The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Samples
2.2. Bacterial Culture
2.3. Antibacterial Activity Test
2.4. Biofilm Proliferation Assay
2.4.1. Effect of the SML Coating on Bacterial Adhesion
2.4.2. Effect of the SML Coating on Biofilm Formation and Maturation
2.4.3. Effect of the SML Coating on Biofilm Dispersal
2.5. Statistics
3. Results
3.1. Antibacterial Activity Test
3.2. Biofilm Proliferation Test
3.2.1. Effect of the SML Coating on Bacterial Adhesion
3.2.2. Effect of the SML Coating on Biofilm Formation and Maturation
CFU Counts
Bacteriostatic and Bactericidal Properties of the SML Coating
Biofilm Biomass Quantification
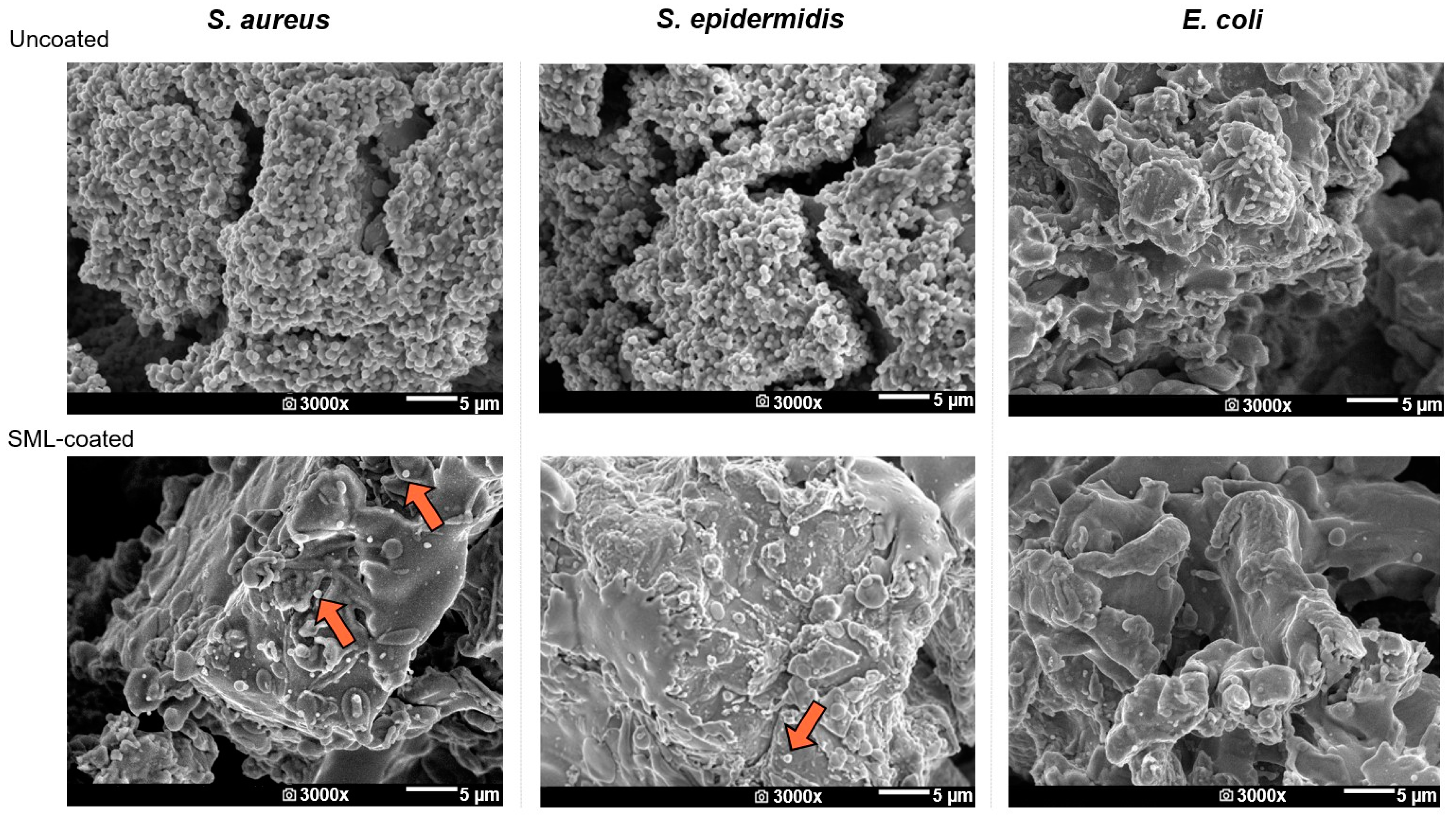
SEM
3.2.3. Effect of the SML Coating on Biofilm Dispersal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Lentino, J.R. Prosthetic Joint Infections: Bane of Orthopedists, Challenge for Infectious Disease Specialists. Clin. Pract. 2003, 36, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Kurtz, S.M.; Lau, E.; Bozic, K.J.; Berry, D.J.; Parvizi, J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J. Arthroplast. 2009, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.J.; Yao, F.M.; He, W.; Wei, Q.S.; He, M.C. Incidence of periprosthetic joint infection after primary total hip arthroplasty is underestimated: A synthesis of meta-analysis and bibliometric analysis. J. Orthop. Surg. Res. 2023, 18, 610. [Google Scholar] [CrossRef] [PubMed]
- Huotari, K.; Lyytikainen, O.; Ollgren, J.; Virtanen, M.J.; Seitsalo, S.; Palonen, R.; Rantanen, P.; Hospital Infection Surveillance, T. Disease burden of prosthetic joint infections after hip and knee joint replacement in Finland during 1999-2004: Capture-recapture estimation. J. Hosp. Infect. 