Nanomaterials for Anti-Infection in Orthopedic Implants: A Review
Abstract
:1. Introduction
2. Antimicrobial Agents Loaded in Nanocontainers
3. Metal Nanoparticles
4. Metal Oxide Nanoparticles
5. Other Inorganic Nanoparticles
6. Organic Nanoparticles
7. Nanoscale Surface with Special Morphology
7.1. Nanopillars
7.2. Nanohole
7.3. Nanomaterials of Other Shapes
| Material | Morphology | Bactericidal Mechanisms | Dimensions | Tested Organism | Biological Activity/Effect/Outcome | Refs. |
|---|---|---|---|---|---|---|
| PDA | Nanoparticles | Release of NOR and CEP | NA | S. aureus and P. aeruginosa | Antibiotic-embedded superhydrophilic coating (PDA/NOR and PDA/CEP) possessed the reinforced antibacterial ability. | [34] |
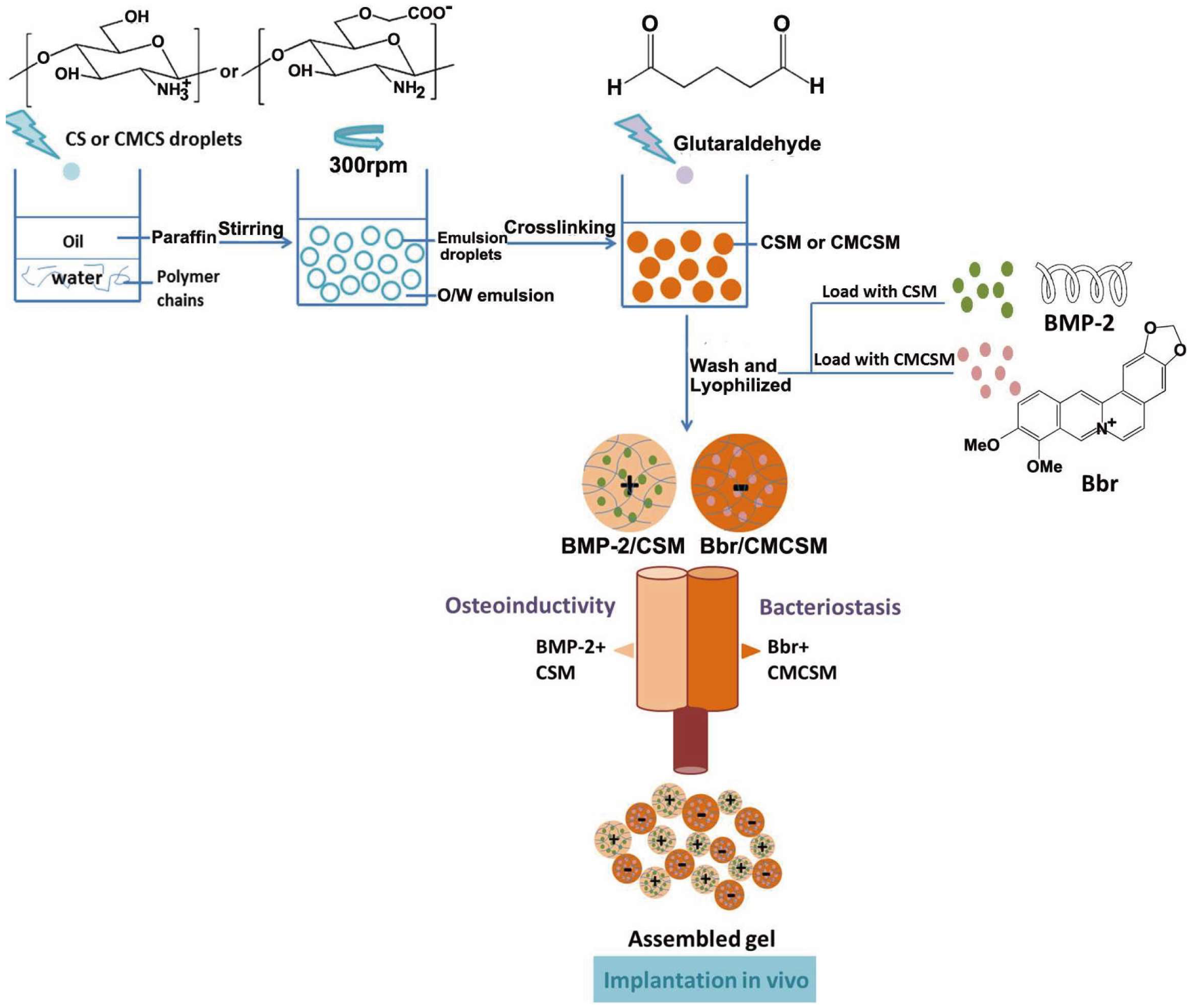
| Chitosan | Nanopores | Release of Bbr | Less than 1 μm | S. aureus | The inhibition area in the Bbr/CMCSM group suggested a significant antimicrobial activity. | [35] |
| LbL films composed of CBSA NPs and OSA | Nanoporous | Release of VAN | Nanoporous structures | S. epidermidis | VAN-loaded LbL films showed excellent antibacterial activity. | [36] |
| Ti-NT | Nanotube | Release of VAN | 100 nm in diameter | S. aureus | The Ti-NT-VAN/pDA/Hya exhibited better antibacterial activity. | [38] |
| Halloysite clay nanotubes | Nanotube | Release of MNA | A diameter of 50 nm and a length of 600 nm | Fn | The nanotube composite inhibits bacterial growth in an area larger than the membrane size and keeps the inhibition zone long. | [41] |
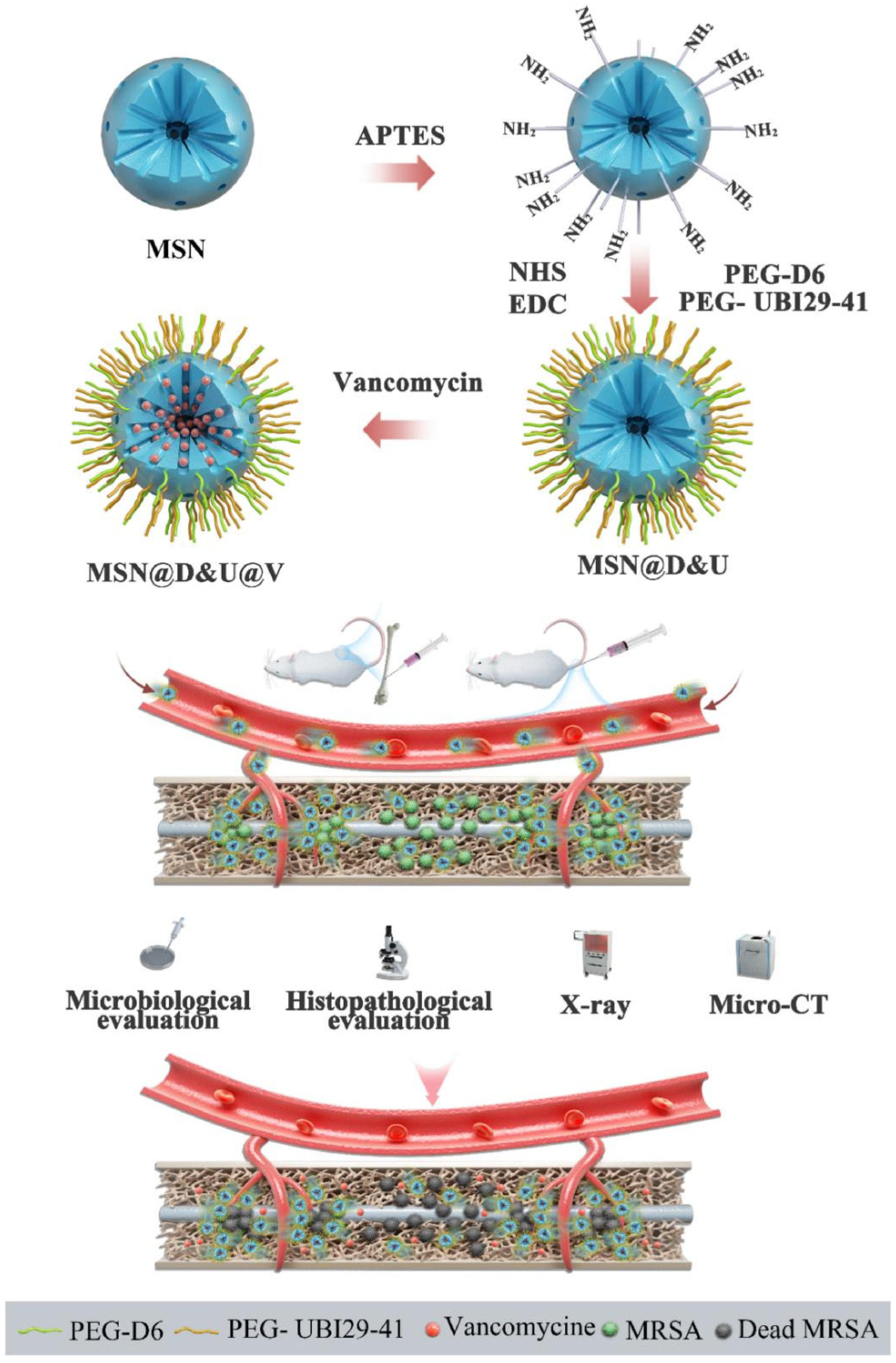
| MSNs | Nanoparticles | Release of VAN | 100 nm in diameter | MRSA | The PEG-D6- and PEG-UBI29-41-modified MSN nanocomposites possessed excellent antibacterial activity. | [48] |
| MSNs | Nanoparticles | Release of Lev | NA | E. coli and S. aureus | The composite scaffold significantly inhibited the growth of both E. coli and S. aureus. | [49] |
| PCL/gelatin | Nanofibers | Release of MNA | 560 nm in average diameter | Fn and Pg | The bacterial inhibition zones are observed around the membrane for the bacterial nanofibers with core-shell structure. | [51] |
| Silver | Nanoparticles | Ionic silver and reactive oxygen species (ROS) | 40–80 nm | E. coli and S. epidermidis | The antibacterial rates for adhered bacteria were maintained at 89.9% and 93.5%, respectively, for S. aureus and E. coli within 12 weeks. | [71] |
| Cu/Ag-PDA-NPs | Nanoparticles | Ionic copper and silver and reactive oxygen species (ROS) | 270 ± 28 nm | S. mutans, P. aeruginosa, E. coli, and S. aureus | The marked antimicrobial activities against S. mutans, P. aeruginosa, E. coli, and S. aureus. | [84] |
| BGz | Nanospheres | Attachment to the cell membrane, accumulation in the cytoplasm, and the production of ROS | 282.8 ± 2.1 nm | P. gingivalis | P. gingivalis was significantly reduced in the BGz group. | [88] |
| Gold | Nanoparticles | Gold ions penetrate the membrane and cell wall, resulting in bacterial death | 33 nm in average diameter | S. epidermidis and Klebsiella spp. | Over 99.99% of the colonies and the colony forming units, including Klebsiella spp. and S. epidermidis, were eliminated at 0.64 wt% of AuNPs. | [96] |
| ZnO | Nanoparticles | Nanoparticle internalization, membranolysis, and ROS generation | 18 ± 5 nm | S. aureus and E. coli | The viability of S. aureus and E. coli bacterial cells was reduced by 65% and 10%, respectively. | [70] |
| CuO | Nanoparticles | Generation of ROS | NA | S. aureus, P. aeruginosa, and E. coli | The bactericidal activity was observed for both Gram-positive and Gram-negative bacteria. | [117] |
| CeO2 | Nanoparticles | Cell membrane damage caused by ROS | ≤20 nm | P. aeruginosa | CNPs in a solution at a concentration of 200 µg/mL had bactericidal activity against P. aeruginosa. | [127] |
| TiO2 | Nanoparticles | NA | 15 nm in diameter | S. aureus, E. coli, and C. albicans | It inhibited the growth of S. aureus and E. coli, as well as C. albicans. | [130] |
| Se | Nanoparticles | Destruction of bacterial membranes to induce rapid cell lysis | 30–70 nm | MRSA and MRSE | The decrease in bacterial cell viability of MRSA and MRSE can be attributed to a decrease in the number of bacterial cells. | [145] |
| MgF2 | Nanoparticles | Direct antimicrobial effects of MgF2-NPs enhancing the phagocytosis of PMNs | NA | MRSA, S. epidermidis, and S. aureus | MgF2 nanoparticle films significantly restricted the biofilm formation in a dose-dependent manner. | [150] |
| Nb2C | Nanosheets | Biofilm inhibition, intrinsic bactericidal and photothermal bacteria ablation | Average size of ~150 nm | MRSA and E. coli | Nb2C@TP can effectively eradicate planktonic bacteria and inhibit biofilm formation. | [164] |
| G-CgQAs | Nanoparticles | Positive charge and suitable hydrophobic–hydrophilic balance | 60 nm | S. aureus, MRSA, and E. coli | It not only has a broad spectrum and high antimicrobial activity against Gram-negative, Gram-positive, and antibiotic-resistant bacteria, but also shows strong anti-infective effects against vancomycin-resistant bacteria. | [179] |
