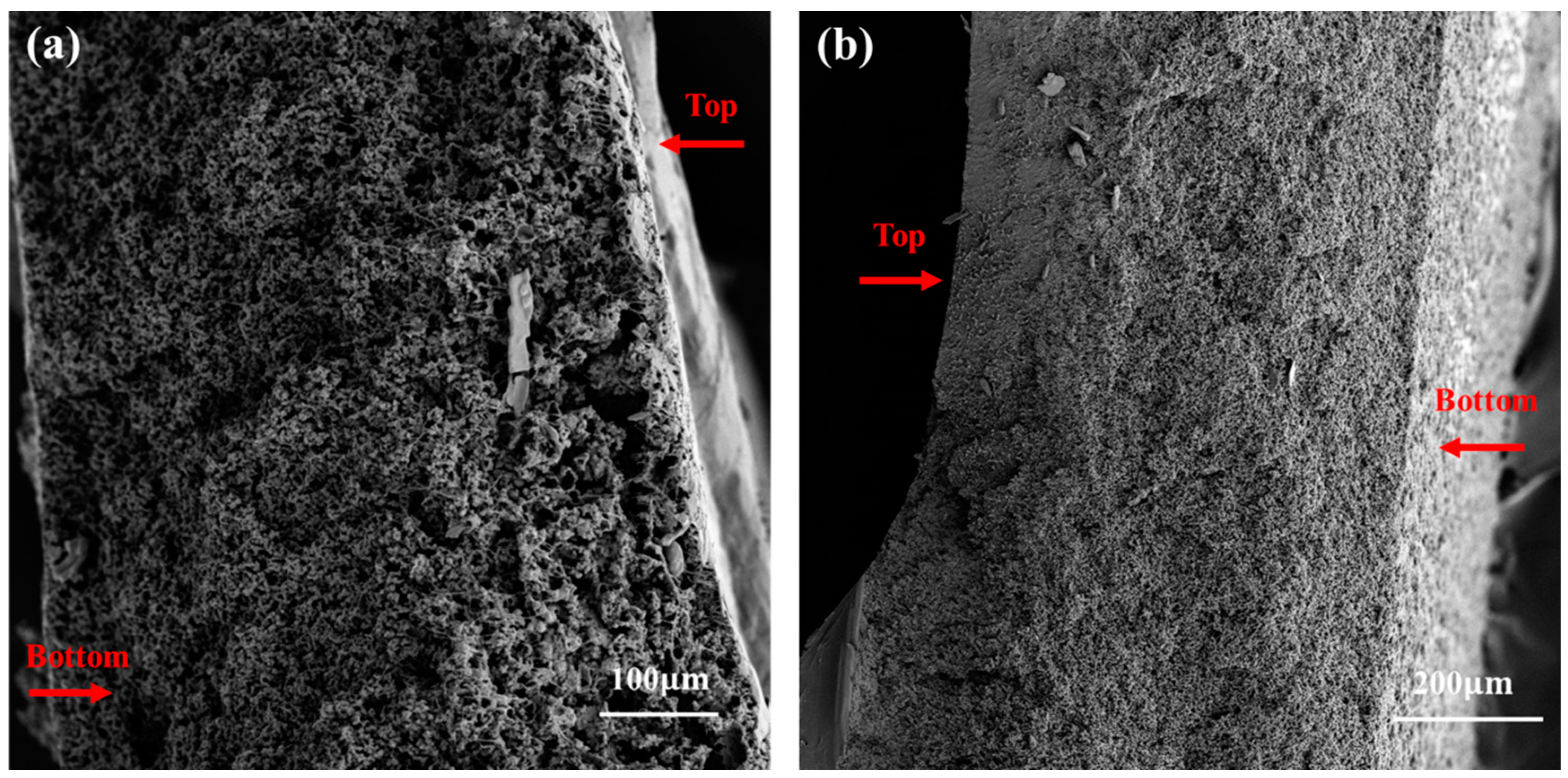
3.1. Microscopic Processes of NIPS and VIPS
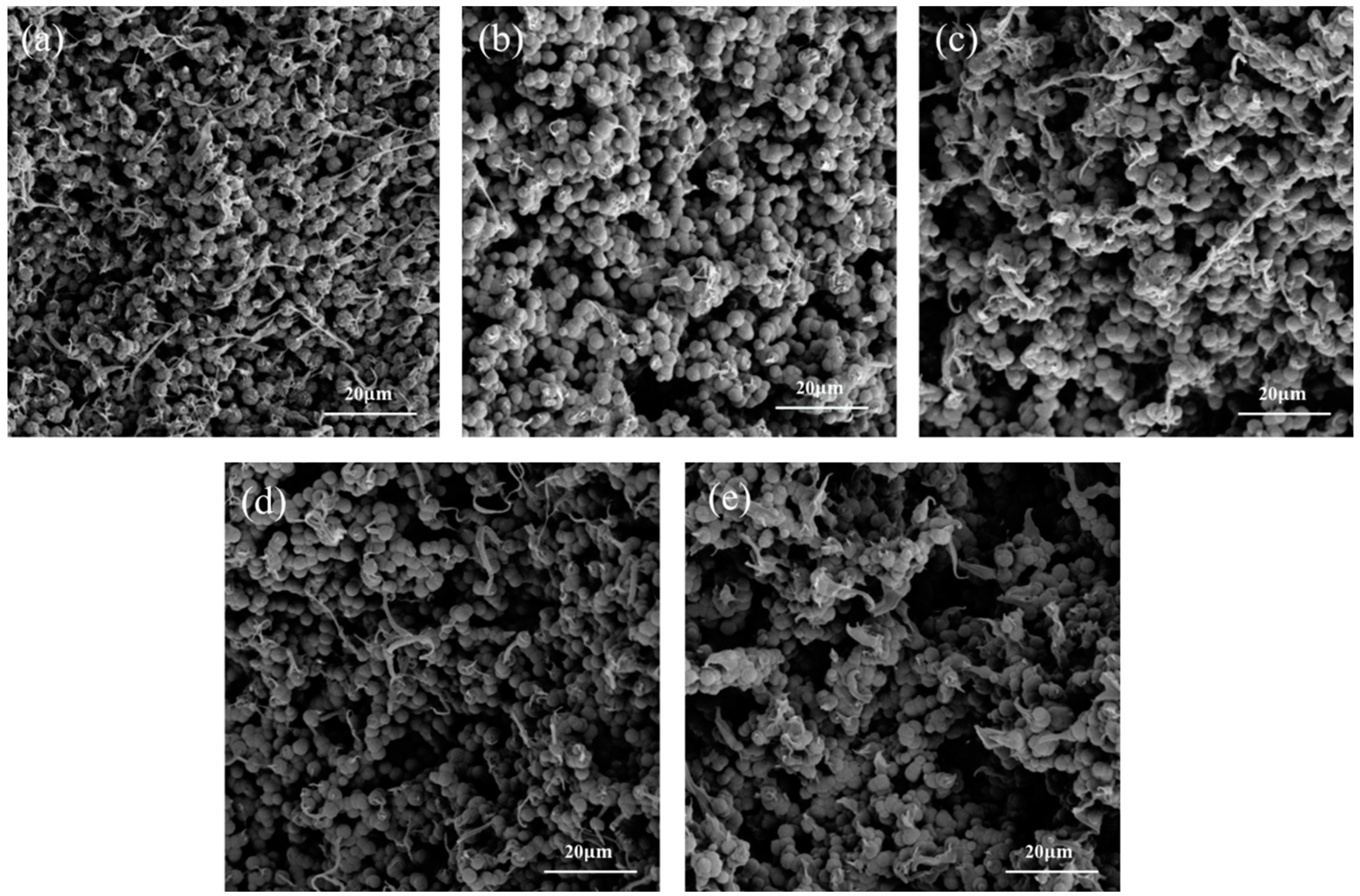
3.2. The Influence of NaCl-ps Size for PVDF-MPF
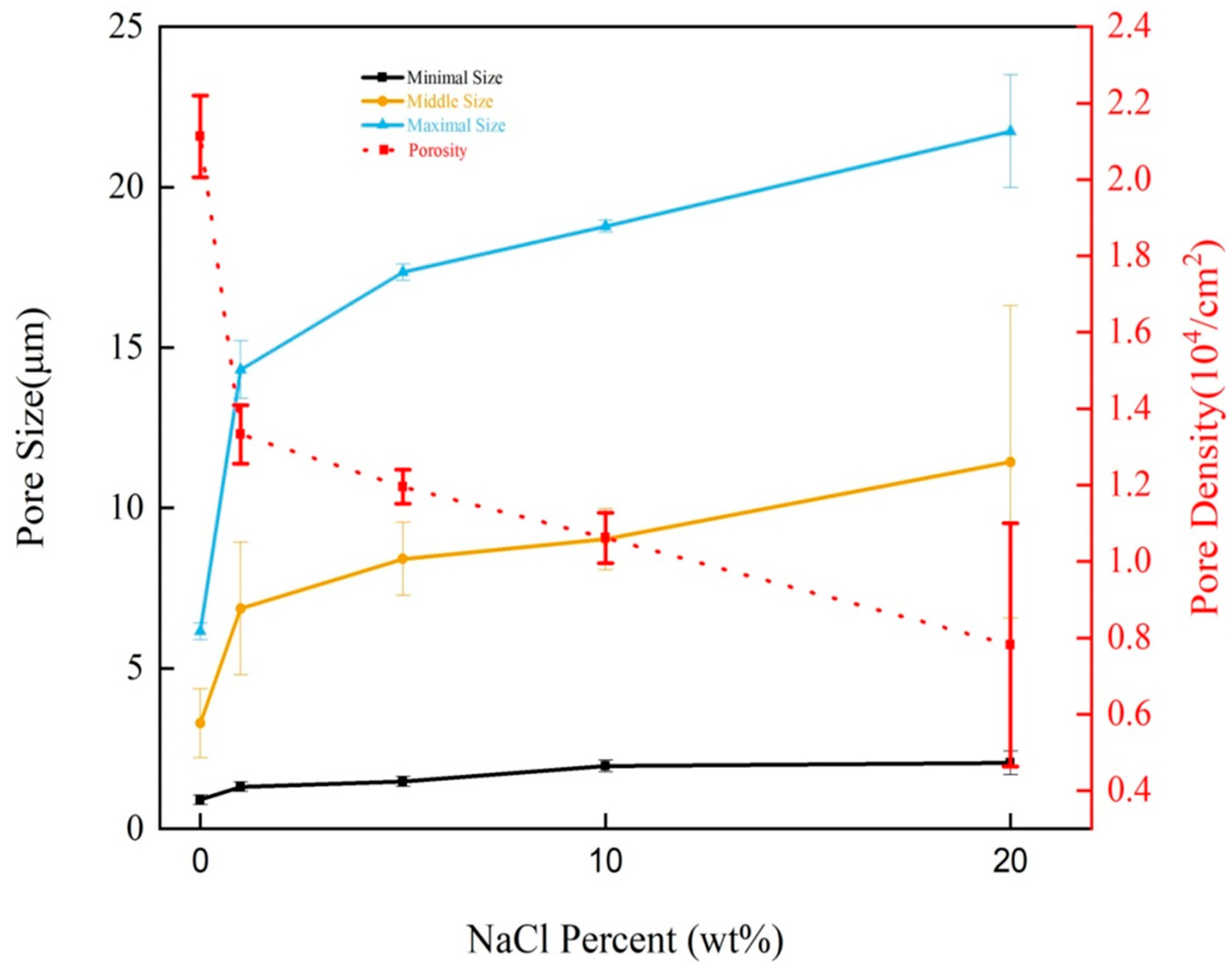
3.3. The Effect of Adding Weight of NaCl-ps on PVDF-MPF
3.4. Water Absorption of PVDF-MPF Added with Different NaCl-ps Weights
| NaCl-ps Concentration (wt%) | Weight of PVDF-MPFs before Being Filled with Water (g) | Weight of PVDF-MPFs after Being Filled with Water (g) | Porosity (%) |
|---|---|---|---|
| 0 | 0.0503 | 0.0504 | 0.35 |
| 1 | 0.0205 | 0.0411 | 64.21 |
| 5 | 0.0230 | 0.0483 | 66.26 |
| 10 | 0.0712 | 0.1488 | 66.05 |
| 20 | 0.0183 | 0.0532 | 77.29 |
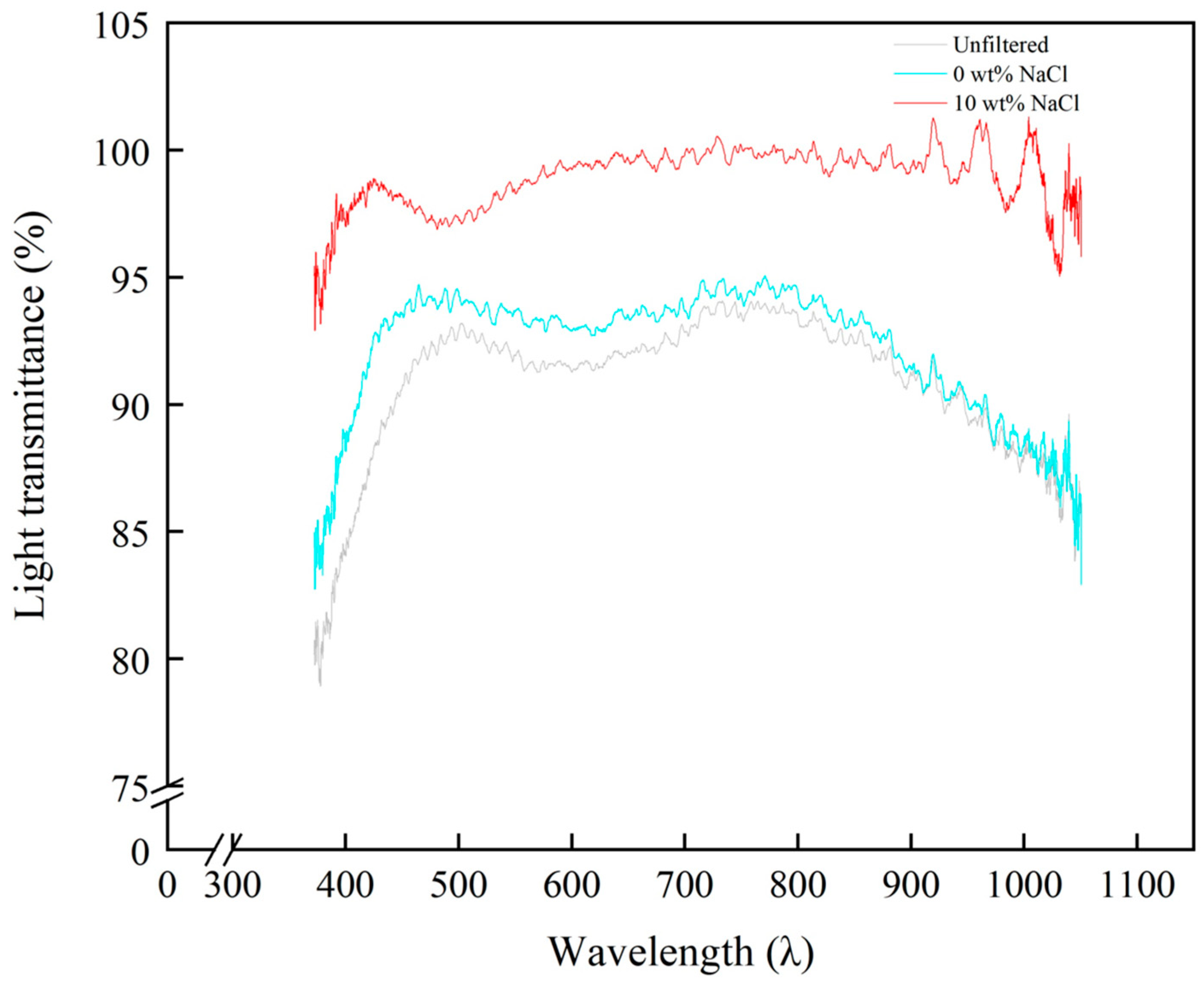
3.5. Performance Test of PVDF-MPF for Carbon Ink Purification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alsulaili, A.; Al-Harbi, M.; Elsayed, K. The influence of household filter types on quality of drinking water. Process Saf. Environ. Protect. 2020, 143, 204–211. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Pararneswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Lindqvist, J.; Malmstrom, E. Surface modification of natural substrates by atom transfer radical polymerization. J. Appl. Polym. Sci. 2006, 100, 4155–4162. [Google Scholar] [CrossRef]
- Lal, R. World Water Resources and Achieving Water Security. Agron. J. 2015, 107, 1526–1532. [Google Scholar] [CrossRef]
- Wotton, R.S. Water purification using sand. Hydrobilogia 2002, 469, 193–201. [Google Scholar] [CrossRef]