2010, 75, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the Medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Day, C.W.; Costi, K.; Pannach, S.; Atkins, G.J.; Hofstaetter, J.G.; Callary, S.A.; Nelson, R.; Howie, D.W.; Solomon, L.B. Long-Term Outcomes of Staged Revision Surgery for Chronic Periprosthetic Joint Infection of Total Hip Arthroplasty. J. Clin. Med. 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Nham, F.; Zalikha, A.K.; El-Othmani, M.M. Epidemiology of total hip arthroplasty: Demographics, comorbidities and outcomes. Arthroplasty 2023, 5, 2. [Google Scholar] [CrossRef]
- Nham, F.H.; Patel, I.; Zalikha, A.K.; El-Othmani, M.M. Epidemiology of primary and revision total knee arthroplasty: Analysis of demographics, comorbidities and outcomes from the national inpatient sample. Arthroplasty 2023, 5, 18. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The Epidemiology of Revision Total Knee Arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Rottier, W.; Seidelman, J.; Wouthuyzen-Bakker, M. Antimicrobial treatment of patients with a periprosthetic joint infection: Basic principles. Arthroplasty 2023, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Chen, A.F. Prosthetic Joint Infection: Treatment; UpToDate: Waltham, MA, USA, 2023. [Google Scholar]
- Anagnostakos, K.; Schmitt, C. Can periprosthetic hip joint infections be successfully managed by debridement and prosthesis retention? World J. Orthop. 2014, 5, 218–224. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489. [Google Scholar] [CrossRef]
- Scheper, H.; Gerritsen, L.M.; Pijls, B.G.; Van Asten, S.A.; Visser, L.G.; De Boer, M.G.J. Outcome of Debridement, Antibiotics, and Implant Retention for Staphylococcal Hip and Knee Prosthetic Joint Infections, Focused on Rifampicin Use: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2021, 8, ofab298. [Google Scholar] [CrossRef]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z. Microbial biofilm. Annu. Rev. Microbial. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Arts, J.; Geurts, J. Management of Periprosthetic Joint Infections (PJIs); Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–306. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sanchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodriguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef]
- Casenaz, A.; Piroth, L.; Labattut, L.; Sixt, T.; Magallon, A.; Guilloteau, A.; Neuwirth, C.; Amoureux, L. Epidemiology and antibiotic resistance of prosthetic joint infections according to time of occurrence, a 10-year study. J. Infect. 2022, 85, 492–498. [Google Scholar] [CrossRef]
- Hays, M.R.; Kildow, B.J.; Hartman, C.W.; Lyden, E.R.; Springer, B.D.; Fehring, T.K.; Garvin, K.L. Increased Incidence of Methicillin-Resistant Staphylococcus aureus in Knee and Hip Prosthetic Joint Infection. J. Arthroplast. 2023, 38, S326–S330. [Google Scholar] [CrossRef]
- Svensson Malchau, K.; Tillander, J.; Zaborowska, M.; Hoffman, M.; Lasa, I.; Thomsen, P.; Malchau, H.; Rolfson, O.; Trobos, M. Biofilm properties in relation to treatment outcome in patients with first-time periprosthetic hip or knee joint infection. J. Orthop. Transl. 2021, 30, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Zhang, W.; Xu, G.; Xu, C.; Liu, W.; Li, J. Potential side effects of antibacterial coatings in orthopaedic implants: A systematic review of clinical studies. Front. Bioeng. Biotechnol. 2023, 11, 1111386. