Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metallic Alloys
2.2. Alkaline Solutions
2.3. Characterization
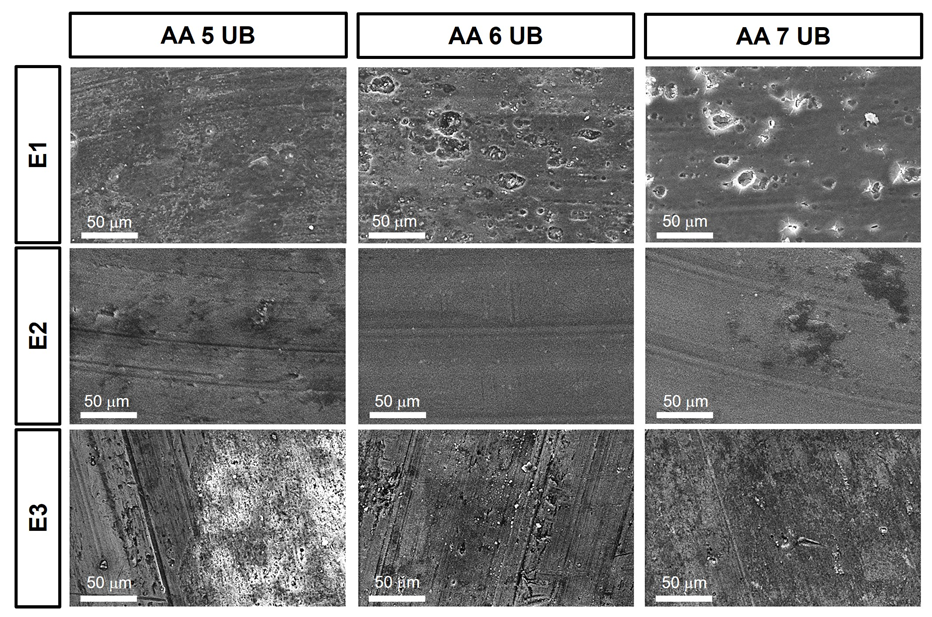
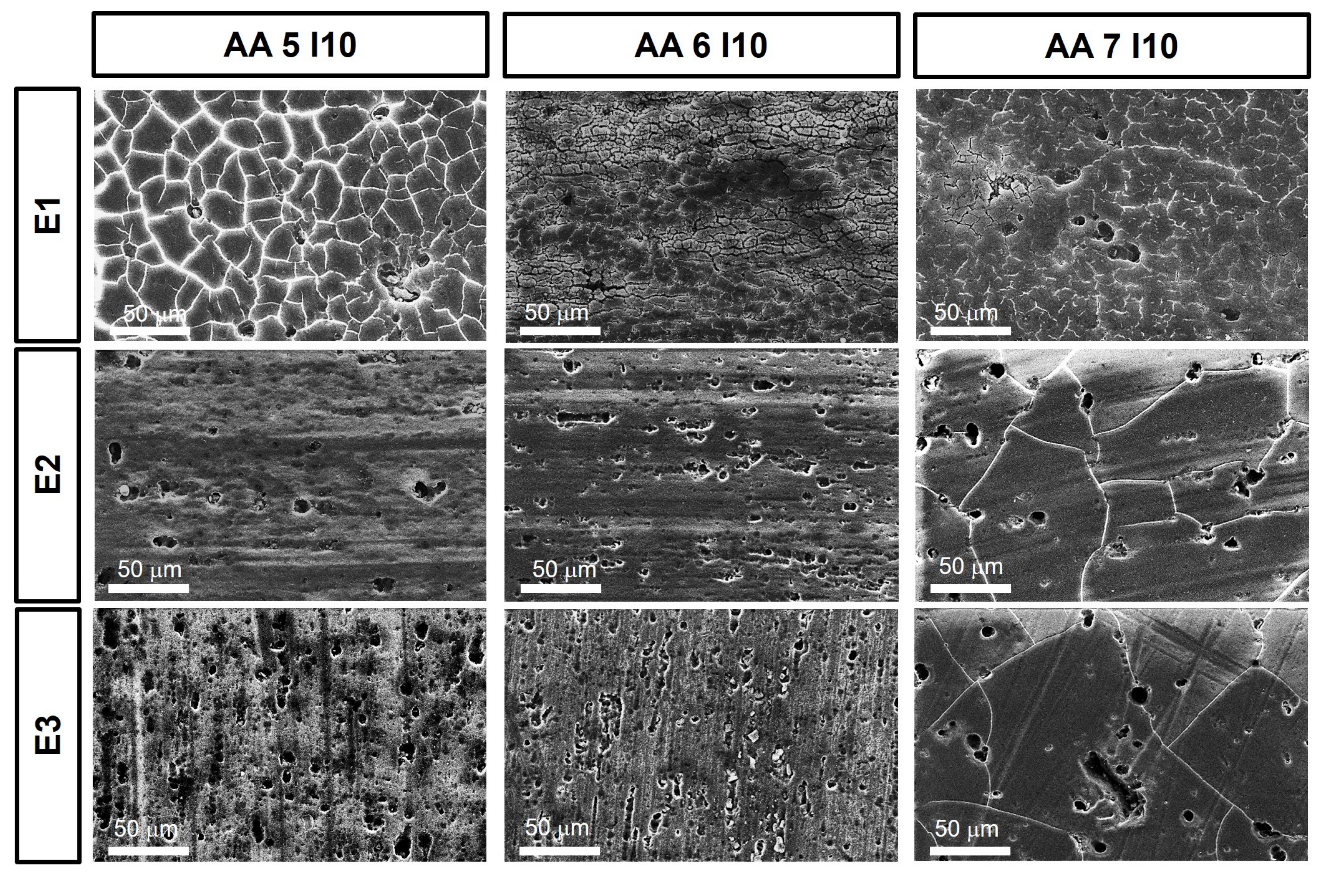
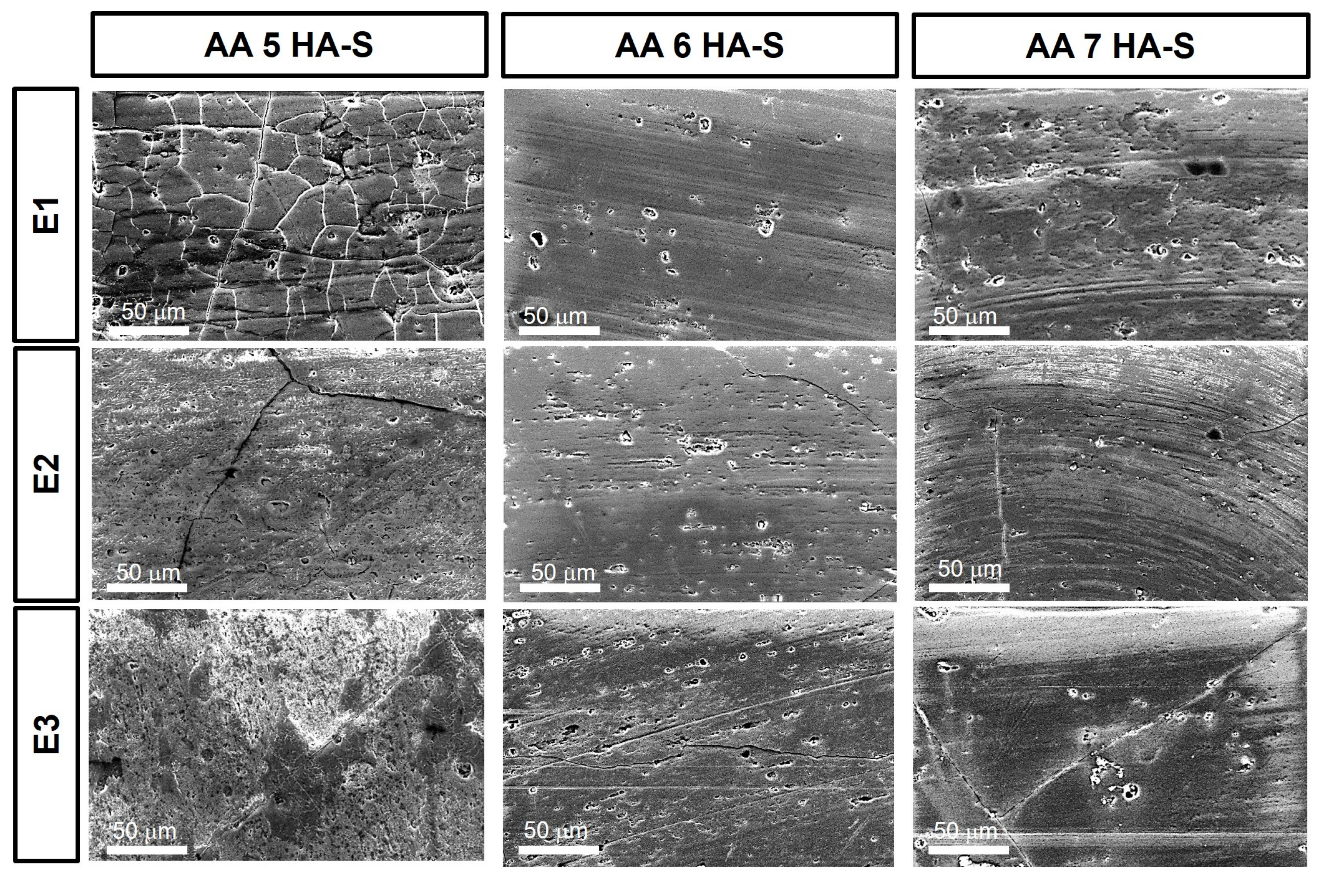
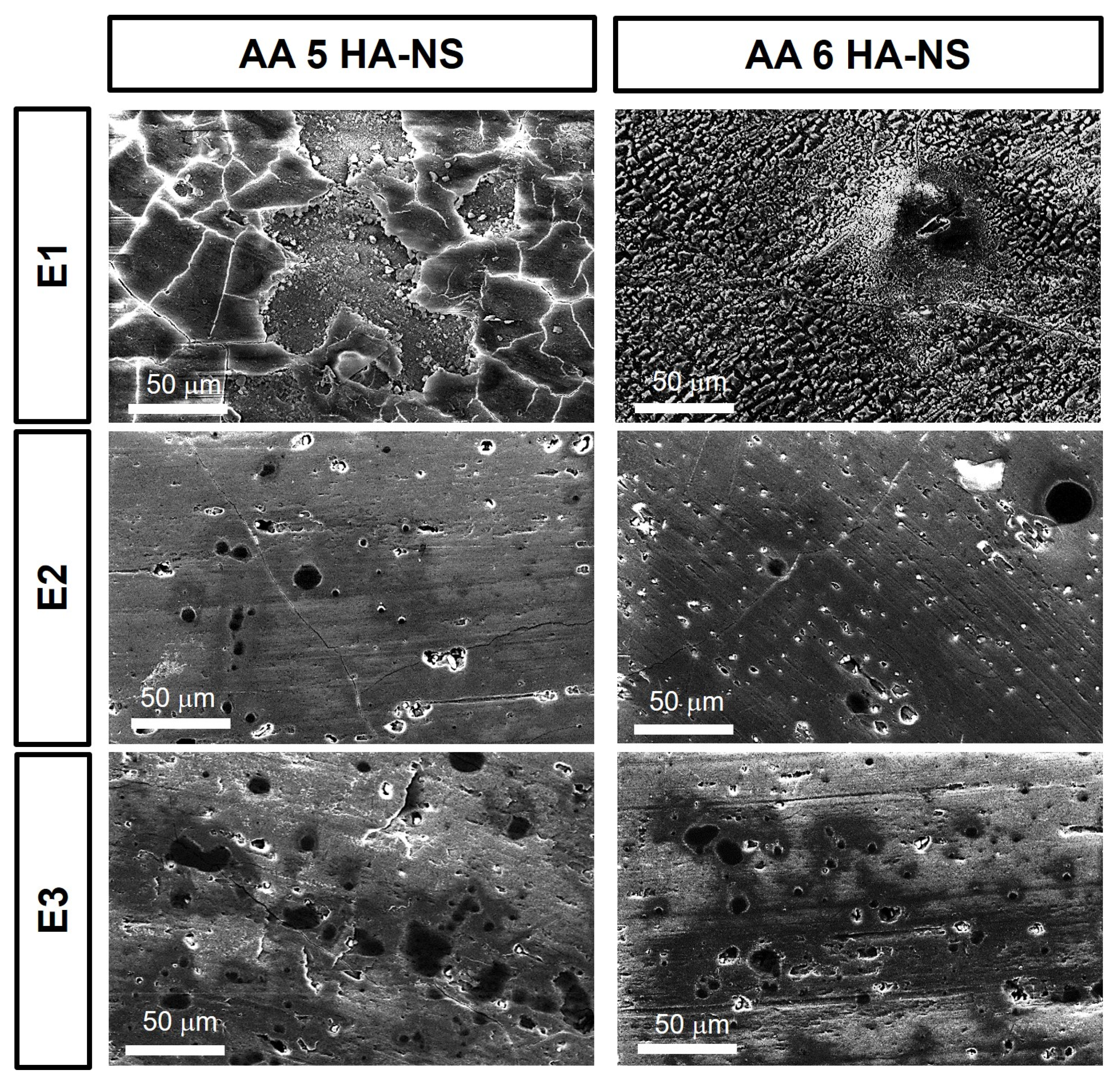
3. Results and Discussion
4. Conclusions
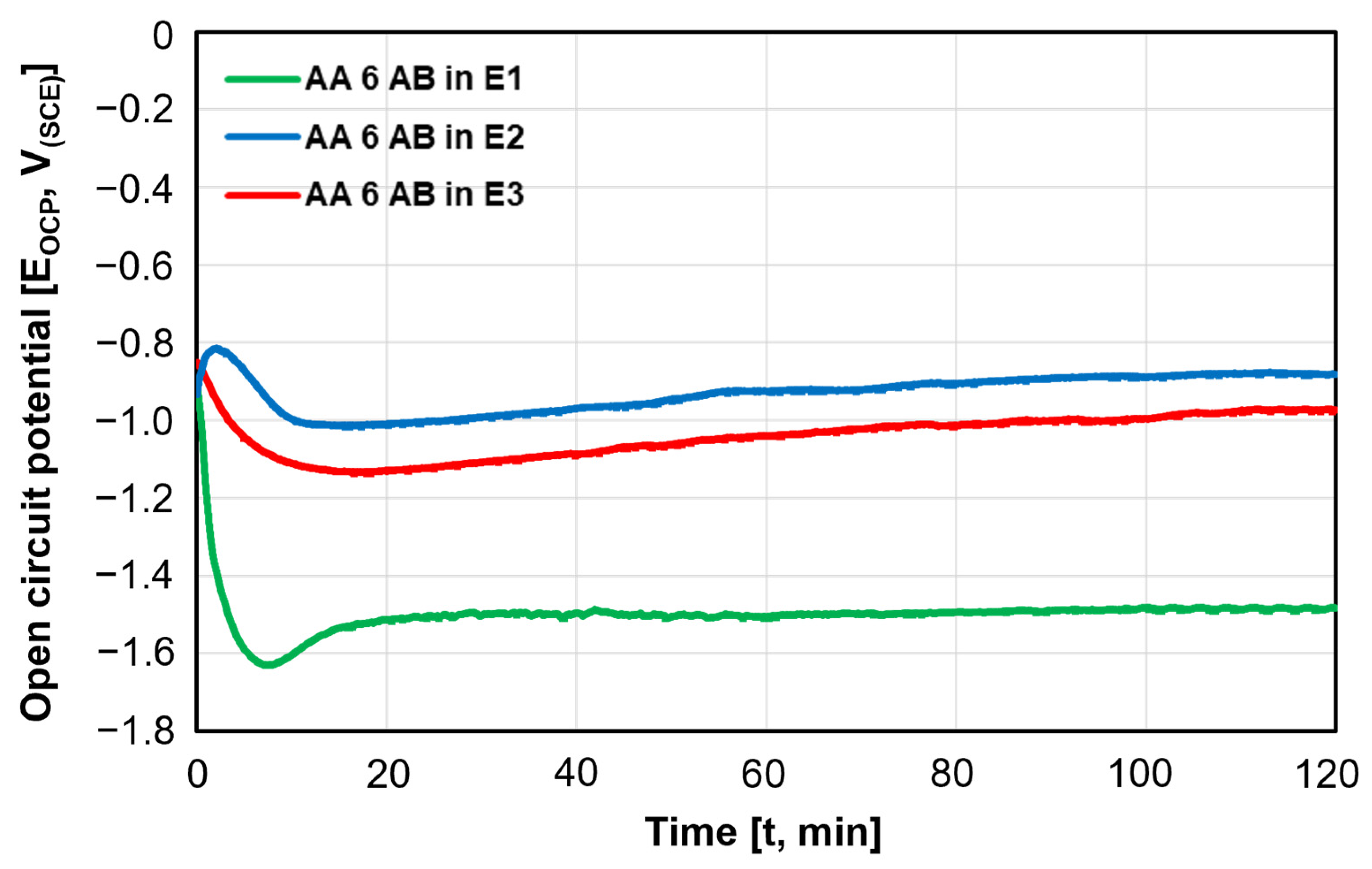
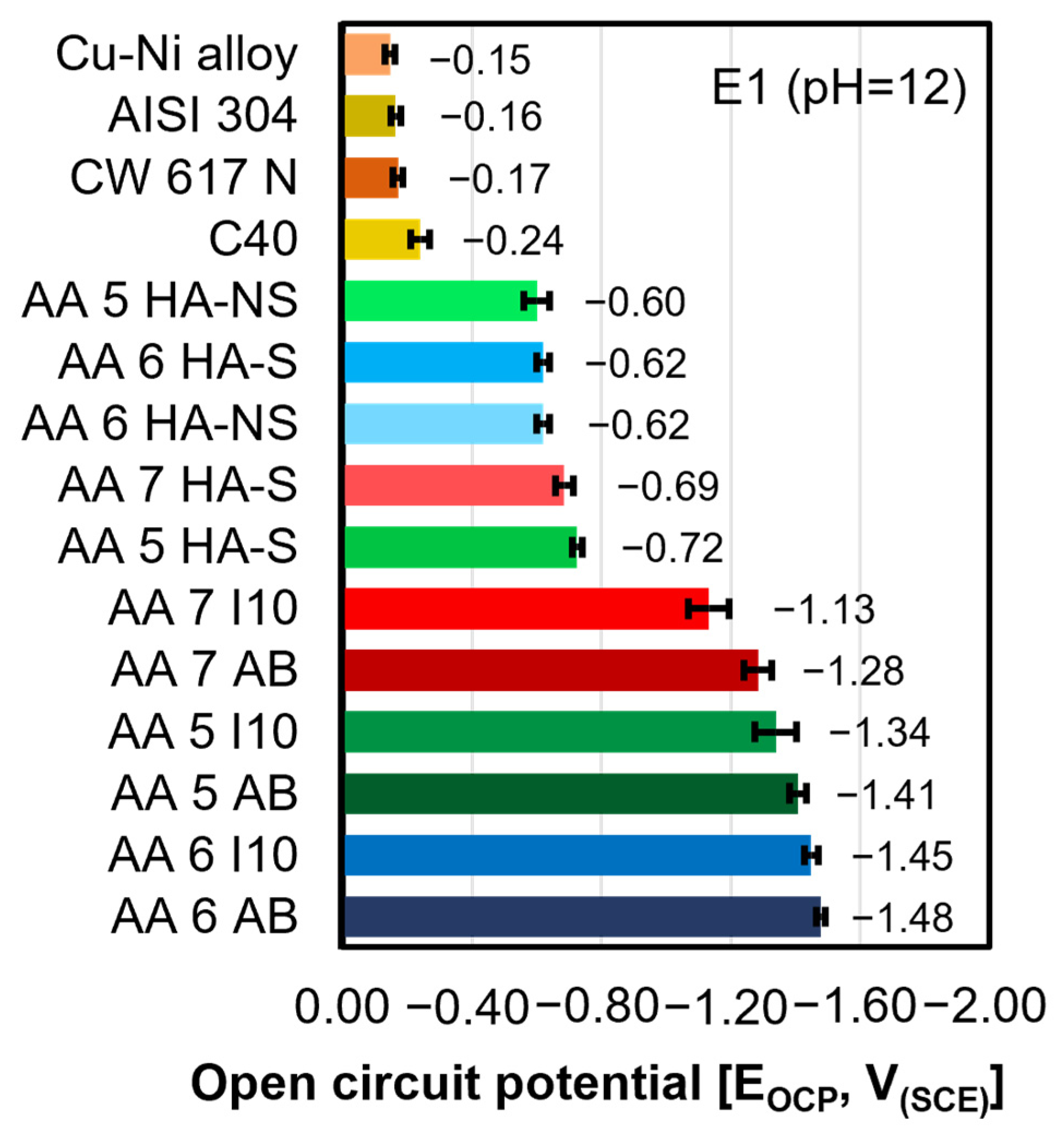
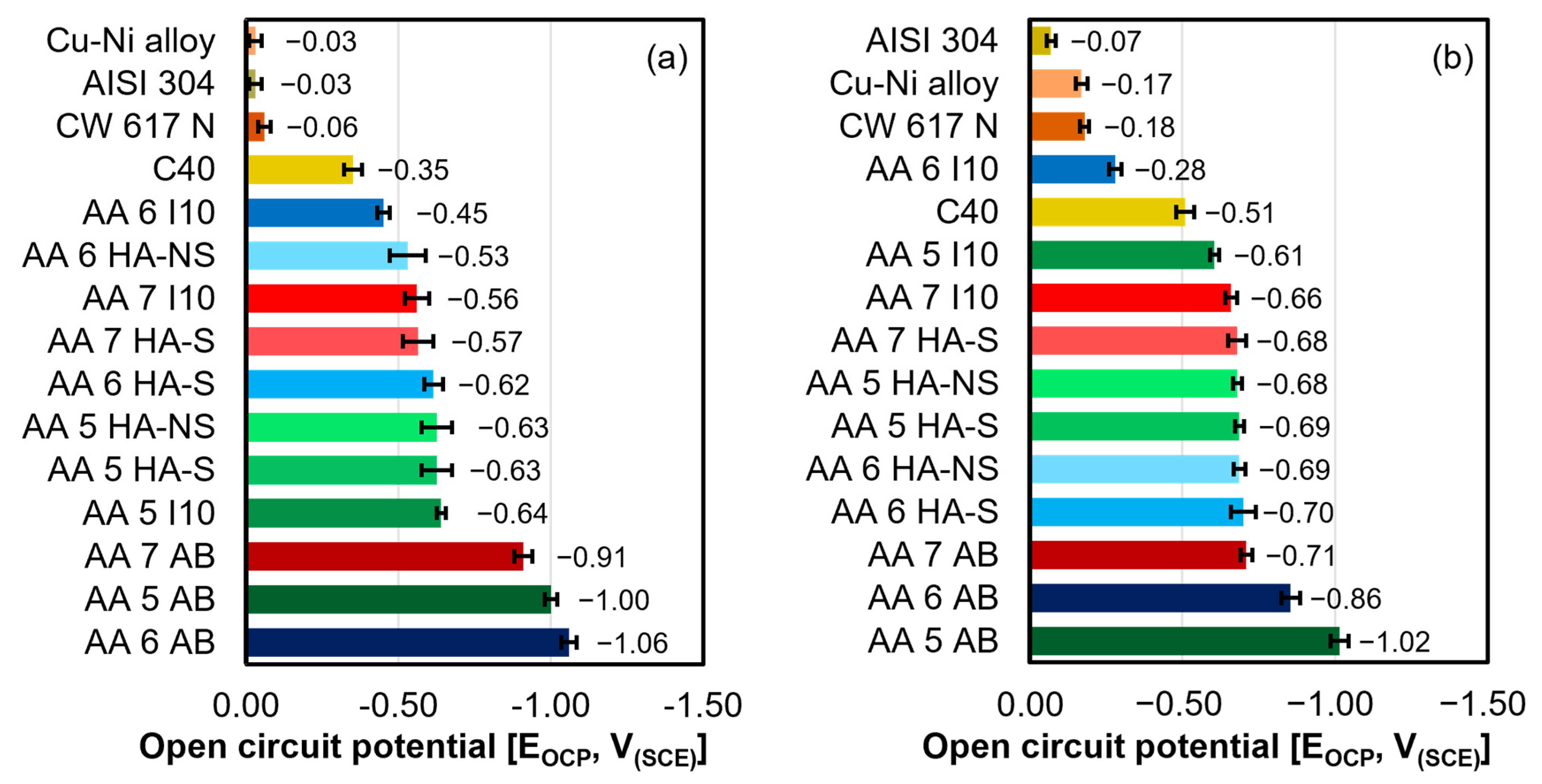
- The as-built samples were found to be the most electronegative ones, holding the lowest positions in the galvanic series. Among the samples, AA 6 AB, tested in E1 and E2 electrolytes, as well as AA 5 AB, tested in E3, exhibited the most electronegative EOCP values. Conversely, AA 7 AB appeared to be the most electropositive in all the considered environments;
- The EOCP values measured for the three alloys in I10 condition increased with the aggressiveness of the testing solution, whereas the HA-S and HA-NS samples exhibited no significant variation in EOCP values across any of the environments considered;
- Comparing the E-log(i) characteristic curves obtained for the three alloys in AB condition in E1 with those recorded in E2 and E3 solutions reveals a variation in their electrochemical behavior. In the former electrolyte, specimens show an active behavior, while in E2 and E3 an active-passive behavior was observed. All the anodized samples in E2 and E3 exhibited the lowest current density and consequently the lowest corrosion rate;
- The electrochemical behavior of AA samples analyzed in all the considered electrolytes was found to be dependent on the pH of the environment as well as the thickness of the anodization treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Liu, G.; Müller, D.B. Addressing sustainability in the aluminum industry: A critical review of life cycle assessments. J. Clean. Prod. 2012, 35, 108–117. [Google Scholar] [CrossRef]
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Davis, J.R. Physical Metallurgy of Aluminum Alloys. In Metals Handbook Desk Edition; ASM International: Detroit, MI, USA, 2018; pp. 437–442. [Google Scholar]
