Enhanced Biocidal Activity of Heterophase Zinc Oxide/Silver Nanoparticles Contained within Painted Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO-Ag NPs
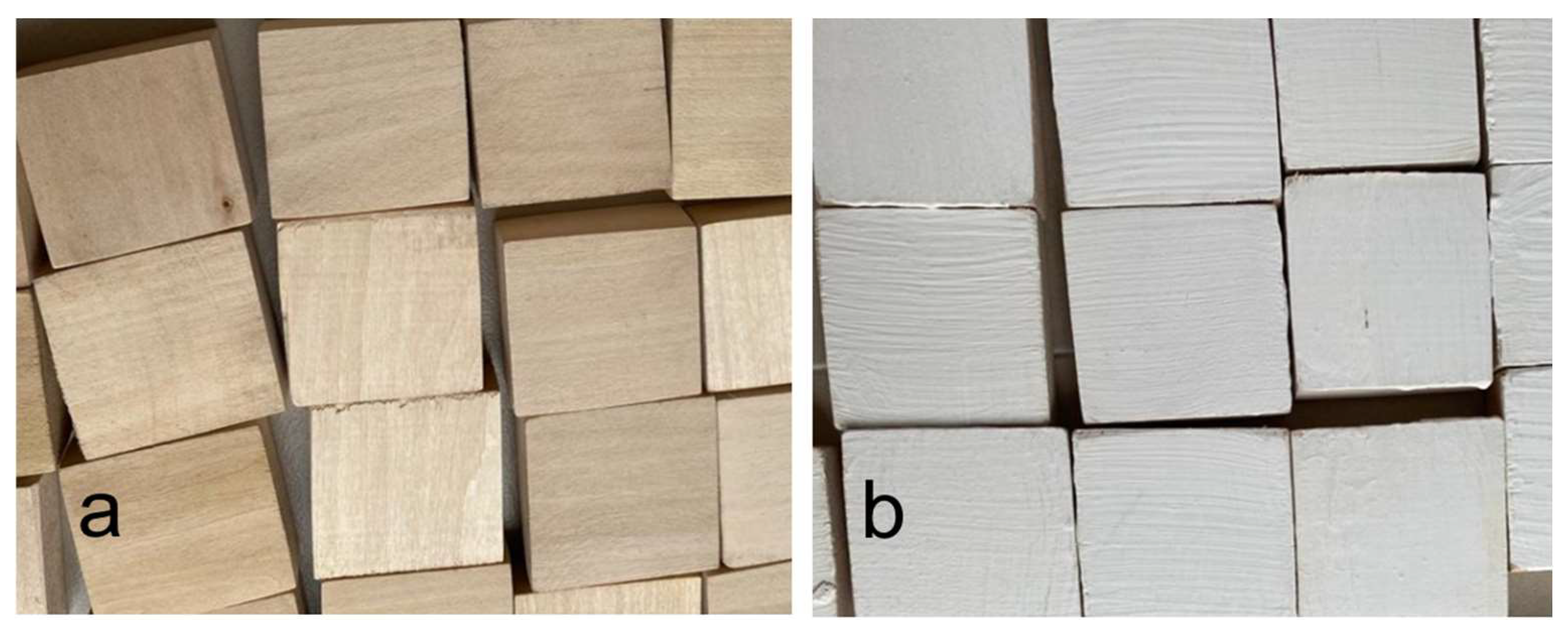
2.2. Preparation of Modified Paint Coating
2.3. Characterization
2.4. Antibacterial, Antifungal, and Antiviral Activity of Modified Paint Coating
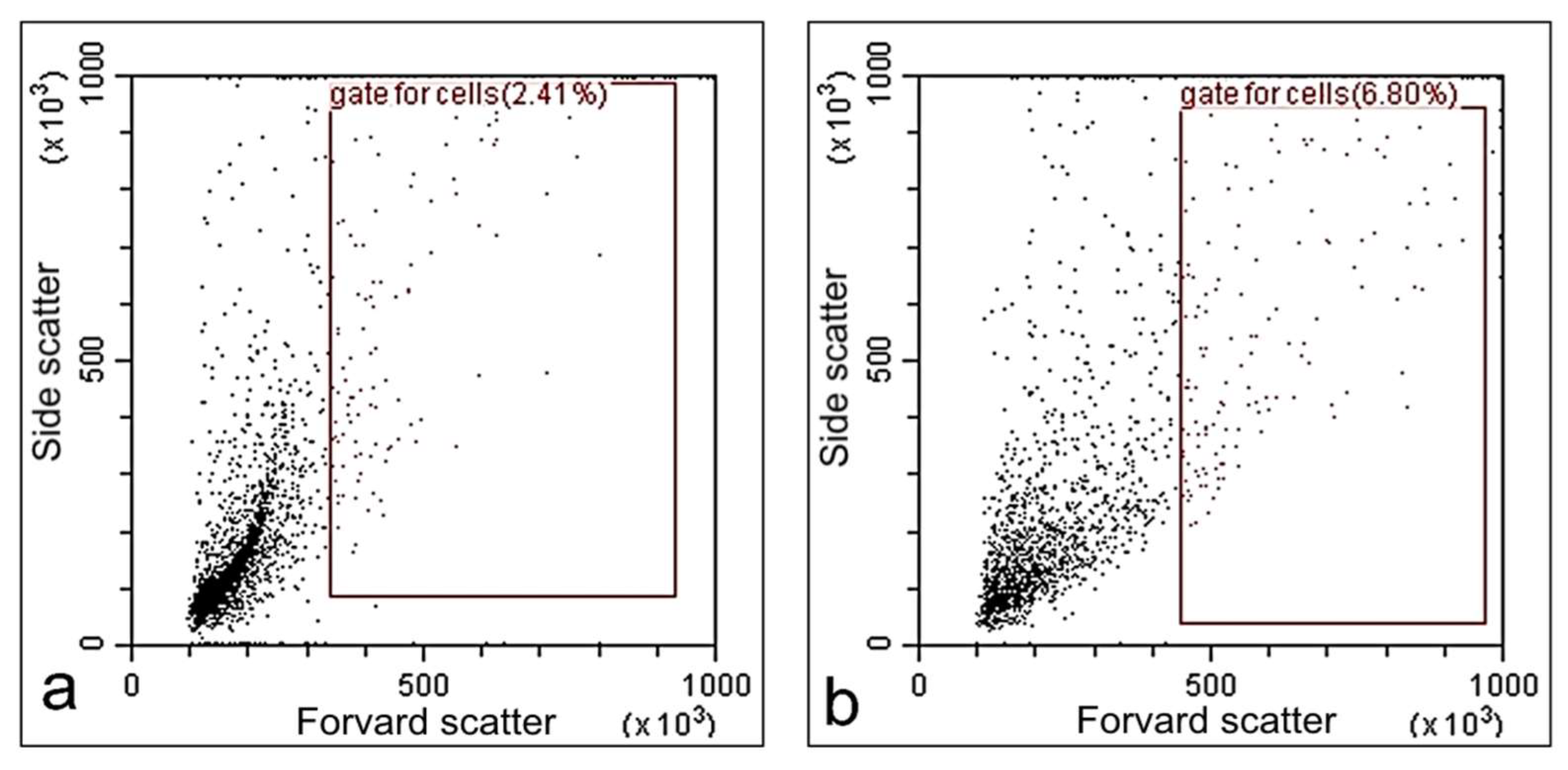
2.5. Cytotoxicity of Modified Paint Coating
3. Results
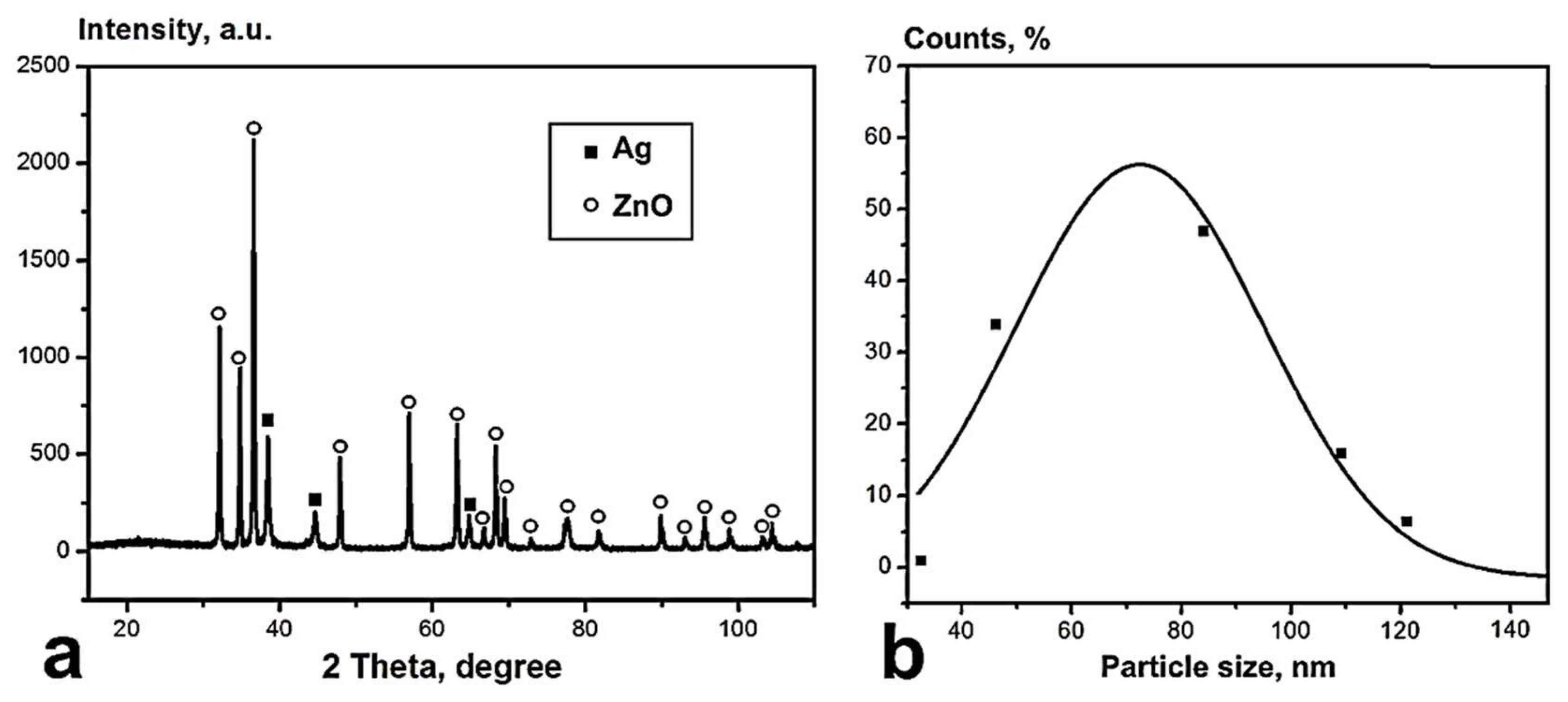
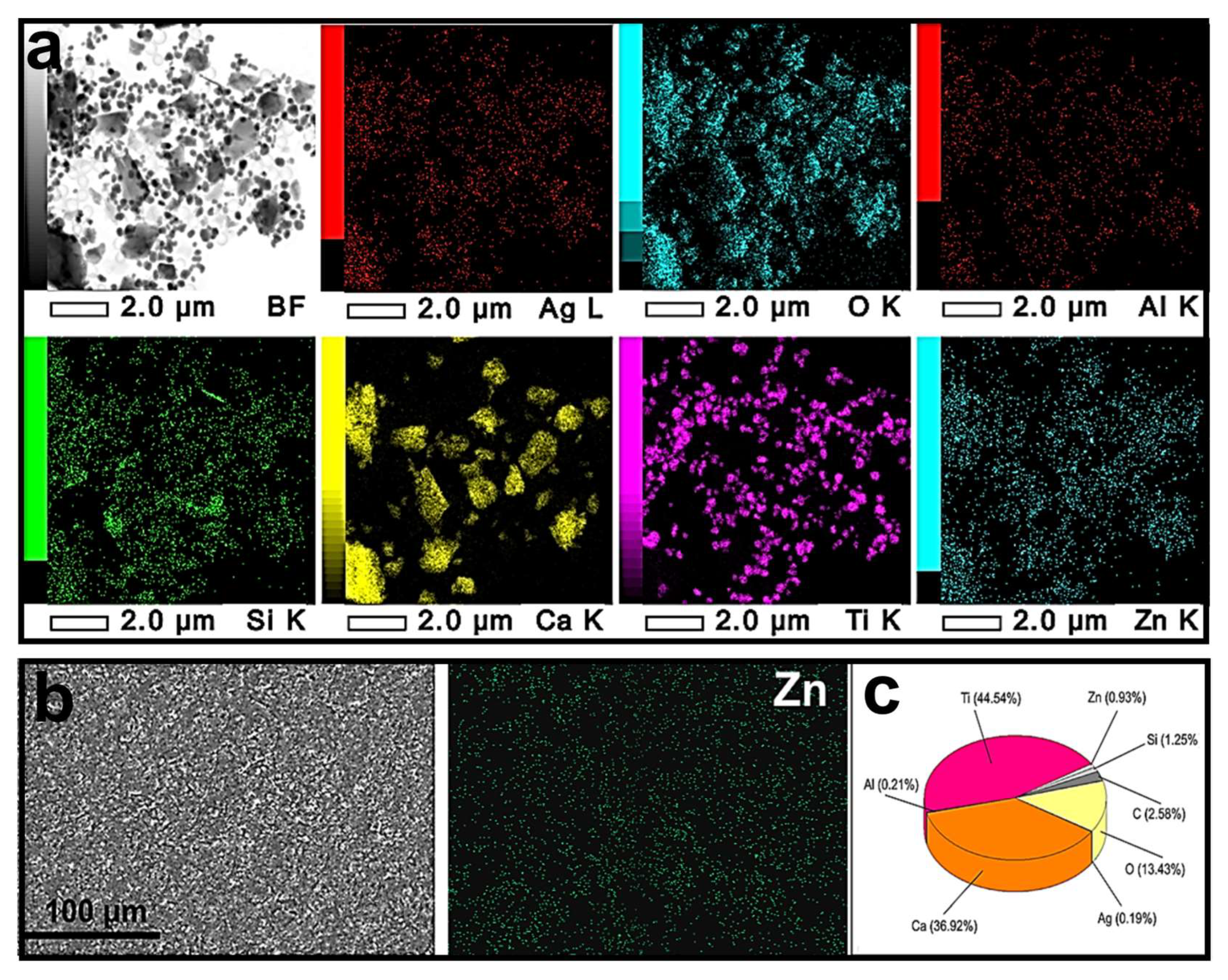
3.1. Preparation and Physicochemical Characteristics of ZnO-Ag NPs
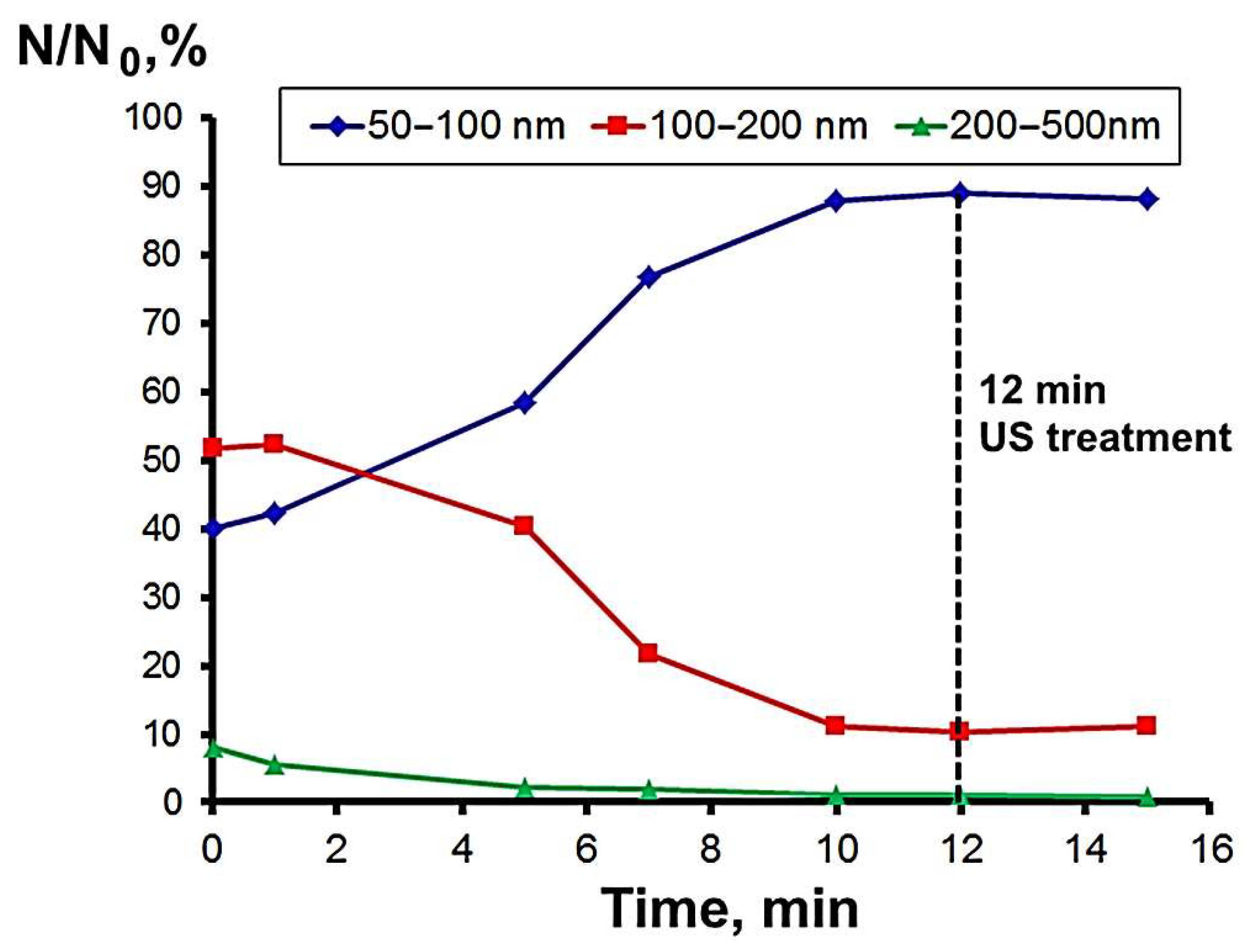
3.2. Nanoparticle Deagglomeration
3.3. Characterization of Modified Paint Coating
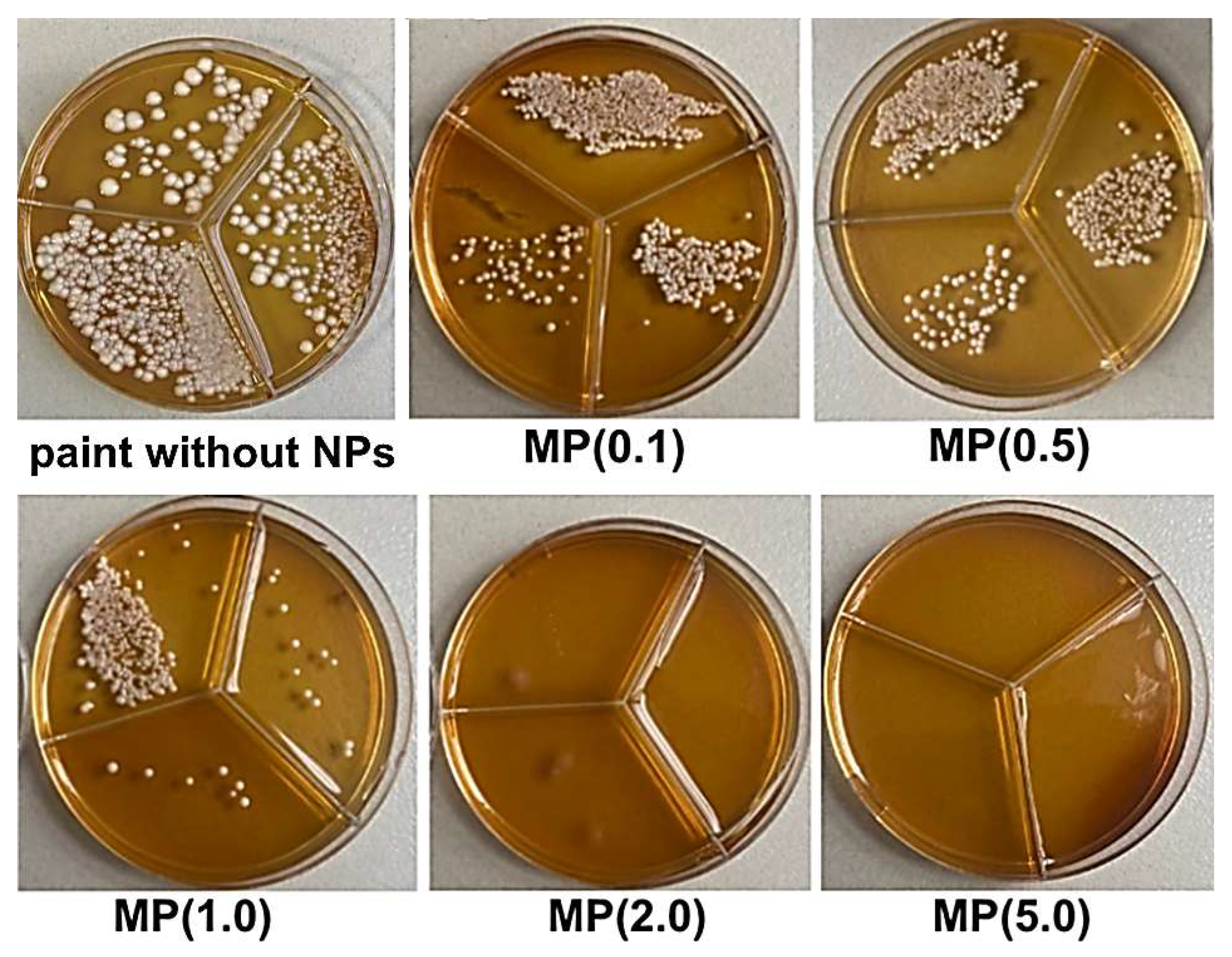
3.4. Biological Studies of Modified Paint Coating
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, J.A. Nosocomial Infections. In Critical Care; Routledge: Abingdon, UK, 2021; pp. 82–83. [Google Scholar]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial use in COVID-19 patients in the first phase of the SARS-CoV-2 pandemic: A scoping