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Alt, V. Antimicrobial coated implants in trauma and orthopaedics—A clinical review and risk-bene fi t analysis. Inj. Int. J. Care Inj. 2017, 48, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Alt, V.; Chen, A.F. Antimicrobial coatings for orthopaedic implants—Ready for use? J. Bone Jt. Infect. 2020, 5, 125–127. [Google Scholar] [CrossRef]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Nag, T.C. A Comparison of Bacterial Adhesion and Biofilm Formation on Commonly Used Orthopaedic Metal Implant Materials: An In vitro Study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Jülke, H.; Zehe, M.; Lemarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial silver multilayer coating for prevention of bacterial colonization of orthopedic implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, P.; Lampe, K.; Wagener, M.; Stigler, B.; Heiss, C.; Ullrich, M.S.; Domann, E.; Schnettler, R.; Alt, V. Ag/SiOxCy plasma polymer coating for antimicrobial protection of fracture fixation devices. J. Biomed. Mater. Res. 2010, 94B, 196–202. [Google Scholar] [CrossRef]
- Azab, M.A.; Allen, M.J.; Daniels, J.B. Evaluation of a silver-impregnated coating to inhibit colonization of orthopaedic implants by biofilm forming methicillin-resistant Staphylococcus pseudintermedius. Vet. Comp. Orthop. Traumatol. 2016, 29, 347–350. [Google Scholar] [CrossRef]
- Stein, S.; Kruck, L.; Warnecke, D.; Seitz, A.; Durselen, L.; Ignatios, A. Osseointegration of titanium implants with a novel silver coating under dynamic loading. Eur. Cells Mater. 2020, 39, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Heiss, C.; Rupp, M. Treatment of a Recurrent Periprosthetic Joint Infection with an Intramedullary Knee Arthrodesis System with Low-Amount Metallic Silver Coating. J. Bone Jt. Infect. 2019, 4, 111–114. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-e.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- ISO 22196:2011(E); Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- ASTM E2180-18; Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018.