| PLC-SO3X | Nanoparticles | The low local pH from sulfonic acid side chains | 50 nm | S. aureus and E. coli | The sterilization rate of the PLC-SO3H coating against S. aureus and E. coli were 99.1% and >99.9%, respectively. | [184] |
| nZMS | Nanoparticles | Release of zinc ions | 100 nm | E. coli | The nZPC revealed good antibacterial activity against E. coli, in which 50nZPC had the strongest antibacterial activity. | [169] |
| Silicon | Nanopillars | Stress-induced deflection of the nanopillars | 360 nm | P. aeruginosa and S. aureus | The 360 nm nanopillar array exhibited the highest antimicrobial activity, resulting in 95 ± 5% and 83 ± 12% cell death of P. aeruginosa and S. aureus, respectively. | [193] |
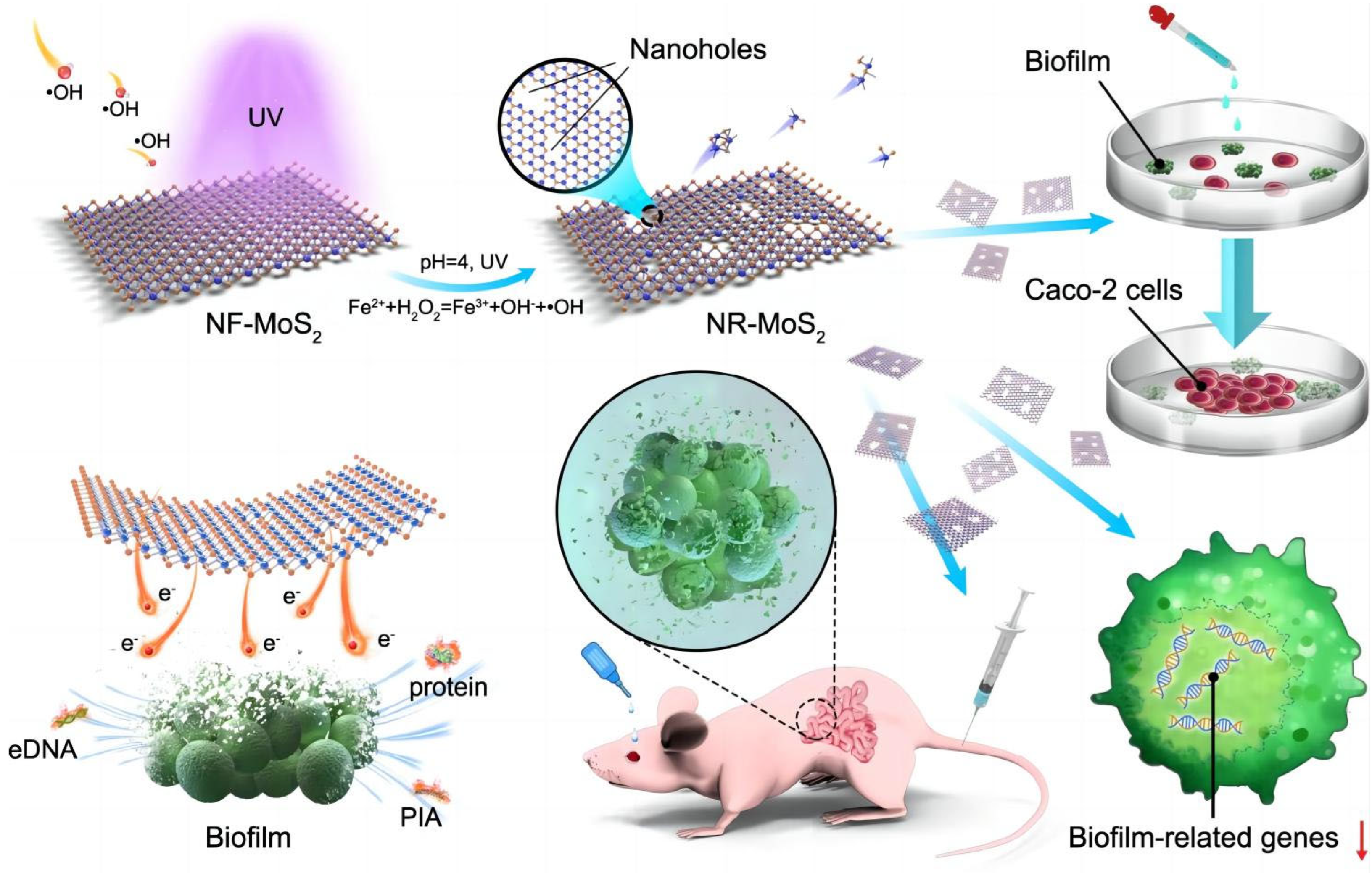
| NR-MoS2 | Nanohole | Facilitation of electron transport | 40 nm | S. aureus | The anti-infection capacity of the NR-MoS2 was found to be excellent, both in vitro and in vivo. | [202] |
| CuFe5O8 | Nanocubes | Generation of ROS; remodeling of the immune microenvironment | A mean side length of about 15 nm | S. aureus and E. coli | CuFe5O8 NCs effectively ruin the bacterial cell wall or membranes of S. aureus and E. coli planktonic bacteria. | [206] |
| MWCNTs | Nanotubes | The physical interaction of the nanomaterials with cell membranes, disruption of cell membranes and DNA structures. | 7–33 nm | E. coli, S. aureus, B. subtilis, C. albicans, and P. aeruginosa | The nanocomposites show obvious antibacterial activity and reduce biofilm formation in multiple associated microbial strains. | [212] |
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCloskey, A.P.; Gilmore, B.F.; Laverty, G. Evolution of antimicrobial peptides to self-assembled peptides for biomaterial applications. Pathogens 2014, 3, 791–821. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Higgins, C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007, 446, 749–757. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef]
- Zou, W.; Zhou, Q.; Zhang, X.; Hu, X. Environmental Transformations and Algal Toxicity of Single-Layer Molybdenum Disulfide Regulated by Humic Acid. Environ. Sci. Technol. 2018, 52, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.-Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? NPJ Biofilms Microbiomes 2015, 1, 15022. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Brugger, J.; Maniura-Weber, K.; Ren, Q. Antibacterial Au nanostructured surfaces. Nanoscale 2016, 8, 2620–2625. [Google Scholar] [CrossRef] [PubMed]
- Jahed, Z.; Lin, P.; Seo, B.B.; Verma, M.S.; Gu, F.X.; Tsui, T.Y.; Mofrad, M.R.K. Responses of Staphylococcus aureus bacterial cells to nanocrystalline nickel nanostructures. Biomaterials 2014, 35, 4249–4254. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ercan, B.; Sun, L.; Ziemer, K.S.; Webster, T.J. Understanding the Role of Polymer Surface Nanoscale Topography on Inhibiting Bacteria Adhesion and Growth. ACS Biomater. Sci. Eng. 2016, 2, 122–130. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.T.; Kim, S.-K.; Lee, J.-H.; Choi, H.S.; Kim, C.-S.; Jeong, M.Y. Nanostructured multifunctional surface with antireflective and antimicrobial characteristics. ACS Appl. Mater. Interfaces 2015, 7, 326–331. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Liu, R.; Yang, K.; Zhang, Y.; Liao, Z.; Liu, W.; Qi, M.; Misra, R. Effect of Heat Treatment on Cu Distribution, Antibacterial Performance and Cytotoxicity of Ti–6Al–4V–5Cu Alloy. J. Mater. Sci. Technol. 2015, 31, 723–732. [Google Scholar] [CrossRef]
- Luo, F.; Tang, Z.; Xiao, S.; Xiang, Y. Study on properties of copper-containing austenitic antibacterial stainless steel. Mater. Technol. 2019, 34, 525–533. [Google Scholar] [CrossRef]
- Nune, K.C.; Somani, M.C.; Spencer, C.T.; Misra, R. Cellular response of Staphylococcus aureus to nanostructured metallic biomedical devices: Surface binding and mechanism of disruption of colonization. Mater. Technol. 2017, 32, 22–31. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.P.; Miller, K.P.; Cash, B.M.; Jones, S.; Glenn, S.; Benicewicz, B.C.; Decho, A.W. Functionalised nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem. Commun. 2014, 50, 12030–12033. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Blanchemain, N.; Laurent, T.; Chai, F.; Neut, C.; Haulon, S.; Krump-konvalinkova, V.; Morcellet, M.; Martel, B.; Kirkpatrick, C.J.; Hildebrand, H.F. Polyester vascular prostheses coated with a cyclodextrin polymer and activated with antibiotics: Cytotoxicity and microbiological evaluation. Acta Biomater. 2008, 4, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Zelken, J.; Wanich, T.; Gardner, M.; Griffith, M.; Bostrom, M. PMMA is superior to hydroxyapatite for colony reduction in induced osteomyelitis. Clin. Orthop. Relat. Res. 2007, 462, 190–194. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Aizenberg, J.; Analoui, M.; Andrews, A.M.; Bisker, G.; Boyden, E.S.; Kamm, R.D.; Karp, J.M.; Mooney, D.J.; Oklu, R.; et al. Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation. ACS Nano 2017, 11, 5195–5214. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Zhan, W.; Chen, H. A Smart Antibacterial Surface for the On-Demand Killing and Releasing of Bacteria. Adv. Healthc. Mater. 