review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, J.; Wang, Y.; Verstraete, W.; Yuan, Z.; Guo, J. Insights of metallic nanoparticles and ions in accelerating the bacterial uptake of antibiotic resistance genes. J. Hazard. Mater. 2022, 421, 126728. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Hussain, C.M.; Abdukhakimov, M.; Khaydarov, R.; Krishnamurthy, P.T.; Evgrafova, S. Silver-Nanoparticle-Embedded Antimicrobial Paints. In Handbook of Consumer Nanoproducts; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–10. [Google Scholar]
- Nemeş, N.S.; Ardean, C.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Duţeanu, N.; Muntean, D. Antimicrobial activity of cellulose based materials. Polymers 2022, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef]
- Mohite, V.S.; Darade, M.M.; Sharma, R.K.; Pawar, S.H. Nanoparticle engineered photocatalytic paints: A roadmap to self-sterilizing against the spread of communicable diseases. Catalysts 2022, 12, 326. [Google Scholar] [CrossRef]
- Tornero, A.C.F.; Blasco, M.G.; Azqueta, M.C. Antimicrobial ecological waterborne paint based on novel hybrid nanoparticles of zinc oxide partially coated with silver. Prog. Org. Coat. 2018, 121, 130–141. [Google Scholar] [CrossRef]