- Melian, J.A.H.; Arnana, J.; Gonzalez Diaz, O.; Aguiar Bujalance, M.E.; Dona Rodriguez, J.M. Effect of stone filters in a pond-wetland system treating raw wastewater from a university campus. Desalination 2009, 237, 277–284. [Google Scholar] [CrossRef]
- Hubbel, L.; Elmore, A.C.; Reidmeyer, M. Comparison of a native clay soil and an engineered clay used in experimental ceramic pot filter fabrication. Water Sci. Technol.-Water Supply 2015, 15, 569–577. [Google Scholar] [CrossRef]
- Masmali, I.; Khalid, A.; Shuaib, U.; Razaq, A.; Garg, H.; Razzaque, A. On Selection of the Efficient Water Purification Strategy at Commercial Scale Using Complex Intuitionistic Fuzzy Dombi Environment. Water 2023, 10, 1907. [Google Scholar] [CrossRef]
- Sarkar, S.; Tribedi, P.; Das Gupta, A.; Saha, T.; Sil, A.K. Microbial Functional Diversity Decreases with Sewage Purification in Stabilization Ponds. Waste Biomass Valorization 2017, 8, 417–423. [Google Scholar] [CrossRef]
- Losch, P.; Anotonyuk, S. Selective particle deposition at cross-flow filtration with constant filtrate flux. Powder Technol. 2021, 388, 305–317. [Google Scholar] [CrossRef]
- Templeton, E.J.; Salin, E.D. A novel filtration method integrated on centrifugal microfluidic devices. Microfluid. Nanofluid. 2014, 17, 245–251. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Kang, P.K.; Shah, D.O. Filtration of nanoparticles with dimethyldioctadecylammonium bromide treated microporous polypropylene filters. Langmuir 1997, 13, 1820–1826. [Google Scholar] [CrossRef]