2016, 5, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, L.; Xiao, S.; Yang, Y.; Chen, F.; Fan, P.; Zhao, Z.; Zhong, M.; Yang, J. Bacteria killing and release of salt-responsive, regenerative, double-layered polyzwitterionic brushes. Chem. Eng. J. 2018, 333, 1–10. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Thappeta, K.R.V.; Subramanian, J.T.L.; Pranantyo, D.; Kang, E.-T.; Duan, H.; Kline, K.; Chan-Park, M.B. In Vivo Anti-Biofilm and Anti-Bacterial Non-Leachable Coating Thermally Polymerized on Cylindrical Catheter. ACS Appl. Mater. Interfaces 2017, 9, 36269–36280. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110040. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Liu, K.; Yang, L.; Zhang, B.; Luo, Q.; Luo, R.; Wang, Y. Nanoparticles-stacked superhydrophilic coating supported synergistic antimicrobial ability for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 132, 112535. [Google Scholar] [CrossRef]
- Cai, B.; Zou, Q.; Zuo, Y.; Mei, Q.; Ma, J.; Lin, L.; Chen, L.; Li, Y. Injectable Gel Constructs with Regenerative and Anti-Infective Dual Effects Based on Assembled Chitosan Microspheres. ACS Appl. Mater. Interfaces 2018, 10, 25099–25112. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Sun, H.; Li, P.; Wang, K.; Ren, F.; Lu, X. Porous titanium scaffolds with self-assembled micro/nano-hierarchical structure for dual functions of bone regeneration and anti-infection. J. Biomed. Mater. Res. A 2017, 105, 3482–3492. [Google Scholar] [CrossRef]
- Yu, X.; Pan, Q.; Zheng, Z.; Chen, Y.; Chen, Y.; Weng, S.; Huang, L. pH-responsive and porous vancomycin-loaded PLGA microspheres: Evidence of controlled and sustained release for localized inflammation inhibition in vitro. RSC Adv. 2018, 8, 37424–37432. [Google Scholar] [CrossRef]
- He, R.; Sui, J.; Wang, G.; Wang, Y.; Xu, K.; Qin, S.; Xu, S.; Ji, F.; Zhang, H. Polydopamine and hyaluronic acid immobilisation on vancomycin-loaded titanium nanotube for prophylaxis of implant infections. Colloids Surf. B Biointerfaces 2022, 216, 112582. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Thieme, M.; Nguyen, J.; Schmehl, T.; Gessler, T.; Seeger, W.; Agarwal, S.; Greiner, A.; Kissel, T. Novel ‘nano in nano’ composites for sustained drug delivery: Biodegradable nanoparticles encapsulated into nanofiber non-wovens. Macromol. Biosci. 2010, 10, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Yu, S.-H. Nanoparticles meet electrospinning: Recent advances and future prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Chorylek, A.; Chytrosz, P.; Brzychczy-Wloch, M.; Jaworska, J.; Kasperczyk, J.; Hakkarainen, M.; Engvall, K.; Kotarba, A. Multifunctional PLGA/Parylene C Coating for Implant Materials: An Integral Approach for Biointerface Optimization. ACS Appl. Mater. Interfaces 2016, 8, 22093–22105. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Yue, Q.; Li, J.; Gao, M.; Yang, X.; Ren, Y.; Cheng, X.; Cui, P.; Deng, Y. Smart Cargo Delivery System based on Mesoporous Nanoparticles for Bone Disease Diagnosis and Treatment. Adv. Sci. 2021, 8, e2004586. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Gao, X.; Li, L.; Cai, X.; Huang, Q.; Xiao, J.; Cheng, Y. Targeting nanoparticles for diagnosis and therapy of bone tumors: Opportunities and challenges. Biomaterials 2021, 265, 120404. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, P.; Peng, X.; Huang, Q.; Ding, M.; Chen, Y.; Jin, R.; Xie, J.; Zhao, C.; Li, J. Hexapeptide-conjugated calcitonin for targeted therapy of osteoporosis. J. Control. Release 2019, 304, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, C.; Chen, D.; Madrid, K.; Peng, S.; Dong, X.; Zhang, M.; Gu, Y. Bacteria-Targeting Conjugates Based on Antimicrobial Peptide for Bacteria Diagnosis and Therapy. Mol. Pharm. 2015, 12, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact. Mater. 2022, 16, 134–148. [Google Scholar] [CrossRef]
- Kuang, Z.; Dai, G.; Wan, R.; Zhang, D.; Zhao, C.; Chen, C.; Li, J.; Gu, H.; Huang, W. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021, 8, 193–202. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhang, Y.; Wen, S.; Wang, Y.; Zhang, H. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 134–139. [Google Scholar] [CrossRef]
- Ansari, N.; Lodha, A.; Menon, S.K. Smart platform for the time since death determination from vitreous humor cystine. Biosens. Bioelectron. 2016, 86, 115–121. [Google Scholar] [CrossRef]
- Tanaka, M.; Saito, S.; Kita, R.; Jang, J.; Choi, Y.; Choi, J.; Okochi, M. Array-Based Screening of Silver Nanoparticle Mineralization Peptides. Int. J. Mol. Sci. 2020, 21, 2377. [Google Scholar] [CrossRef] [PubMed]