- Yazid, S.A.; Mohd, Z.; Mohamad, J. Effect of titanium (IV) isopropoxide molarity on the crystallinity and photocatalytic activity of titanium dioxide thin film deposited via green sol-gel route. J. Mater. Res. Technol. 2018, 8, 1434–1439. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Hńfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Bolaina-Lorenzo, E.; Puente-Urbina, B.A.; Espinosa-Neira, R.; Ledezma, A.; Rodríguez-Fernández, O.; Betancourt-Galindo, R. A simple method to improve antibacterial properties in commercial face masks via incorporation of ZnO and CuO nanoparticles through chitosan matrix. Mater. Chem. Phys. 2022, 287, 126299. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic Potential of Micro-Nano Filopodia-Like ZnO Structures against Herpes Simplex Virus-1. Antivir. Res. 2011, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Irfan, M.; Ramgir, N.; Muthe, K.P.; Debnath, A.K.; Ansari, S.; Surjit, M. Antiviral activity of zinc oxide nanoparticles and tetrapods against the Hepatitis E and Hepatitis C viruses. Front. Microbiol. 2022, 13, 881595. [Google Scholar] [CrossRef] [PubMed]
- Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M.A.; Selim, N.M. Antiviral activity of zinc oxide nanoparticles mediated by Plumbago indica L. extract against Herpes Simplex Virus Type 1 (HSV-1). Int. J. Nanomed. 2021, 16, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Wolfgruber, S.; Rieger, J.; Cardozo, O.; Punz, B.; Himly, M.; Stingl, A.; Zatloukal, K. Antiviral Activity of Zinc Oxide Nanoparticles against SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 8425. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abol-Fotouh, D.; Salama, E.; Elkady, M.F. Assessment of antimicrobial, cytotoxicity, and antiviral impact of a green zinc oxide/activated carbon nanocomposite. Sci. Rep. 2022, 12, 8774. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B Biol. 2021, 219, 112206. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Cao, G.; Qu, J.; Deng, Y.; Tang, J. Antibacterial activity of Cu2O and Ag co-modified rice grains-like ZnO nanocomposites. J. Mater. Sci. Technol. 2018, 34, 2359–2367. [Google Scholar] [CrossRef]