- Hong, S.K.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohammad, A.W.; Abu Arabi, M. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Aryanti, P.T.; Nugroho, F.A.; Widiasa, I.N.; Sutrisna, P.D.; Wenten, I.G. Preparation of highly selective PSf/ZnO/PEG400 tight ultrafiltration membrane for dyes removal. J. Membr. Sci. 2022, 139, e52779. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Li, J.Q.; Yuan, X.Y.; Li, X.; Zhang, Y.Y.; Sheng, J. Preparation and properties of electrospun poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2005, 97, 466–474. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.C.; Matsuura, I.; Gopal, R.; Kaur, S.; Rarnakrishna, S.; Khayet, M. Production of drinking water from saline water by air-gap membrane distillation using polyvinylidene fluoride nanofiber membrane. J. Membr. Sci. 2008, 311, 1–6. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.Q.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2012, 425, 30–39. [Google Scholar] [CrossRef]
- Liu, P.; Wang, J.H.; Chen, J.; Liu, L.M.; Wang, H.H.; Zeng, B.Q.; Chi, F. A new structure of flexible oled with copper nanowire anode and graphere oxide/PEDOT: PSS anode buffer layer. Surf. Rev. Lett. 2020, 27, 1950171. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, P.; Wang, H.H.; Zeng, B.Q.; Wang, J.H.; Chi, F. Flexible organic light-emitting devices with copper nanowire composite transparent conductive electrode. J. Mater. Sci. 2018, 54, 2343–2350. [Google Scholar] [CrossRef]