- Sjollema, J.; Zaat, S.A.J.; Fontaine, V.; Ramstedt, M.; Luginbuehl, R.; Thevissen, K.; Li, J.; van der Mei, H.C.; Busscher, H.J. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018, 70, 12–24. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.F.; Wang, L.; Jiang, N.; Qin, C.H.; Hu, Y.J.; Yu, B. A rabbit model of implant-related osteomyelitis inoculated with biofilm after open femoral fracture. Exp. Ther. Med. 2017, 14, 4995–5001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernatova, S.; Samek, O.; Pilat, Z.; Sery, M.; Jezek, J.; Jakl, P.; Siler, M.; Krzyzanek, V.; Zemanek, P.; Hola, V.; et al. Following the mechanisms of bacteriostatic versus bactericidal action using Raman spectroscopy. Molecules 2013, 18, 13188–13199. [Google Scholar] [CrossRef] [PubMed]
- Ommen, P.; Zobek, N.; Meyer, R.L. Quantification of biofilm biomass by staining: Non-toxic safranin can replace the popular crystal violet. J. Microbiol. Methods 2017, 141, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Bruenke, J.; Roschke, I.; Agarwal, S.; Riemann, T.; Greiner, A. Quantitative Comparison of the Antimicrobial Efficiency of Leaching versus Nonleaching Polymer Materials. Macromol. Biosci. 2016, 16, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bechert, T.; Steinrücke, P.; Guggenbichler, J.P. A new method for screening anti-infective biomaterials. Nat. Med. 2000, 6, 1053–1056. [Google Scholar] [CrossRef]