- Vikal, S.; Gautam, Y.K.; Ambedkar, A.K.; Gautam, D.; Singh, J.; Pratap, D.; Singh, B.P. Structural, optical and antimicrobial properties of pure and Ag-doped ZnO nanostructures. J. Semicond. 2022, 43, 032802. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.E.; Craciun, V.; Popa, M.; Chifiriuc, M.C.; Swart, H. Influence of Ag, Au and Pd noble metals doping on structural, optical and antimicrobial properties of zinc oxide and titanium dioxide nanomaterials. Heliyon 2019, 5, e01333. [Google Scholar] [CrossRef]
- Bakina, O.V.; Glazkova, E.A.; Pervikov, A.V.; Rodkevich, N.G.; Vornakova, E.A.; Chzhou, V.R.; Lerner, M.I. Electric explosion of wires as versatile method for antibacterial Janus-like ZnO–Ag nanoparticles preparation. J. Mater. Sci. Mater. Electron. 2021, 32, 10623–10634. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Kazantsev, S.O.; Kondranova, A.M.; Fomenko, A.N.; Pervikov, A.V.; Rodkevich, N.G.; Bakina, O.V. Design of antimicrobial composite nanoparticles ZnxMe (100-x)/O by electrical explosion of two wires in the oxygen-containing atmosphere. Mater. Des. 2019, 183, 108099. [Google Scholar] [CrossRef]
- ISO 21702; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019.