- Totten, G.; MacKenzie, D.S. Handbook of Aluminum: Physical Metallurgy and Processes, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; Volume 1, ISBN 0824704940. [Google Scholar]
- Qbau, N.; Nam, N.; Hien, N.; Ca, N. Development of light weight high strength aluminum alloy for selective laser melting. J. Mater. Res. Technol. 2020, 9, 14075–14081. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Iqbal, A.P.; Ahmed, A.F.; Islam, S. Corrosion behavior of aluminum alloy in NaOH and Syzygium Samarangense solution for environmental sustainability. Curr. Res. Green Sustain. Chem. 2022, 5, 100254. [Google Scholar] [CrossRef]
- Mogucheva, A.; Babich, E.; Kaibyshev, R. Microstructure and Mechanical Properties of An Al-Mg-Sc-Zr Alloy Subjected to Extensive Cold Rolling. In Proceedings of the 13th International Conference on Aluminum Alloys (ICAA13), Pittsburgh, PA, USA, 3–7 June 2012; pp. 1773–1778. [Google Scholar]
- Brinksmeier, E.; Meyer, D.; Huesmann-Cordes, A.; Herrmann, C. Metalworking fluids—Mechanisms and performance. CIRP Ann. Manuf. Technol. 2015, 64, 605–628. [Google Scholar] [CrossRef]
- El Sachat, A.; Meristoudi, A.; Markos, C.; Sakellariou, A.; Papadopoulos, A.; Katsikas, S.; Riziotis, C. Characterization of Industrial Coolant Fluids and Continuous Ageing Monitoring by Wireless Node—Enabled Fiber Optic Sensors. Sensors 2017, 17, 568. [Google Scholar] [CrossRef]
- Bird, R.W. Aqueous alkaline cleaners: A better alternative. Met. Finish. 1995, 93, 10–20. [Google Scholar] [CrossRef]
- Quadri, T.W.; Akpan, E.D.; Olasunkanmi, L.O.; Fayemi, O.E.; Ebenso, E.E. Fundamentals of Corrosion Chemistry. In Environmentally Sustainable Corrosion Inhibitors: Fundamentals and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–45. ISBN 9780323854054. [Google Scholar]
- Kisasoz, A. Corrosion behavior of alloy AA6063-T4 in HCl and NaOH solutions. Mater. Test. 2018, 60, 478–482. [Google Scholar] [CrossRef]