- Schreurs, W.J.A.; Rosenberg, H. Effect of Silver Ions on Transport and Retention of Phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Wiegand, C.; Völpel, A.; Ewald, A.; Remesch, M.; Kuever, J.; Bauer, J.; Griesheim, S.; Hauser, C.; Thielmann, J.; Tonndorf-Martini, S.; et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE 2018, 13, e0194339. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of GramPositive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: “the race for the surface” experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur. Cell Mater. 2015, 27, 196–212. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Debefve, L.; Boure, L.; Campoccia, D.; Schlegel, U.; Richards, R.G. Influence of material and microtopography on the development of local infection in vivo: Experimental investigation in rabbits. Int. J. Artif. Organs 2009, 32, 663–670. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Campoccia, D.; Nees, S.K.; Boure, L.P.; Richards, R.G. In vivo evaluation of the effect of intramedullary nail microtopography on the development of local infection in rabbits. Int. J. Artif. Organs 2010, 33, 667–675. [Google Scholar] [CrossRef]
- Elek, S.D.; Conen, P.E. The virulence of staphylococcus pyogenes for man. A study of the problems of wound infection. Br. J. Exp. Pathol. 1957, 38, 573–586. [Google Scholar] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; NMandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Standar, K.; Kreikemeyer, B.; Redanz, S.; Munter, W.L.; Laue, M.; Podbielski, A. Setup of an in vitro test system for basic studies on biofilm behavior of mixed-species cultures with dental and periodontal pathogens. PLoS ONE 2010, 5, e13135. [Google Scholar] [CrossRef]