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- Lamb, R.N.; Ngamsom, B.; Trimm, D.L.; Gong, B.; Silveston, P.L.; Praserthdam, P. Surface characterisation of Pd–Ag/Al2O3 catalysts for acetylene hydrogenation using an improved XPS procedure. Appl. Catal. A Gen. 2004, 268, 43–50. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Stakheev, A.Y.; Mytareva, A.I.; Prosvirin, I.P.; Bukhtiyarov, V.I. In situ XPS study of the size effect in the interaction of NO with the surface of the model Ag/Al2O3/FeCrAl catalysts. Russ. Chem. Bull. 2015, 64, 2780–2785. [Google Scholar] [CrossRef]
- Wang, H.; Luo, S.; Li, X.; Liu, W.; Wu, X.; Weng, D.; Liu, S. Thermally stable Ag/Al2O3 confined catalysts with high diffusion-induced oxidation activity. Catal. Today 2019, 332, 189–194. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD synthesis and characterization of iron and copper oxides modified ZnO structured films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Neykova, N.; Stuchlik, J.; Hruska, K.; Poruba, A.; Remes, Z.; Pop-Georgievski, O. Study of the surface properties of ZnO nanocolumns used for thin-film solar cells. Beilstein J. Nanotechnol. 2017, 8, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Holubnycha, V.; Myronov, P.; Bugaiov, V.; Opanasyuk, A.; Dobrozhan, O.; Yanovska, A.; Pogorielov, M.; Kalinkevichet, O. Effect of ultrasound treatment on chitosan-silver nanoparticles antimicrobial activity. In Proceedings of the IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; pp. 1–4. [Google Scholar]
- Sharma, R.K.; Ghose, R. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Djearamane, S.; Xiu, L.J.; Wong, L.S.; Rajamani, R.; Bharathi, D.; Kayarohanam, S.; Selvaraj, S. Antifungal Properties of Zinc Oxide Nanoparticles on Candida albicans. Coatings 2022, 12, 1864. [Google Scholar] [CrossRef]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.A.; Mocan, L. Review on silver nanoparticles as a novel class of antibacterial solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Ashraf, M.S.; Alomrani, S.O.; Alreshidi, M.; Patel, M. Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics 2023, 12, 1415. [Google Scholar] [CrossRef]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. JBIC J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Yilmaz, E. Antibacterial, antiviral, and self-cleaning mats with sensing capabilities based on electrospun nanofibers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Kandula, S.; Jeevanandam, P. Sun-Light-Driven Photocatalytic Activity by ZnO/Ag Heteronanostructures Synthesized via a Facile Thermal Decomposition Approach. RSC Adv. 2015, 5, 76150–76159. [Google Scholar] [CrossRef]
- Rokesh, K.; Mohan, S.C.; Karuppuchamy, S.; Jothivenkatachalam, K. Photo-assisted advanced oxidation processes for Rhodamine B degradation using ZnO–Ag nanocomposite materials. J. Environ. Chem. Eng. 2018, 6, 3610–3620. [Google Scholar] [CrossRef]
- Mohapatra, B.; Mohapatra, S.; Sharma, N. Biosynthesized Ag–ZnO nanohybrids exhibit strong antibacterial activity by inducing oxidative stress. Ceram. Int. 2023, 49, 20218–20233. [Google Scholar] [CrossRef]