- Xu, H.R.; Liu, P.; Huang, B.; Jiang, X.W.; Gao, Q.G.; Liu, L.M. Preparation of Double-Layer Crossed Silver Nanowire Film and Its Application to OLED. Coatings 2022, 12, 26. [Google Scholar] [CrossRef]
- Trommer, K.; Palitzsch, W.; Morgenstern, B.; Loser, U. Microporous PVC—New Membrane Material for Application in Microfiltration. Chem. Ing. Tech. 2012, 84, 63–69. [Google Scholar] [CrossRef]
- Kostov, G.; Christova, N.; Charadjiev, P. Microporous membrane filters on the basis of poly(tetrafluoroethylene). Acta Polym. 1991, 42, 499–503. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Ariono, D.; Aryanti, P.T.P.; Hakim, A.N.; Subagjo, S.; Wenten, I.G. Determination of Thermodynamic Properties of polysulfone/PEG Membrane Solutions Based on Flory-Huggins Model. AIP Conf. Proc. 2017, 1840, 090008. [Google Scholar]
- Jin, T.T.; Zhao, Z.P.; Chen, K.C. Preparation of a poly(vinyl chloride) ultrafiltration membrane through the combination of thermally induced phase separation and non-solvent-induced phase separation. J. Appl. Polym. Sci. 2015, 133, 42953. [Google Scholar] [CrossRef]
- Matsuyama, H.; Teramoto, M.; Nakatani, R.; Maki, T. Membrane formation via phase separation induced by penetration of nonsolvent from vapor phase. I. Phase diagram and mass transfer process. J. Appl. Polym. Sci. 1999, 74, 159–170. [Google Scholar] [CrossRef]
- Gu, M.H.; Zhang, J.; Wang, X.L.; Ma, W.Z. Crystallization behavior of PVDF in PVDF-DMP system via thermally induced phase separation. J. Appl. Polym. Sci. 2006, 102, 3714–3719. [Google Scholar] [CrossRef]