- Stiefel, P.; Rosenberg, U.; Schneider, J.; Mauerhofer, S.; Maniura-Weber, K.; Ren, Q. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 2016, 100, 4135–4145. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Dassanayake, R.P.; Falkenberg, S.M.; Stasko, J.A.; Shircliff, A.L.; Lippolis, J.D.; Briggs, R.E. Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PLoS ONE 2020, 15, e0233973. [Google Scholar] [CrossRef]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How do we determine the efficacy of an antibacterial surface? A review of standardised antibacterial material testing methods. Antibiotics 2021, 10, 1069. [Google Scholar] [CrossRef]
- Zmistowski, B.; Fedorka, C.J.; Sheehan, E.; Deirmengian, G.; Austin, M.S.; Parvizi, J. Prosthetic joint infection caused by gram-negative organisms. J. Arthroplast. 2011, 26, 104–108. [Google Scholar] [CrossRef]
- Perni, S.; Bojan, B.; Prokopovich, P. A retrospective study of risk factors, causative micro-organisms and healthcare resources consumption associated with prosthetic joint infections (PJI) using the Clinical Practice Research Datalink (CPRD) Aurum database. PLoS ONE 2023, 18, e0282709. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the Protocol for the LIVE/DEAD((R)) BacLight(TM) Bacterial Viability Kit for Rapid Determination of Bacterial Load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowics, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Durham Brooks, T.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 1–25. [Google Scholar]
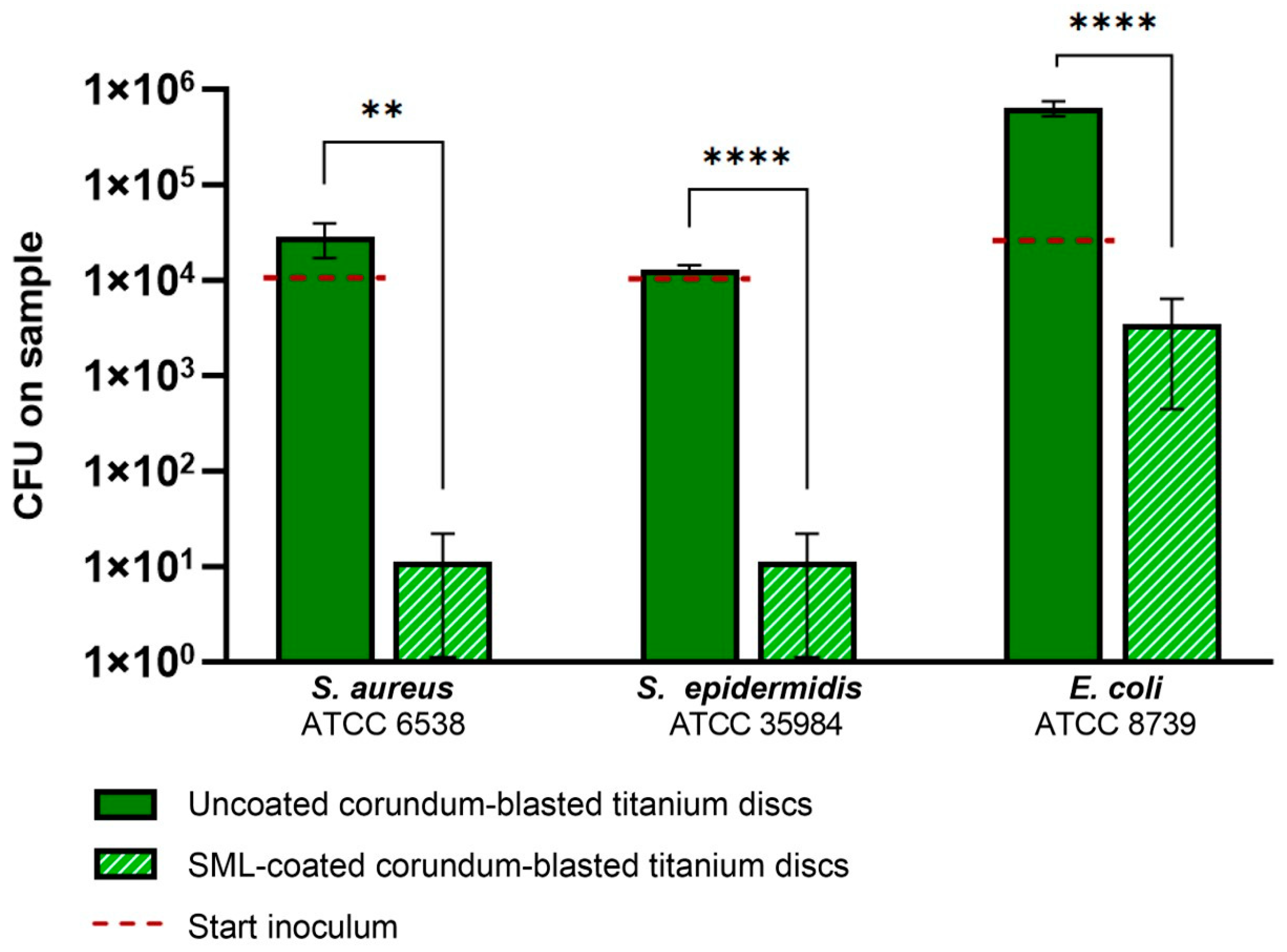
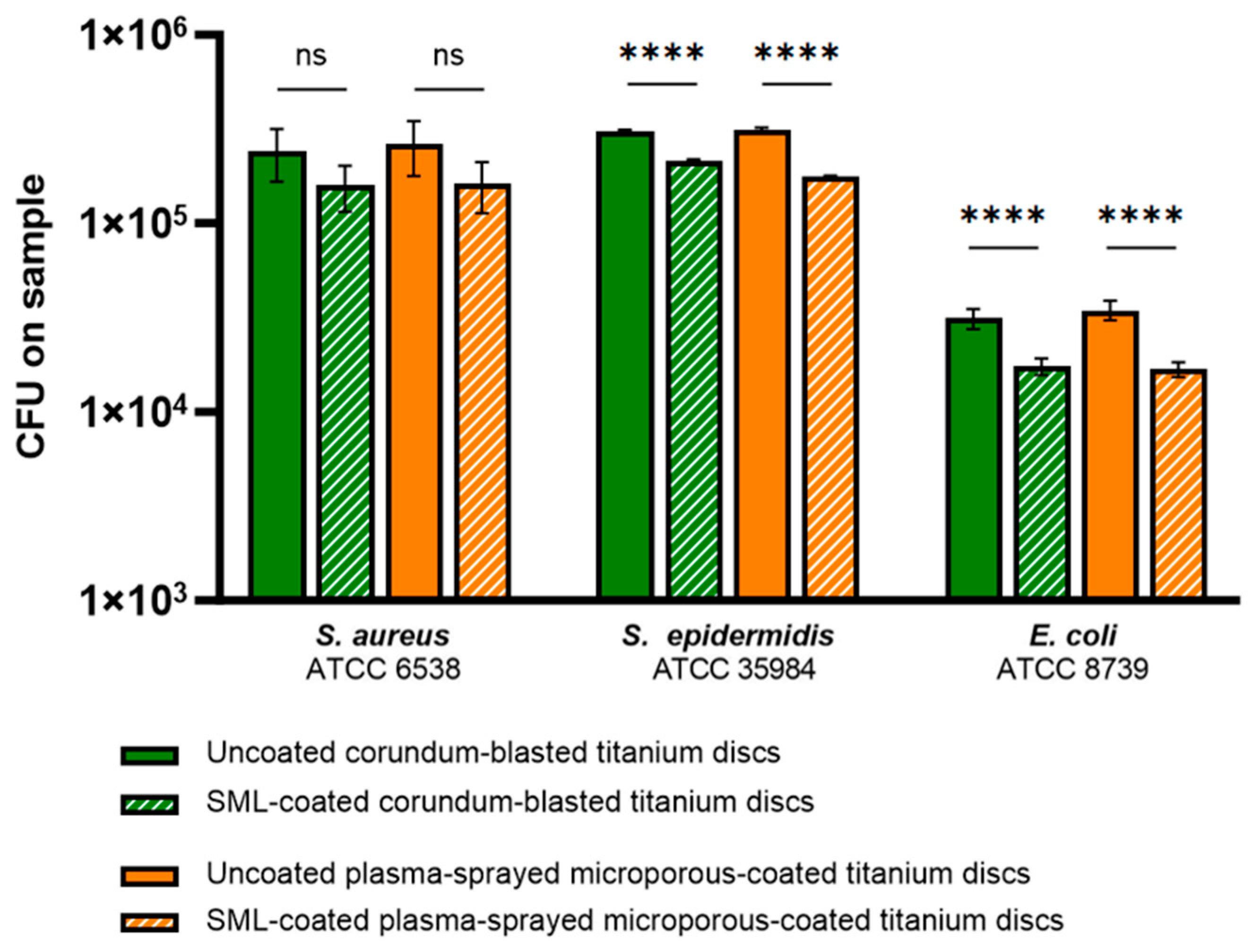
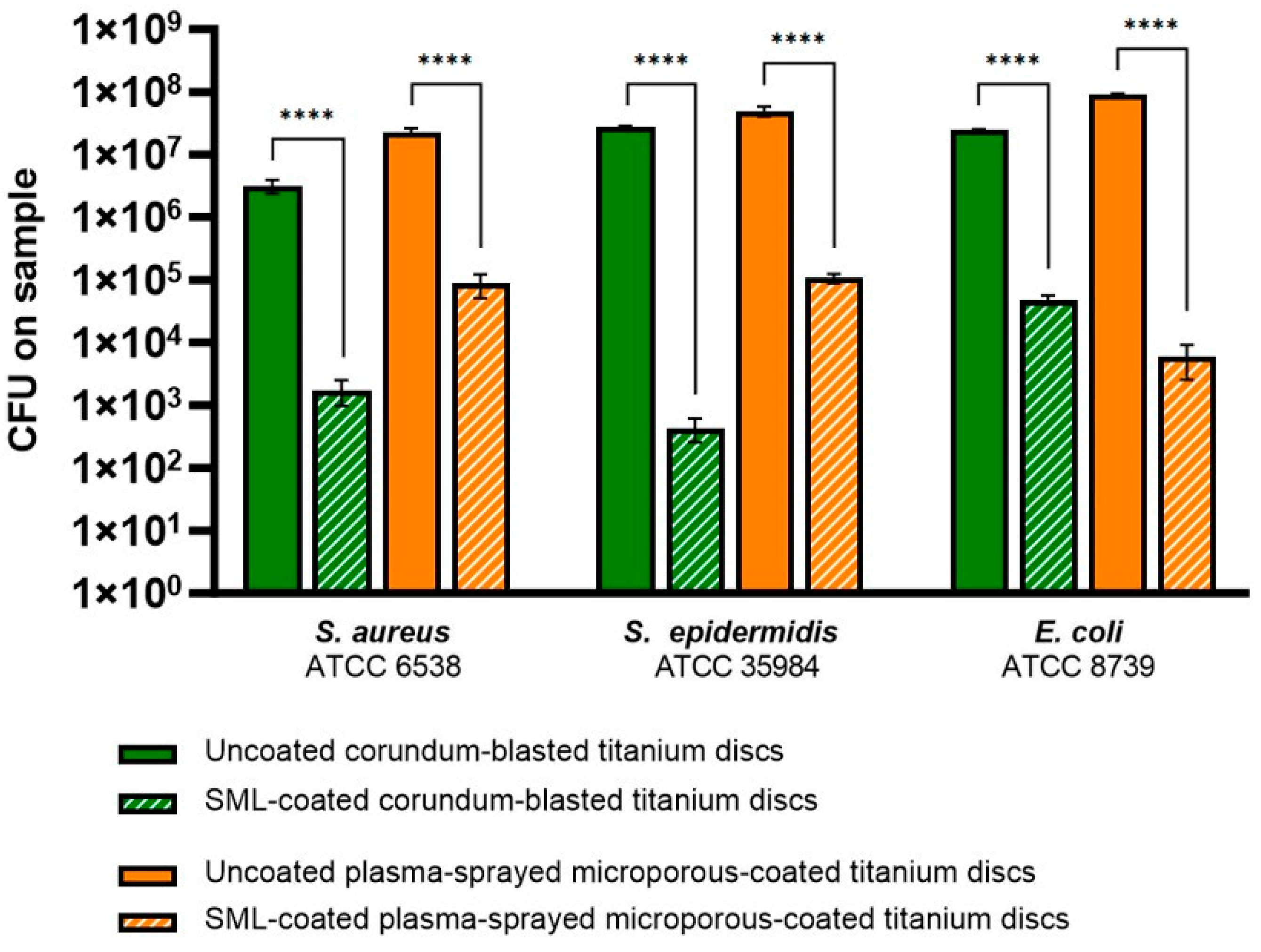


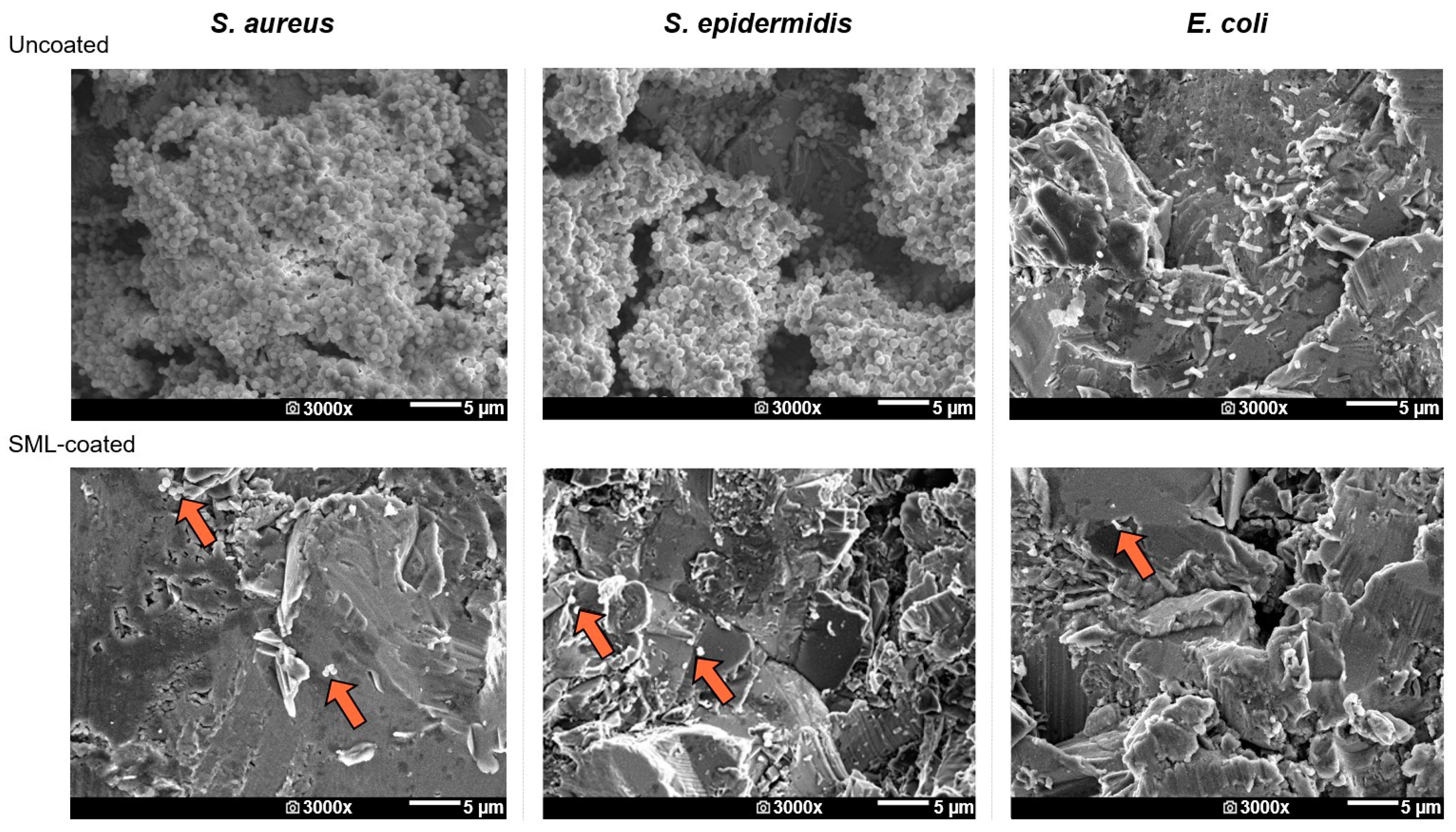
| Bacterial Strain | Antibacterial Activity R | Reduction in CFUs (%) |
|---|---|---|
| S. aureus | 4.0 | >99.9 |
| S. epidermidis | 3.7 | >99.9 |
| E. coli | 2.8 | >99.4 |
| Bacterial Strain | Log10 (±SD) Reduction | |
|---|---|---|
| Corundum-Blasted | Plasma-Sprayed Microporous-Coated | |
| S. aureus | 3.42 (±0.33) | 2.48 (±0.15) |