- Reboul, M.C.; Baroux, B. Metallurgical aspects of corrosion resistance of aluminium alloys. Mater. Corros. 2011, 62, 215–233. [Google Scholar] [CrossRef]
- Adams, F.V.; Akinwamide, S.O.; Obadele, B.; Olubambi, P.A. Comparison study on the corrosion behavior of aluminum alloys in different acidic media. Mater. Today Proc. 2021, 38, 1040–1043. [Google Scholar] [CrossRef]
- Pruthviraj, R.D.; Rashmi, M. Electrochemical Studies of Aluminium 7075 Alloy in Different Concentration of Acid Chloride Medium. J. Mater. Sci. Eng. 2016, 5, 221. [Google Scholar] [CrossRef]
- Shahidi, M.; Gholamhosseinzadeh, M. Electrochemical evaluation of AA6061 aluminum alloy corrosion in citric acid solution without and with chloride ions. J. Electroanal. Chem. 2015, 757, 8–17. [Google Scholar] [CrossRef]
- Kruger, J. Passivity. In Corrosion: Fundamentals, Testing, and Protection; ASM International: Detroit, MI, USA, 2003; Volume 13A, pp. 61–67. [Google Scholar]
- Kumari, P.R.; Nayak, J.; Shetty, A.N. Corrosion behavior of 6061/Al-15 vol. pct. SiC(p) composite and the base alloy in sodium hydroxide solution. Arab. J. Chem. 2016, 9, S1144–S1154. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Dobryden, I.; Sefer, B.; Ryl, J.; Wrzesińska, A.; Makarova, I.V.; Bobowska, I.; Kurilo, I.I.; Claesson, P.M. Surface and corrosion properties of AA6063-T5 aluminum alloy in molybdate-containing sodium chloride solutions. Corros. Sci. 2020, 171, 108658. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Shih, T.-S.; Wu, C.-E. Electrochemical behavior of anodized AA6063-T6 alloys affected by matrix structures. Appl. Surf. Sci. 2013, 264, 410–418. [Google Scholar] [CrossRef]
- Joshi, S.; Fahrenholtz, W.G.; O’keefe, M.J. Electrochemical Characterization of Al 7075-T6 Surface Oxide after Alkaline Treatments. J. Electrochem. Soc. 2011, 158, C296–C301. [Google Scholar] [CrossRef]
- Andreatta, F.; Lohrengel, M.; Terryn, H.; de Wit, J. Electrochemical characterisation of aluminium AA7075-T6 and solution heat treated AA7075 using a micro-capillary cell. Electrochimica Acta 2003, 48, 3239–3247. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Lee, K.; Gao, L. Performance of AA5052 alloy anode in alkaline ethylene glycol electrolyte with dicarboxylic acids additives for aluminium-air batteries. J. Power Sources 2015, 297, 464–471. [Google Scholar] [CrossRef]