- Granados, A.; Pleixats, R.; Vallribera, A. Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules 2021, 26, 3008. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.; Jang, W.Y.; Park, K.; Lee, J.; Kim, H.; Lee, K.; Lee, C.K.; Lee, Y.; Lee, S.H.; Seo, J. Antibacterial infection and immune-evasive coating for orthopedic implants. Sci. Adv. 2020, 6, eabb0025. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, J.; Kim, J.; Jang, Y.; Shin, K.; Ha, E.; Ryu, S.; Kim, B.-G.; Wooh, S.; Char, K. Development of Multimodal Antibacterial Surfaces Using Porous Amine-Reactive Films Incorporating Lubricant and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 6550–6560. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Chang, M.-H.; Hsiao, Y.-P.; Hsu, C.-Y.; Lai, P.-S. Photo-Crosslinked Polymeric Matrix with Antimicrobial Functions for Excisional Wound Healing in Mice. Nanomaterials 2018, 8, 791. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of zinc oxide nanoparticles by cell-biomass and supernatant of Lactobacillus plantarum TA4 and its antibacterial and biocompatibility properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Shi, M.; Kwon, H.S.; Peng, Z.; Elder, A.; Yang, H. Effects of surface chemistry on the generation of reactive oxygen species by copper nanoparticles. ACS Nano 2012, 6, 2157–2164. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Dayaghi, E.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.F.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Coelho, C.C.; Padrão, T.; Costa, L.; Pinto, M.T.; Costa, P.C.; Domingues, V.F.; Quadros, P.A.; Monteiro, F.J.; Sousa, S.R. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci. Rep. 2020, 10, 19098. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Dong, Z.; He, C.; Yang, W.; Peng, S.; Yang, Y.; Qi, F. A peritectic phase refines the microstructure and enhances Zn implants. J. Mater. Res. Technol. 2020, 9, 2623–2634. [Google Scholar] [CrossRef]
- Sui, J.; Liu, S.; Chen, M.; Zhang, H. Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review. Coatings 2022, 12, 1125. [Google Scholar] [CrossRef]
- Auger, S.; Henry, C.; Péchoux, C.; Suman, S.; Lejal, N.; Bertho, N.; Larcher, T.; Stankic, S.; Vidic, J. Exploring multiple effects of Zn0.15Mg0.85O nanoparticles on Bacillus subtilis and macrophages. Sci. Rep. 2018, 8, 12276. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Castillo, K.; Torres-Huerta, A.M.; Del Ángel-López, D.; Domínguez-Crespo, M.A.; Dorantes-Rosales, H.; Palma-Ramírez, D.; Willcock, H. In Situ Growth of Silver Nanoparticles on Chitosan Matrix for the Synthesis of Hybrid Electrospun Fibers: Analysis of Microstructural and Mechanical Properties. Polymers 2022, 14, 674. [Google Scholar] [CrossRef]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Bandeira, M.; Chee, B.S.; Frassini, R.; Nugent, M.; Giovanela, M.; Roesch-Ely, M.; Da Crespo, J.S.; Devine, D.M. Antimicrobial PAA/PAH Electrospun Fiber Containing Green Synthesized Zinc Oxide Nanoparticles for Wound Healing. Materials 2021, 14, 2889. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Xiong, P.; Zhou, W.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; Liu, Z.; et al. Additively Manufactured Macroporous Titanium with Silver-Releasing Micro-/Nanoporous Surface for Multipurpose Infection Control and Bone Repair—A Proof of Concept. ACS Appl. Mater. Interfaces 2016, 8, 28495–28510. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Pérez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis Sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 745. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Xue, C.; Song, X.; Liu, M.; Ai, F.; Liu, M.; Shang, Q.; Shi, X.; Li, F.; He, X.; Xie, L.; et al. A highly efficient, low-toxic, wide-spectrum antibacterial coating designed for 3D printed implants with tailorable release properties. J. Mater. Chem. B 2017, 5, 4128–4136. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lv, L.; Bai, Y.; Yang, H.; Zhou, H.; Li, C.; Yang, L. Nanotechnology-enabled materials for hemostatic and anti-infection treatments in orthopedic surgery. Int. J. Nanomed. 2018, 13, 8325–8338. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, Z.; Wu, X.; Li, X.; Xu, Y. Sunlight-Induced Synthesis of Non-Target Biosafety Silver Nanoparticles for the Control of Rice Bacterial Diseases. Nanomaterials 2020, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Qiao, Y.; Meng, F.; Liu, X. Spacing-Dependent Antimicrobial Efficacy of Immobilized Silver Nanoparticles. J. Phys. Chem. Lett. 2014, 5, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; de Stefano, M.A.; Luongo, C.; et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Cicuéndez, M.; Vallet-Regí, M.; Salinas, A.J. Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Yeroslavsky, G.; Lavi, R.; Alishaev, A.; Rahimipour, S. Sonochemically-Produced Metal-Containing Polydopamine Nanoparticles and Their Antibacterial and Antibiofilm Activity. Langmuir 2016, 32, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Sitthisak, S.; Sengupta, M.; Johnson, M.; Jayaswal, R.K.; Morrissey, J.A. Copper stress induces a global stress response in Staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl. Environ. Microbiol. 2010, 76, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Maertens, L.; Coninx, I.; Claesen, J.; Leys, N.; Matroule, J.-Y.; van Houdt, R. Copper Resistance Mediates Long-Term Survival of Cupriavidus metallidurans in Wet Contact with Metallic Copper. Front. Microbiol. 2020, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.; Wan, L.; Yang, K.; Li, X.; Yuwen, L.; Dong, H.; Mou, Y.; Yang, D.; Wang, L. Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections. Nat. Commun. 2022, 13, 3875. [Google Scholar] [CrossRef]