| S. epidermidis | 4.87 (±0.20) | 2.67 (±0.01) |
| E. coli | 2.72 (±0.10) | 4.32 (±0.31) |
| Bacterial Strain | Reduction in Biomass (OD540) | |
|---|---|---|
| Corundum-Blasted | Plasma-Sprayed Microporous-Coated | |
| S. aureus | 97.9% | 81.6% |
| S. epidermidis | 93.4% | 76.2% |
| E. coli | 60.0% | 100% |
| Bacterial Strain | ΔOD600 (±SD) | |
|---|---|---|
| Corundum-Blasted | Plasma-Sprayed Microporous-Coated | |
| S. aureus | 0.357 (±0.015) | 0.366 (±0.002) |
| S. epidermidis | 0.434 (±0.000) | 0.449 (±0.001) |
| E. coli | 0.496 (±0.001) | 0.469 (±0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hoogstraten, S.W.G.; Fechter, J.; Bargon, R.; van Agtmaal, J.L.; Peeters, L.C.W.; Geurts, J.; Arts, J.J.C. The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants—An In Vitro Study. Coatings 2024, 14, 216. https://doi.org/10.3390/coatings14020216
van Hoogstraten SWG, Fechter J, Bargon R, van Agtmaal JL, Peeters LCW, Geurts J, Arts JJC. The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants—An In Vitro Study. Coatings. 2024; 14(2):216. https://doi.org/10.3390/coatings14020216
Chicago/Turabian Stylevan Hoogstraten, Sanne W. G., Janine Fechter, Rainer Bargon, Julia L. van Agtmaal, Laura C. W. Peeters, Jan Geurts, and Jacobus J. C. Arts. 2024. "The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants—An In Vitro Study" Coatings 14, no. 2: 216. https://doi.org/10.3390/coatings14020216
APA Stylevan Hoogstraten, S. W. G., Fechter, J., Bargon, R., van Agtmaal, J. L., Peeters, L. C. W., Geurts, J., & Arts, J. J. C. (2024). The Antibacterial Properties of a Silver Multilayer Coating for the Prevention of Bacterial Biofilm Formation on Orthopedic Implants—An In Vitro Study. Coatings, 14(2), 216. https://doi.org/10.3390/coatings14020216