- Eid, S.; Abdallah, M.; Kamar, E.; El-Etre, A.Y. Corrosion Inhibition of Aluminum and Aluminum Silicon Alloys in Sodium Hydroxide Solutions by Methyl Cellulose. J. Mater. Environ. Sci. 2015, 11, 892–901. [Google Scholar]
- Xhanari, K.; Finšgar, M. Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab. J. Chem. 2019, 12, 4646–4663. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Liu, J.; Zhang, D.; Gao, L.; Tong, L. Evaluation of AA5052 alloy anode in alkaline electrolyte with organic rare-earth complex additives for aluminium-air batteries. J. Power Sources 2015, 293, 484–491. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Qin, W.; Sun, J.; Huang, Z.; Li, Y.; Li, B.; Sun, J. A robust superhydrophobic surface on AA3003 aluminum alloy with intermetallic phases in-situ pinning effect for corrosion protection. J. Alloys Compd. 2022, 898, 163038. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, C.; Hashimoto, T.; Yuan, Y.; Kang, D.; Hunter, J.; Zhou, X. Alkaline etching and desmutting of aluminium alloy: The behaviour of Mg2Si particles. J. Alloys Compd. 2020, 842, 155834. [Google Scholar] [CrossRef]
- Eckermann, F.; Suter, T.; Uggowitzer, P.J.; Afseth, A.; Schmutz, P. The influence of MgSi particle reactivity and dissolution processes on corrosion in Al–Mg–Si alloys. Electrochim. Acta 2008, 54, 844–855. [Google Scholar] [CrossRef]
- Buchheit, R.G. A Compilation of Corrosion Potentials Reported for Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 1995, 142, 3994. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Fleming, K.M.; Gibson, M.A.; Birbilis, N. Electrochemical Behavior and Localized Corrosion Associated with Mg2Si Particles in Al and Mg Alloys. ECS Electrochem. Lett. 2012, 1, C1–C3. [Google Scholar] [CrossRef]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the Influences of Alloying Elements on the