- Li, Z.; Xie, K.; Yang, S.; Yu, T.; Xiao, Y.; Zhou, Y. Multifunctional Ca-Zn-Si-based micro-nano spheres with anti-infective, anti-inflammatory, and dentin regenerative properties for pulp capping application. J. Mater. Chem. B 2021, 9, 8289–8299. [Google Scholar] [CrossRef]
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au Nanocomposite for Antibacterial Applications. Nanomaterials 2022, 12, 3832. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating superhydrophobic and antibacterial surfaces on gold by femtosecond laser pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers 2017, 9, 154. [Google Scholar] [CrossRef]
- Tamayo, L.; Acuña, D.; Riveros, A.L.; Kogan, M.J.; Azócar, M.I.; Páez, M.; Leal, M.; Urzúa, M.; Cerda, E. Porous Nanogold/Polyurethane Scaffolds with Improved Antibiofilm, Mechanical, and Thermal Properties and with Reduced Effects on Cell Viability: A Suitable Material for Soft Tissue Applications. ACS Appl. Mater. Interfaces 2018, 10, 13361–13372. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Shareena Dasari, T.P.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4, 199. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Alkahtani, S.; Almeer, R.S. Silver-doped graphene oxide nanocomposite triggers cytotoxicity and apoptosis in human hepatic normal and carcinoma cells. Int. J. Nanomed. 2018, 13, 5685–5699. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Lek. Listy 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.-C.; Hsiao, C.-D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Canta, M.; Cauda, V. The investigation of the parameters affecting the ZnO nanoparticle cytotoxicity behaviour: A tutorial review. Biomater. Sci. 2020, 8, 6157–6174. [Google Scholar] [CrossRef]
- d’Amora, M.; Schmidt, T.J.N.; Konstantinidou, S.; Raffa, V.; de Angelis, F.; Tantussi, F. Effects of Metal Oxide Nanoparticles in Zebrafish. Oxid. Med. Cell. Longev. 2022, 2022, 3313016. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef]
- Mellor, R.D.; Uchegbu, I.F. Ultrasmall-in-Nano: Why Size Matters. Nanomaterials 2022, 12, 2467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Chen, J.; Yang, J.; Wang, H.; Shen, X.; Sun, Y.-M.; Guo, M.; Zhang, X.-D. Effects of surface charges of gold nanoclusters on long-term in vivo biodistribution, toxicity, and cancer radiation therapy. Int. J. Nanomed. 2016, 11, 3475–3485. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349. [Google Scholar] [CrossRef] [PubMed]
- Nava, O.J.; Soto-Robles, C.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Olivas, A.; Luque, P.A. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Hui, A.; Ding, J.; Liu, X.; Wang, A. Synergistic Effect of Glycyrrhizic Acid and ZnO/Palygorskite on Improving Chitosan-Based Films and Their Potential Application in Wound Healing. Polymers 2021, 13, 3878. [Google Scholar] [CrossRef]
- Kalemtas, A.; Kocer, H.B.; Aydin, A.; Terzioglu, P.; Aydin, G. Mechanical and antibacterial properties of ZnO/chitosan bio-composite films. J. Polym. Eng. 2022, 42, 35–47. [Google Scholar] [CrossRef]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel design of bandages using cotton pads, doped with chitosan, glycogen and ZnO nanoparticles, having enhanced antimicrobial and wounds healing effects. Int. J. Biol. Macromol. 2022, 197, 121–130. [Google Scholar] [CrossRef]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Brouki Milan, P.; Amoupour, M. Using artificial neural network for design and development of PVA/chitosan/starch/heparinized nZnO hydrogels for enhanced wound healing. J. Ind. Eng. Chem. 2022, 108, 88–100. [Google Scholar] [CrossRef]
- Keerthana, S.; Kumar, A. Potential risks and benefits of zinc oxide nanoparticles: A systematic review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-C.; Huang, C.-M.; Yu, X.-W.; Chen, Y.-Y. Effect of nano zinc oxide on proliferation and toxicity of human gingival cells. Hum. Exp. Toxicol. 2022, 41, 9603271221080236. [Google Scholar] [CrossRef] [PubMed]
- Zeghoud, S.; Hemmami, H.; Ben Seghir, B.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A review on biogenic green synthesis of ZnO nanoparticles by plant biomass and their applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Ramalingam, R.; Mohamed Khalith, S.B.; Musthafa, S.A.; Dowlath, M.J.H.; Munuswamy-Ramanujam, G.; Arunachalam, K.D. Copper oxide nanoparticles infused electrospun polycaprolactone/gelatin scaffold as an antibacterial wound dressing. Mater. Lett. 2021, 294, 129787. [Google Scholar] [CrossRef]
- Raba-Páez, A.M.; D Malafatti, J.O.; Parra-Vargas, C.A.; Paris, E.C.; Rincón-Joya, M. Effect of tungsten doping on the structural, morphological and bactericidal properties of nanostructured CuO. PLoS ONE 2020, 15, e0239868. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-Y.; Huang, H.-M.; Li, Z.-L.; Chen, C.-Y.; Shih, Y.-J.; Whang-Peng, J.; Cheng, R.H.; Mo, J.-K.; Lin, H.-Y.; et al. Toxicologic Concerns with Current Medical Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7597. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, O.; Bhagat, M.; Gopalakrishnan, C.; Arunachalam, K.D. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J. Exp. Nanosci. 2008, 3, 185–193. [Google Scholar] [CrossRef]