- Hernandez, A.; Calvo, J.I.; Pradanos, P.; Tejerina, F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J. Membr. Sci. 1996, 112, 1–12. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Arul, H.; Bosak, V.Z.; Burban, A.F.; Voice, T.C.; Bruening, M.L.; Tarabara, V.V. Effect of filler incorporation route on the properties of polysulfone-silver nanocomposite membranes of different porosities. J. Membr. Sci. 2008, 325, 58–68. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Luque-Alled, J.M.; Leaper, S.; Alberto, M.; Fan, X.; Vijayaraghavan, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Szekely, G.; Ahmed, S.I.A.; et al. PVDF membranes containing reduced graphene oxide: Effect of degree of reduction on membrane distillation performance. Desalination 2019, 452, 196–207. [Google Scholar] [CrossRef]
- Julian, H.; Sutrisna, P.D.; Hakim, A.N.; Harsono, H.O.; Hugo, Y.A.; Wenten, I.G. Nano-silica/polysulfone asymmetric mixed-matrix membranes (MMMs) with high CO2 permeance in the application of CO2/N2 separation. Polym. Plast. Technol. Mater. 2019, 58, 678–689. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water Desalination Using Carbon-Nanotube-Enhanced Membrane Distillation. ACS Appl. Mater. Interfaces 2011, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mondal, P.; Chakraborty, A.; Kuttippurath, J.; Purkait, M. Effect of different molecular weight polyethylene glycol on flat sheet cellulose acetate membranes for evaluating power density performance in pressure retarded osmosis study. J. Water Process Eng. 2019, 30, 100632. [Google Scholar] [CrossRef]