Microstructure and Mechanical Properties of Aluminum Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Birbilis, N.; Buchheit, R.G. Investigation and Discussion of Characteristics for Intermetallic Phases Common to Aluminum Alloys as a Function of Solution pH. J. Electrochem. Soc. 2008, 155, C117. [Google Scholar] [CrossRef]
- de Miera, M.S.; Curioni, M.; Skeldon, P.; Thompson, G. The behaviour of second phase particles during anodizing of aluminium alloys. Corros. Sci. 2010, 52, 2489–2497. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Linardi, E.; Haddad, R.; Lanzani, L. Stability Analysis of the Mg2Si Phase in AA 6061 Aluminum Alloy. Procedia Mater. Sci. 2012, 1, 550–557. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J. Effect of solution heat treatment on galvanic coupling between intermetallics and matrix in AA7075-T6. Corros. Sci. 2003, 45, 1733–1746. [Google Scholar] [CrossRef]
- De Wit, J.H.W. Local Potential Measurements with the SKPFM on Aluminium Alloys. Electrochim. Acta 2004, 49, 2841–2850. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, B.; Tian, B.; Zhou, Y.; Wang, J.Q.; Han, E.H.; Ke, W. SVET Study of Galvanic Corrosion of Al/Mg2Si Couple in Aqueous Solutions at Different pH. J. Electrochem. Soc. 2017, 164, C240–C249. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.; Wang, Y.-M.; Kuo, H.-H.; Kia, S. Effect of alloy types on the anodizing process of aluminum. Surf. Coatings Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Influence of anodizing process on fatigue life of machined aluminium alloy. Procedia Eng. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Mashtalyar, D.V.; Gnedenkov, A.S.; Filonina, V.S.; Vyaliy, I.E.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Izotov, N.V.; Sinebryukhov, S.L.; et al. Icephobic Performance of Combined Fluorine-Containing Composite Layers on Al-Mg-Mn–Si Alloy Surface. Polymers 2021, 13, 3827. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, J.; Zhang, D.; Wang, Y.; He, Z.; Guo, P. Properties of Micro-Arc Oxidation Coatings on 5052 Al Alloy Sealed by SiO2 Nanoparticles. Coatings 2022, 12, 373. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Belov, E.A.; Imshinetskiy, I.M.; Sinebrukhov, S.L.; Gnedenkov, S.V. Features of Composite Layers Created Using an Aqueous Suspension of a Fluoropolymer. Polymers 2022, 14, 4667. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Dikici, B.; Dafali, A.; Fattah-Alhosseini, A. Self-assembly of coumarin molecules on plasma electrolyzed layer for optimizing the electrochemical performance of AZ31 Mg alloy. J. Magnes. Alloys 2023, 11, 1618–1628. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Yu, L. Investigation on Anodized 5052 Aluminum Alloy and its Corrosion Resistance in Simulated Acid Rain. Int. J. Electrochem. Sci. 2023, 18, 100336. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, G.; Zhang, Z.; Wang, Y. Research on corrosion resistance of anodized and sealed 6061 aluminum alloy in 3.5% sodium chloride solution. Int. J. Electrochem. Sci. 2023, 18, 100092. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Corrosion Testing of Anodized Aerospace Alloys: Comparison Between Immersion and Salt Spray Testing using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2020, 167, 041505. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Bian, G.; Zhang, Y. Electrochemical behavior and corrosion mechanism of anodized 7B04 aluminum alloy in acid NaCl environments. J. Alloys Compd. 2021, 886, 161231. [Google Scholar] [CrossRef]
- Thompson, G.E.; Zhang, L.; Smith, C.J.E.; Skeldon, P. Boric/Sulfuric Acid Anodizing of Aluminum Alloys 2024 and 7075: Film Growth and Corrosion Resistance. Corrosion 1999, 55, 1052–1061. [Google Scholar] [CrossRef]
- Emmanuel, A.; Fayomi, O.; Akande, I. Aluminium Alloys as Advanced Materials: A short communication. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012024. [Google Scholar] [CrossRef]
- Huang, C.; Wu, Z.; Huang, R.; Wang, W.; Li, L. Mechanical Properties of AA5083 in Different Tempers at Low Temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 279, 012002. [Google Scholar] [CrossRef]