- Mersian, H.; Alizadeh, M.; Hadi, N. Synthesis of zirconium doped copper oxide (CuO) nanoparticles by the Pechini route and investigation of their structural and antibacterial properties. Ceram. Int. 2018, 44, 20399–20408. [Google Scholar] [CrossRef]
- Thakur, N.; Anu; Kumar, K. Effect of (Ag, Co) co-doping on the structural and antibacterial efficiency of CuO nanoparticles: A rapid microwave assisted method. J. Environ. Chem. Eng. 2020, 8, 104011. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra Mathpal, M.; Prakash, J.; Viljoen, B.C.; Roos, W.D.; Swart, H.C. Band gap tailoring of cauliflower-shaped CuO nanostructures by Zn doping for antibacterial applications. J. Alloys Compd. 2020, 832, 154968. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Zamani, K.; Allah-Bakhshi, N.; Akhavan, F.; Yousefi, M.; Golmoradi, R.; Ramezani, M.; Bach, H.; Razavi, S.; Irajian, G.-R.; Gerami, M.; et al. Antibacterial effect of cerium oxide nanoparticle against Pseudomonas aeruginosa. BMC Biotechnol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Iordache, F.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Teodorescu, S.; et al. Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications. Nanomaterials 2022, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Bu, J.; Song, C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials 2022, 15, 5820. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Polash, S.A.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossain, S.; Hasan, M.A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Niloy, M.S.; Hossain, M.M.; Takikawa, M.; Shakil, M.S.; Polash, S.A.; Mahmud, K.M.; Uddin, M.F.; Alam, M.; Shubhra, R.D.; Shawan, M.M.A.K.; et al. Synthesis of Biogenic Silver Nanoparticles Using Caesalpinia digyna and Investigation of Their Antimicrobial Activity and In Vivo Biocompatibility. ACS Appl. Bio Mater. 2020, 3, 7722–7733. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e10. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Zhang, W.; Pu, Z.; Jiang, L.; Chen, Y. Surface interactions affect the toxicity of engineered metal oxide nanoparticles toward Paramecium. Chem. Res. Toxicol. 2012, 25, 1675–1681. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, D.; Guo, G.; Yeung, K.W.K.; Zhang, X.; Liu, X. Band Gap Engineering of Titania Film through Cobalt Regulation for Oxidative Damage of Bacterial Respiration and Viability. ACS Appl. Mater. Interfaces 2017, 9, 27475–27490. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- Truong, L.B.; Medina-Cruz, D.; Mostafavi, E.; Rabiee, N. Selenium Nanomaterials to Combat Antimicrobial Resistance. Molecules 2021, 26, 3611. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Arbab, S.; Urbankova, L.; Horky, P. Toxicological effects of nanoselenium in animals. J. Anim. Sci. Biotechnol. 2022, 13, 72. [Google Scholar] [CrossRef]
- Biswas, D.P.; O’Brien-Simpson, N.M.; Reynolds, E.C.; O’Connor, A.J.; Tran, P.A. Comparative study of novel in situ decorated porous chitosan-selenium scaffolds and porous chitosan-silver scaffolds towards antimicrobial wound dressing application. J. Colloid Interface Sci. 2018, 515, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, H.; Xia, W. Progress in the Surface Functionalization of Selenium Nanoparticles and Their Potential Application in Cancer Therapy. Antioxidants 2022, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Shahverdi, A.R.; Faramarzi, M.A.; Hassanzadeh, G.R.; Rahimi, H.R.; Sabzevari, O. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013, 51, 58–63. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2016, 27, 45101. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, S.; Gadd, G.M.; McGrath, J.; Rooney, D.W.; Zhao, Q. Fungal-derived selenium nanoparticles and their potential applications in electroless silver coatings for preventing pin-tract infections. Regen. Biomater. 2022, 9, rbac013. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, H.; Wang, Q.; Wang, J.; Tan, J.; Li, J.; Jin, P.; Shen, H. Nano-layered magnesium fluoride reservoirs on biomaterial surfaces strengthen polymorphonuclear leukocyte resistance to bacterial pathogens. Nanoscale 2017, 9, 875–892. [Google Scholar] [CrossRef]
- Pramoda Kumari, J. Fluoride and Its Interaction with Human Life—A Review. Word J. Pharm. Res. 2017, 6, 292–315. [Google Scholar] [CrossRef]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Saïd-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Breaker, R.R. New insight on the response of bacteria to fluoride. Caries Res. 2012, 46, 78–81. [Google Scholar] [CrossRef]
- Zheng, T.-X.; Li, W.; Gu, Y.-Y.; Zhao, D.; Qi, M.-C. Classification and research progress of implant surface antimicrobial techniques. J. Dent. Sci. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.M.; Hill, R.G.; Brauer, D.S. Surface properties and ion release from fluoride-containing bioactive glasses promote osteoblast differentiation and mineralization in vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, J.; Friedman, A.; Lellouche, J.-P.; Gedanken, A.; Banin, E. Improved antibacterial and antibiofilm activity of magnesium fluoride nanoparticles obtained by water-based ultrasound chemistry. Nanomedicine 2012, 8, 702–711. [Google Scholar] [CrossRef]