- Chen, F.Z.; Lu, Y.; Liu, X.; Song, J.L.; He, G.J.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P. Table Salt as a Template to Prepare Reusable Porous PVDF-MWCNT Foam for Separation of Immiscible Oils/Organic Solvents and Corrosive Aqueous Solutions. Adv. Funct. Mater. 2017, 27, 1702926. [Google Scholar] [CrossRef]
- Bian, W.B.; Zhang, R.X.; Chen, X.H.; Zhang, C.X.; Meng, M. Three-Dimensional Porous PVDF Foam Imprinted Membranes with High Flux and Selectivity toward Artemisinin/Artemether. J. Mol. 2023, 28, 7452. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, X.Y.; Wu, H.B. Adsorption and Desorption Properties of Modified Feather and Feather/PP Melt-Blown Filter Cartridge of Lead Ion (Pb2+). J. Appl. Polym. Sci. 2015, 132, 41555. [Google Scholar] [CrossRef]
- Arteta, S.M.; Vera, R.; Perez, L.D. Hydrophobic cellulose fibers via ATRP and their performance in the removal of pyrene from water. J. Appl. Polym. Sci. 2017, 134, 44482. [Google Scholar] [CrossRef]
- Wu, Y.L.; Kong, Y.; Lin, X.; Liu, W.H.; Xu, J.P. Surface-modified hydrophilic membranes in membrane distillation. J. Membr. Sci. 1992, 72, 189–196. [Google Scholar] [CrossRef]
- Jung, J.; Shin, Y.; Choi, Y.J.; Sohn, J.; Lee, S.; An, K. Hydrophobic surface modification of membrane distillation (MD) membranes using water-repelling polymer based on urethane rubber. Desalination Water Treat. 2016, 57, 10031–10041. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zong, Y.; Yang, N.; Zhang, N.; Jiang, B.; Zhang, L.H.; Xiao, X.M. Surface hydrophilic modification of PVDF membranes based on tannin and zwitterionic substance towards effective oil-in-water emulsion separation. Sep. Purif. Technol. 2019, 234, 116015. [Google Scholar] [CrossRef]
- Ruan, L.X.; Yao, X.N.; Chang, Y.F.; Zhou, L.Q.; Qin, G.W.; Zhang, X.M. Properties and Applications of the beta Phase Poly(vinylidene fluoride). Polymer 2018, 10, 228. [Google Scholar] [CrossRef]
- Li, W.B.A.; Li, X.Z.; Xie, X.H.; Yuan, A.B.; Xia, B.J. Effect of drying temperature on a thin PVDF-HFP/PET composite nonwoven separator for lithium-ion batteries. Ionics 2017, 23, 929–935. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological study of poly(vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Liu, P.; Hao, J.; Gao, Q.; Yang, J. Preparation of NaCl Particles Added Polyvinylidene Fluoride Microporous Filter and a Simple Filtration Device. Coatings 2024, 14, 196. https://doi.org/10.3390/coatings14020196
Peng L, Liu P, Hao J, Gao Q, Yang J. Preparation of NaCl Particles Added Polyvinylidene Fluoride Microporous Filter and a Simple Filtration Device. Coatings. 2024; 14(2):196. https://doi.org/10.3390/coatings14020196
Chicago/Turabian StylePeng, Lei, Ping Liu, Jian Hao, Qingguo Gao, and Jianjun Yang. 2024. "Preparation of NaCl Particles Added Polyvinylidene Fluoride Microporous Filter and a Simple Filtration Device" Coatings 14, no. 2: 196. https://doi.org/10.3390/coatings14020196