- Shang, J.; Sun, Y.; Zhang, T.; Liu, Z.; Zhang, H. Enhanced antibacterial activity of Ag nanoparticle-decorated ZnO nanorod arrays. J. Nanomater. 2019, 2019, 3281802. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Zeng, Y.; Jiang, Y. Synthesis and properties of Ag/ZnO core/shell nanostructures prepared by excimer laser ablation in liquid. APL Mater. 2015, 3, 086103. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.S. Easy one-pot low-temperature synthesized Ag-ZnO nanoparticles and their activity against clinical isolates of methicillin-resistant Staphylococcus aureus. Front. Bioeng. Biotechnol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zhi, L.; Liu, X.; Jiang, W.; Sun, Y.; Yang, J. The synergetic antibacterial activity of Ag islands on ZnO (Ag/ZnO) heterostructure nanoparticles and its mode of action. J. Inorg. Biochem. 2014, 130, 74–83. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Gavioli, L. Ag functionalization of Al-doped ZnO nanostructured coatings on PLA substrate for antibacterial applications. Coatings 2020, 10, 1238. [Google Scholar] [CrossRef]
- Agnihotri, S.; Bajaj, G.; Mukherji, S.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef]
- de Lucas-Gil, E.; Menendez, J.; Pascual, L.; Fernandez, J.F.; Rubio-Marcos, F. The benefits of the ZnO/clay composite formation as a promising antifungal coating for paint applications. Appl. Sci. 2020, 10, 1322. [Google Scholar] [CrossRef]
- Olson, V.A.; Shchelkunov, S.N. Are we prepared in case of a possible smallpox-like disease emergence? Viruses 2017, 9, 242. [Google Scholar] [CrossRef]
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988; p. 1460. [Google Scholar]
- World Health Organization. 2022–2023 Mpox (Monkeypox) Outbreak: Global Trends; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Merkl, P.; Long, S.; McInerney, G.M.; Sotiriou, G.A. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Spisak, W.; Kaszczyszyn, M.; Szar, M.; Kozak, J.; Stachowicz, K. Antiviral activity of galvanic microcells of zinc and copper contained within painted surfaces. Sci. Rep. 2022, 12, 1368. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Behzadinasab, S.; Chin, A.W.; Poon, L.L.; Ducker, W.A. Reduction of infectivity of SARS-CoV-2 by zinc oxide coatings. ACS Biomater. Sci. Eng. 2021, 7, 5022–5027. [Google Scholar] [CrossRef]
- Primo, J.D.O.; Correa, J.D.S.; Horsth, D.F.; Das, A.; Zając, M.; Umek, P.; Bittencourt, C. Antiviral Properties against SARS-CoV-2 of Nanostructured ZnO Obtained by Green Combustion Synthesis and Coated in Waterborne Acrylic Coatings. Nanomaterials 2022, 12, 4345. [Google Scholar] [CrossRef] [PubMed]
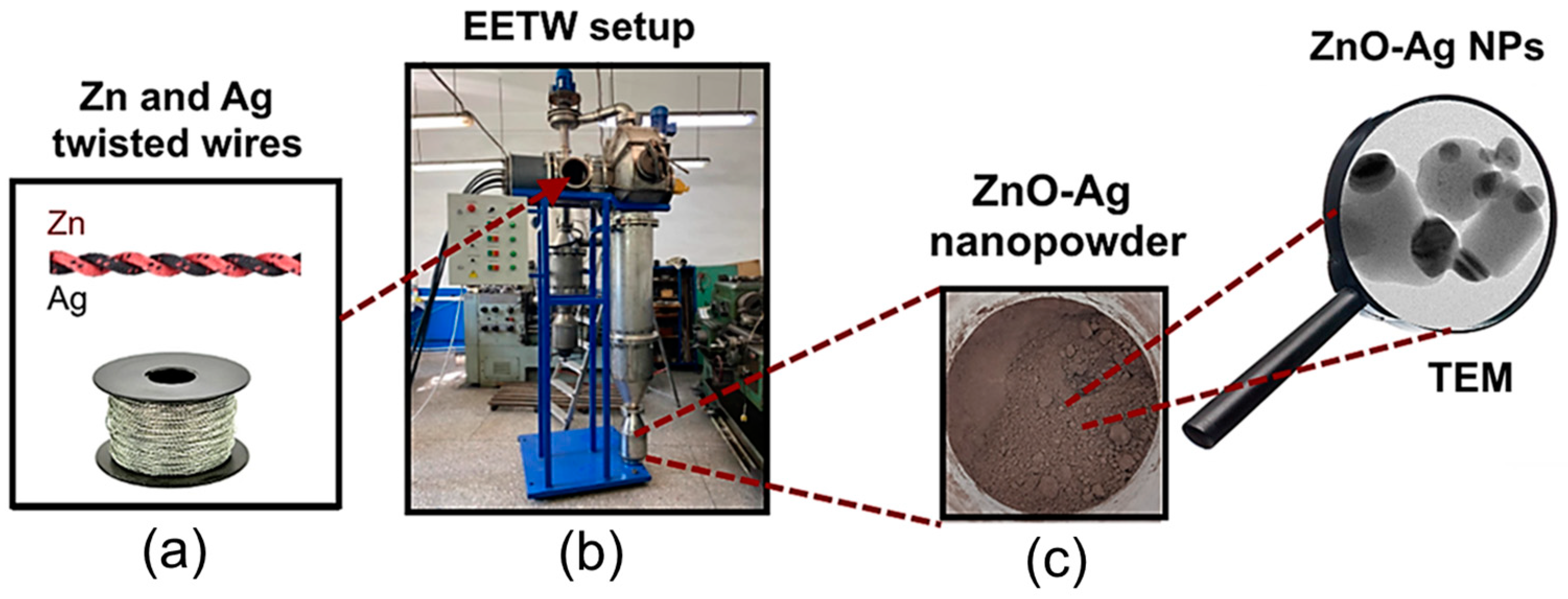




| NP Sample | Photo | Metal Wire | dw, mm | lw, mm | C, μF | U0, kV |
|---|---|---|---|---|---|---|
| ZnO-Ag |  | Zn | 0.38 | 90 | 3.2 | 23 |
| Ag | 0.15 |
| Color of Coating | Density, g/cm3 | Solids Content by Weight, % | pH | Dynamic Viscosity, mPa∙s (100 rpm) |
|---|---|---|---|---|
| white | 1.50−1.60 | 53−63 | 8.0−9.5 | 4500−7000 |
| Ingredients | MP(0.1) | MP(0.5) | MP(1.0) | MP(2.0) | MP(5.0) |