- Ahmad, Z.; Senior, A.E. Inhibition of the ATPase activity of Escherichia coli ATP synthase by magnesium fluoride. FEBS Lett. 2006, 580, 517–520. [Google Scholar] [CrossRef]
- Lellouche, J.; Friedman, A.; Gedanken, A.; Banin, E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611–5624. [Google Scholar] [CrossRef]
- Koyappayil, A.; Chavan, S.G.; Roh, Y.-G.; Lee, M.-H. Advances of MXenes; Perspectives on Biomedical Research. Biosensors 2022, 12, 454. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Lin, H.; Ge, M.; Shi, J.; Zhang, X. Niobium Carbide MXene Augmented Medical Implant Elicits Bacterial Infection Elimination and Tissue Regeneration. ACS Nano 2021, 15, 1086–1099. [Google Scholar] [CrossRef]
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011, 111, 117–151. [Google Scholar] [CrossRef]
- García-Betancur, J.-C.; Goñi-Moreno, A.; Horger, T.; Schott, M.; Sharan, M.; Eikmeier, J.; Wohlmuth, B.; Zernecke, A.; Ohlsen, K.; Kuttler, C.; et al. Cell differentiation defines acute and chronic infection cell types in Staphylococcus aureus. Elife 2017, 6, e28023. [Google Scholar] [CrossRef]
- Arabi Shamsabadi, A.; Sharifian Gh, M.; Anasori, B.; Soroush, M. Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 16586–16596. [Google Scholar] [CrossRef]
- Kang, K.-T.; Koh, Y.-G.; Son, J.; Yeom, J.S.; Park, J.-H.; Kim, H.-J. Biomechanical evaluation of pedicle screw fixation system in spinal adjacent levels using polyetheretherketone, carbon-fiber-reinforced polyetheretherketone, and traditional titanium as rod materials. Compos. Part B Eng. 2017, 130, 248–256. [Google Scholar] [CrossRef]
- Tang, X.; Dai, J.; Sun, H.; Nabanita, S.; Petr, S.; Wang, D.; Cheng, Q.; Wei, J. Mechanical Strength, Surface Properties, Cytocompatibility and Antibacterial Activity of Nano Zinc-Magnesium Silicate/Polyetheretherketone Biocomposites. J. Nanosci. Nanotechnol. 2019, 19, 7615–7623. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Xia, F. Macromol. Rapid Commun. 19/2017. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Chin, W.; Yang, C.; Ng, V.W.L.; Huang, Y.; Cheng, J.; Tong, Y.W.; Coady, D.J.; Fan, W.; Hedrick, J.L.; Yang, Y.Y. Biodegradable Broad-Spectrum Antimicrobial Polycarbonates: Investigating the Role of Chemical Structure on Activity and Selectivity. Macromolecules 2013, 46, 8797–8807. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Jiang, Q.; Zhang, W.; Yue, X.; Guo, S.; Xiao, P.; Du, Q.; Xing, J.; Deng, L.; et al. Structural contributions of blocked or grafted poly(2-dimethylaminoethyl methacrylate) on PEGylated polycaprolactone nanoparticles in siRNA delivery. Biomaterials 2011, 32, 8730–8742. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ma, S.; Peng, G.; He, D. TAT-modified self-assembled cationic peptide nanoparticles as an efficient antibacterial agent. Nanomedicine 2018, 14, 365–372. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Wang, C.; Liu, W.; Zhang, Y.; Deng, L.; Zhang, J.; Huang, P.; Wang, W.; Dong, A. Combating drug-resistant bacterial infection using biodegradable nanoparticles assembled from comb-like polycarbonates grafted with amphiphilic polyquaternium. J. Mater. Chem. B 2021, 9, 357–365. [Google Scholar] [CrossRef]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.H.; Chen, Y.-Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Kwan, R.J.; Nguyen, T.-K.; Yeow, J.; Byrne, F.L.; Oehlers, S.H.; Wong, E.H.H.; Boyer, C. The effects of polymer topology and chain length on the antimicrobial activity and hemocompatibility of amphiphilic ternary copolymers. Polym. Chem. 2018, 9, 1735–1744. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Y.; Wei, T.; Qu, Y.; Wang, Y.; Zhan, W.; Zhang, Y.; Pan, G.; Li, D.; Yu, Q.; et al. Multistimulus Responsive Biointerfaces with Switchable Bioadhesion and Surface Functions. ACS Appl. Mater. Interfaces 2020, 12, 5447–5455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Li, X.; Yu, H.; Che, C.; Luan, S.; Ren, Y.; Li, S.; Liu, P.; Yu, X.; et al. Multifunctional Antibacterial Materials Comprising Water Dispersible Random Copolymers Containing a Fluorinated Block and Their Application in Catheters. ACS Appl. Mater. Interfaces 2020, 12, 7617–7630. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, X.; Wei, Y.; Zhao, Z.; Liu, H.; Yang, F.; Yin, L.; Song, Z.; Luan, S.; Tang, H. A sulfonate-based polypeptide toward infection-resistant coatings. Biomater. Sci. 2021, 9, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Peddinti, B.S.T.; Scholle, F.; Vargas, M.G.; Smith, S.D.; Ghiladi, R.A.; Spontak, R.J. Inherently self-sterilizing charged multiblock polymers that kill drug-resistant microbes in minutes. Mater. Horiz. 2019, 6, 2056–2062. [Google Scholar] [CrossRef]
- Muralidharan, S.K.; Bauman, L.; Anderson, W.A.; Zhao, B. Recyclable antimicrobial sulphonated poly (ether ether ketone)—Copper films: Flat vs. micro-pillared surfaces. Mater. Today Commun. 2020, 25, 101485. [Google Scholar] [CrossRef]