- MacKenzie, D.S. Metallurgy of Heat Treatable Aluminum Alloys. In Aluminum Science and Technology; ASM International: Detroit, MI, USA, 2018; pp. 411–437. [Google Scholar]
- ASTM E407−23; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2023.
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- ASTM G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2023.
- Berne, C.; Andrieu, E.; Reby, J.; Sobrino, J.-M.; Blanc, C. The Electrochemical Behavior of α,β′-Brass in Basic NaNO3 Solutions. J. Electrochem. Soc. 2015, 162, C648–C656. [Google Scholar] [CrossRef]
- Assouli, B.; Srhiri, A.; Idrissi, H. Characterization and control of selective corrosion of α,β′-brass by acoustic emission. NDT E Int. 2003, 36, 117–126. [Google Scholar] [CrossRef]
- Berne, C.; Andrieu, E.; Reby, J.; Sobrino, J.-M.; Blanc, C. Dissolution Kinetics of α,β′-Brass in Basic NaNO3 Solutions. J. Electrochem. Soc. 2016, 163, C7–C15. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.; Ferreira, M.; Montemor, M. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 55, 6174–6181. [Google Scholar] [CrossRef]
- Song, G.D.; Jeon, S.-H.; Son, Y.-H.; Kim, J.G.; Hur, D.H. Galvanic effect of magnetite on the corrosion behavior of carbon steel in deaerated alkaline solutions under flowing conditions. Corros. Sci. 2018, 131, 71–80. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, J.-G.; Li, W.; Poon, C.S. Influence of chloride ion on depassivation of passive film on galvanized steel bars in concrete pore solution. Constr. Build. Mater. 2018, 166, 572–580. [Google Scholar] [CrossRef]
- Deepa, P.; Padmalatha, R. Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234–S2244. [Google Scholar] [CrossRef]
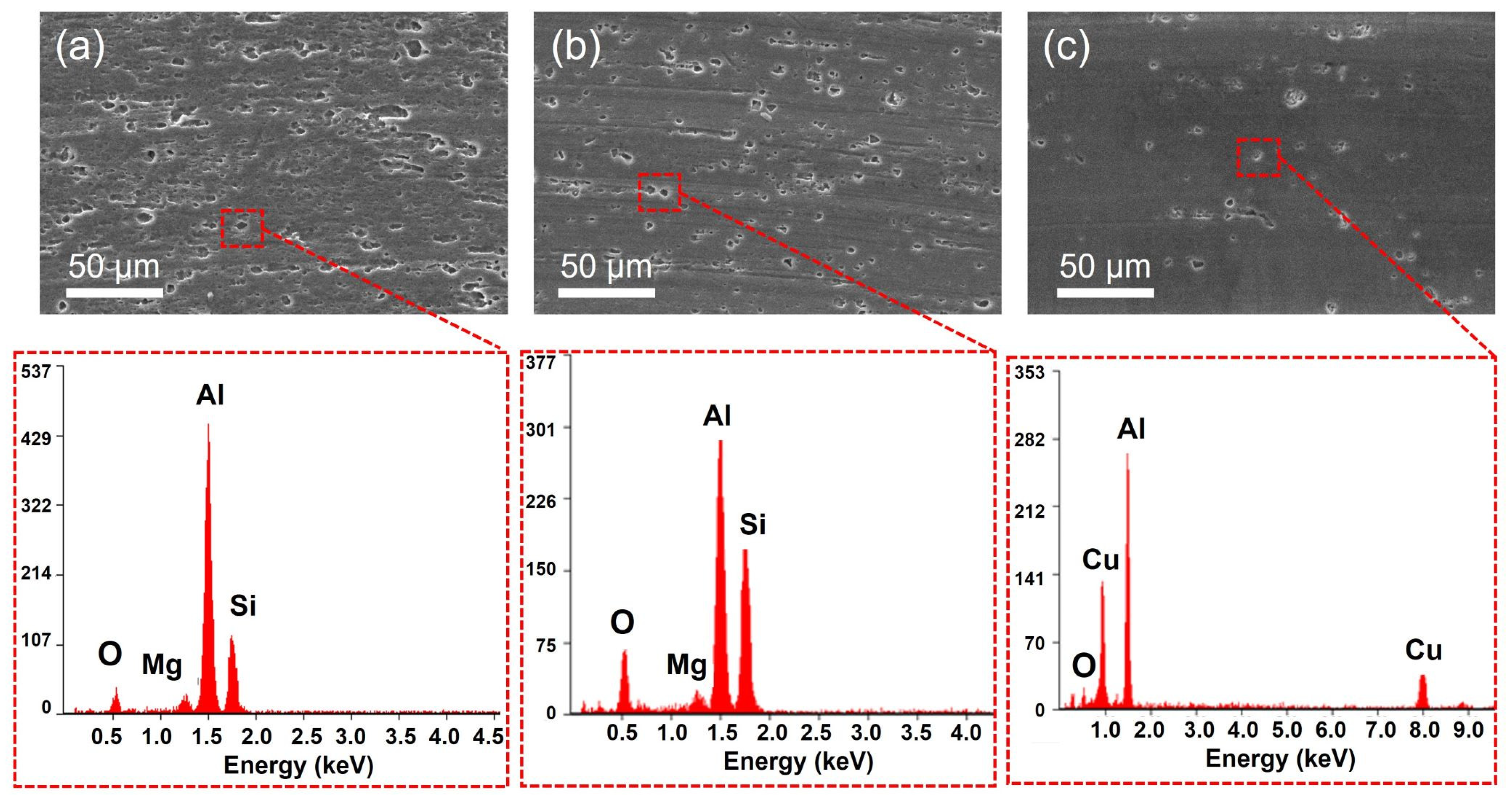


| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | |
|---|---|---|---|---|---|---|---|---|
| AA 5 AB | 0.40 | 0.40 | 0.10 | 0.40–1.00 | 4.00–4.90 | 0.05–0.25 | 0.25 | 0.15 |
| AA 6 AB | 0.70–1.30 | <0.50 | <0.10 | 0.40–1.00 | 0.60–1.20 | <0.25 | <0.2 | <0.1 |
| AA 7 AB | <0.40 | <0.50 | 1.20–2.00 | <0.3 | 2.10–2.90 | 0.18–0.28 | 5.10–6.10 | <0.2 |