|---|---|---|---|---|---|
| Paint matrix | 99.89 wt.% | 99.45 wt.% | 98.90 wt.% | 97.80 wt.% | 94.50 wt.% |
| PVP | 0.01 wt.% | 0.05 wt.% | 0.10 wt.% | 0.20 wt.% | 0.50 wt.% |
| ZnO-Ag NPs | 0.1 wt.% | 0.5 wt.% | 1.0 wt.% | 2.0 wt.% | 5.0 wt.% |
| Different Concentration of Virus-Containing Washing Fluid | Virus Suspension Dilution | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| A | Test sample | Control sample | Virus control | initial | |||||||||
| B | 10−1 | ||||||||||||
| C | 10−2 | ||||||||||||
| D | 10−3 | ||||||||||||
| E | 10−4 | ||||||||||||
| F | 10−5 | ||||||||||||
| G | 10−6 | ||||||||||||
| H | 10−7 | ||||||||||||
| MB | CR | ||||
|---|---|---|---|---|---|
| k, min−1 | R2 | D, % | k, min−1 | R2 | D, % |
| 0.1832 | 0.9761 | 99.5 | 0.117 | 0.942 | 99.7 |
| Sample | S. aureus | MRSA | E. coli | P. aeruginosa | ||||
|---|---|---|---|---|---|---|---|---|
| CFU/mL | R, % | CFU/mL | R, % | CFU/mL | R, % | CFU/mL | R, % | |
| MP(0.1) | (5.17 ± 0.12) × 103 | 97.41 | 2.56 ± 0.35 × 103 | 98.72 | 1.17 ± 0.15 × 103 | 99.41 | 1.47 ± 0.12 × 103 | 99.26 |
| MP(0.5) | (0.87 ± 0.04) × 103 | 99.57 | 0.57 ± 0.06 × 103 | 99.71 | 0.20 ± 0.01 × 103 | 99.90 | 0.55 ± 0.05 × 103 | 99.74 |
| MP(1.0) | 0.23 ± 0.02 × 102 | 99.99 | 0 | 100 | 0 | 100 | 0.16 ± 0.00 × 102 | 99.99 |
| MP(2.0) | 0.10 ± 0.00 × 102 | 99.99 | 0 | 100 | 0 | 100 | 0 | 100 |
| MP(5.0) | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| Sample | Total Amount of Virus | Viral Clearance, log(10) | |||
|---|---|---|---|---|---|
| Control Surface | Test Surface | ||||
| Mean Titer, PFU/0.2 mL | log(10) | Mean Titer, PFU/0.2 mL | log(10) | ||
| Paint without NPs | 1.74 × 108 | 8.2 | 5.6 × 106 | 6.7 | 1.5 |
| MP(0.1) | 1.74 × 108 | 8.2 | 5.6 × 103 | 3.7 | 4.5 |
| MP(0.5) | 1.74 × 108 | 8.2 | 3.0 × 103 | 3.5 | 4.7 |
| MP(1.0) | 1.74 × 108 | 8.2 | less than 102 | less than 2 | more than 6.2 |
| MP(2.0) | 1.74 × 108 | 8.2 | less than 102 | less than 2 | more than 6.2 |
| MP(5.0) | 1.74 × 108 | 8.2 | less than 102 | less than 2 | more than 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakina, O.; Pikuschak, E.; Prokopchuk, A.; Evplonova, E.; Plaksina, T.; Avgustinovich, A.; Spirina, L.; Vorozhtsov, A.; Yakovlev, N.; Lerner, M. Enhanced Biocidal Activity of Heterophase Zinc Oxide/Silver Nanoparticles Contained within Painted Surfaces. Coatings 2024, 14, 241. https://doi.org/10.3390/coatings14020241
Bakina O, Pikuschak E, Prokopchuk A, Evplonova E, Plaksina T, Avgustinovich A, Spirina L, Vorozhtsov A, Yakovlev N, Lerner M. Enhanced Biocidal Activity of Heterophase Zinc Oxide/Silver Nanoparticles Contained within Painted Surfaces. Coatings. 2024; 14(2):241. https://doi.org/10.3390/coatings14020241
Chicago/Turabian StyleBakina, Olga, Elizaveta Pikuschak, Anna Prokopchuk, Elena Evplonova, Tatiana Plaksina, Alexandra Avgustinovich, Liudmila Spirina, Alexander Vorozhtsov, Nikolay Yakovlev, and Marat Lerner. 2024. "Enhanced Biocidal Activity of Heterophase Zinc Oxide/Silver Nanoparticles Contained within Painted Surfaces" Coatings 14, no. 2: 241. https://doi.org/10.3390/coatings14020241