- Zhu, S.; Xue, R.; Yu, Z.; Zhang, X.; Luan, S.; Tang, H. Transition of Conformation and Solubility in β-Sheet-Structured Poly(l-cysteine)s with Methylthio or Sulfonium Pendants. Biomacromolecules 2021, 22, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Evans, M.D.M.; Bean, P.; Saleh-Mghir, A.; Crémieux, A.C.; Castner, D.G.; Falentin-Daudré, C.; Migonney, V. Grafting of Bioactive Polymers with Various Architectures: A Versatile Tool for Preparing Antibacterial Infection and Biocompatible Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 1480–1491. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’Reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K.; et al. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef]
- Truong, V.K.; Geeganagamage, N.M.; Baulin, V.A.; Vongsvivut, J.; Tobin, M.J.; Luque, P.; Crawford, R.J.; Ivanova, E.P. The susceptibility of Staphylococcus aureus CIP 65.8 and Pseudomonas aeruginosa ATCC 9721 cells to the bactericidal action of nanostructured Calopteryx haemorrhoidalis damselfly wing surfaces. Appl. Microbiol. Biotechnol. 2017, 101, 4683–4690. [Google Scholar] [CrossRef]
- Michalska, M.; Gambacorta, F.; Divan, R.; Aranson, I.S.; Sokolov, A.; Noirot, P.; Laible, P.D. Tuning antimicrobial properties of biomimetic nanopatterned surfaces. Nanoscale 2018, 10, 6639–6650. [Google Scholar] [CrossRef]
- Modaresifar, K.; Kunkels, L.B.; Ganjian, M.; Tümer, N.; Hagen, C.W.; Otten, L.G.; Hagedoorn, P.-L.; Angeloni, L.; Ghatkesar, M.K.; Fratila-Apachitei, L.E.; et al. Deciphering the Roles of Interspace and Controlled Disorder in the Bactericidal Properties of Nanopatterns against Staphylococcus aureus. Nanomaterials 2020, 10, 347. [Google Scholar] [CrossRef]
- Linklater, D.P.; de Volder, M.; Baulin, V.A.; Werner, M.; Jessl, S.; Golozar, M.; Maggini, L.; Rubanov, S.; Hanssen, E.; Juodkazis, S.; et al. High Aspect Ratio Nanostructures Kill Bacteria via Storage and Release of Mechanical Energy. ACS Nano 2018, 12, 6657–6667. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, C.; Wu, S.; Ben, Y.; Li, X.; Ge, Z.; Zou, H.; Tian, D.; Yu, Y.; Ding, K. Electrophoresis-Deposited Mesoporous Graphitic Carbon Nitride Surfaces with Efficient Bactericidal Properties. ACS Appl. Bio Mater. 2020, 3, 2255–2262. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Luo, Y.; Ge, M.; Lin, H.; He, R.; Yuan, X.; Yang, C.; Wang, W.; Zhang, X. Anti-Infective Application of Graphene-Like Silicon Nanosheets via Membrane Destruction. Adv. Healthc. Mater. 2020, 9, e1901375. [Google Scholar] [CrossRef]
- Sun, W.; Wu, F.-G. Two-Dimensional Materials for Antimicrobial Applications: Graphene Materials and beyond. Chem. Asian J. 2018, 13, 3378–3410. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Hou, X.; Guo, S.; Zhang, L.; Wei, C.; Peng, T.; Hu, X. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano-bio interactions. Nat. Commun. 2021, 12, 493. [Google Scholar] [CrossRef]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Yamada, K.J.; Kielian, T. Biofilm-Leukocyte Cross-Talk: Impact on Immune Polarization and Immunometabolism. J. Innate Immun. 2019, 11, 280–288. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, H.; Shen, H.; Zhu, C.; He, R.; Tang, J.; Wang, Y.; Jiang, X.; Wang, J.; Bu, W.; et al. Space-Selective Chemodynamic Therapy of CuFe5O8 Nanocubes for Implant-Related Infections. ACS Nano 2020, 14, 13391–13405. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed Engl. 2016, 55, 2101–2106. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- González-Lavado, E.; Valdivia, L.; García-Castaño, A.; González, F.; Pesquera, C.; Valiente, R.; Fanarraga, M.L. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-Fluorouracil. Oncotarget 2019, 10, 2022–2029. [Google Scholar] [CrossRef]
- Seo, Y.; Hwang, J.; Kim, J.; Jeong, Y.; Hwang, M.P.; Choi, J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int. J. Nanomed. 2014, 9, 4621–4629. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Ganciarov, M.; Vasilievici, G.; et al. Hybrid Materials Based on Multi-Walled Carbon Nanotubes and Nanoparticles with Antimicrobial Properties. Nanomaterials 2021, 11, 1415. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, J.; Hou, Y.; Chen, M.; Zheng, Z.; Meng, X.; Liu, L.; Huo, S.; Liu, S.; Zhang, H. Nanomaterials for Anti-Infection in Orthopedic Implants: A Review. Coatings 2024, 14, 254. https://doi.org/10.3390/coatings14030254
Sui J, Hou Y, Chen M, Zheng Z, Meng X, Liu L, Huo S, Liu S, Zhang H. Nanomaterials for Anti-Infection in Orthopedic Implants: A Review. Coatings. 2024; 14(3):254. https://doi.org/10.3390/coatings14030254
Chicago/Turabian StyleSui, Junhao, Yijin Hou, Mengchen Chen, Zhong Zheng, Xiangyu Meng, Lu Liu, Shicheng Huo, Shu Liu, and Hao Zhang. 2024. "Nanomaterials for Anti-Infection in Orthopedic Implants: A Review" Coatings 14, no. 3: 254. https://doi.org/10.3390/coatings14030254
APA StyleSui, J., Hou, Y., Chen, M., Zheng, Z., Meng, X., Liu, L., Huo, S., Liu, S., & Zhang, H. (2024). Nanomaterials for Anti-Infection in Orthopedic Implants: A Review. Coatings, 14(3), 254. https://doi.org/10.3390/coatings14030254