| C | Si | P | Mn | Cr | Mo | Ni | Fe | |
|---|---|---|---|---|---|---|---|---|
| C 40 | 0.37–0.44 | 0.4 | 0.03 | 0.5–0.8 | 0.4 | 0.1 | 0.4 | 97.4–97.8 |
| AISI 304 | 0.07 | 1.0 | 0.045 | 2.0 | 17.5–19.5 | - | 8.0–10.5 | 66.9–71.4 |
| Sn | Fe | Zn | Pb | Ni | Si | Cu | |
|---|---|---|---|---|---|---|---|
| CW 617 N | <0.3 | <0.3 | 38.0–40.0 | 1.6–2.2 | <0.1 | <0.03 | 57.0–59.0 |
| Cu-Ni alloy | - | - | - | - | 1.50–2.50 | - | 88.9–91.5 |
| Alloys | Electrolyte | EOCP | icor | CR | Ref. |
|---|---|---|---|---|---|
| [V(SCE)] | [μA/cm2] | [μm/yr] | |||
| AA 5052 | 4 M NaOH in C2H6O2 | −1.56 | 70,000 | 767,522 | [24] |
| AA 5052 | 4 M NaOH | −1.54 | 70,020 | 767,742 | [27] |
| AA 6061 | 0.05 M NaOH | −1.48 | 676 | 7410 | [19] |
| 0.1 M NaOH | −1.48 | 1750 | 13,750 | ||
| 0.25 M NaOH | −1.49 | 2340 | 25,690 | ||
| 0.5 M NaOH | −1.50 | 5570 | 65,150 | ||
| AA 6063 | 0.05 M NaOH | −1.55 | 404 | 4400 | [67] |
| 0.25 M NaOH | −1.57 | 2090 | 22,780 | ||
| 0.5 M NaOH | −1.59 | 3260 | 35,530 |
| Sample | icor [µA/cm2] | CR [µm/yr] |
|---|---|---|
| AA 5 AB | 4.03 ± 0.04 | 45.0 ± 0.4 |
| AA 6 AB | 8.05 ± 0.07 | 87.6 ± 0.8 |
| AA 7 AB | 3.71 ± 0.03 | 41.4 ± 0.4 |
| AA 5 I10 | 2.87 ± 0.03 | 32.1 ± 0.4 |
| AA 6 I10 | 1.99 ± 0.02 | 21.7 ± 0.2 |
| AA 7 I10 | 0.93 ± 0.04 | 10.4 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, R.; Masi, G.; Bignozzi, M.C. Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments. Coatings 2024, 14, 240. https://doi.org/10.3390/coatings14020240
Fabris R, Masi G, Bignozzi MC. Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments. Coatings. 2024; 14(2):240. https://doi.org/10.3390/coatings14020240
Chicago/Turabian StyleFabris, Riccardo, Giulia Masi, and Maria Chiara Bignozzi. 2024. "Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments" Coatings 14, no. 2: 240. https://doi.org/10.3390/coatings14020240
APA StyleFabris, R., Masi, G., & Bignozzi, M. C. (2024). Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments. Coatings, 14(2), 240. https://doi.org/10.3390/coatings14020240








