Effect of Temperature, Vacuum Condition and Surface Roughness on Oxygen Boost Diffusion of Ti–6Al–4V Alloy
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
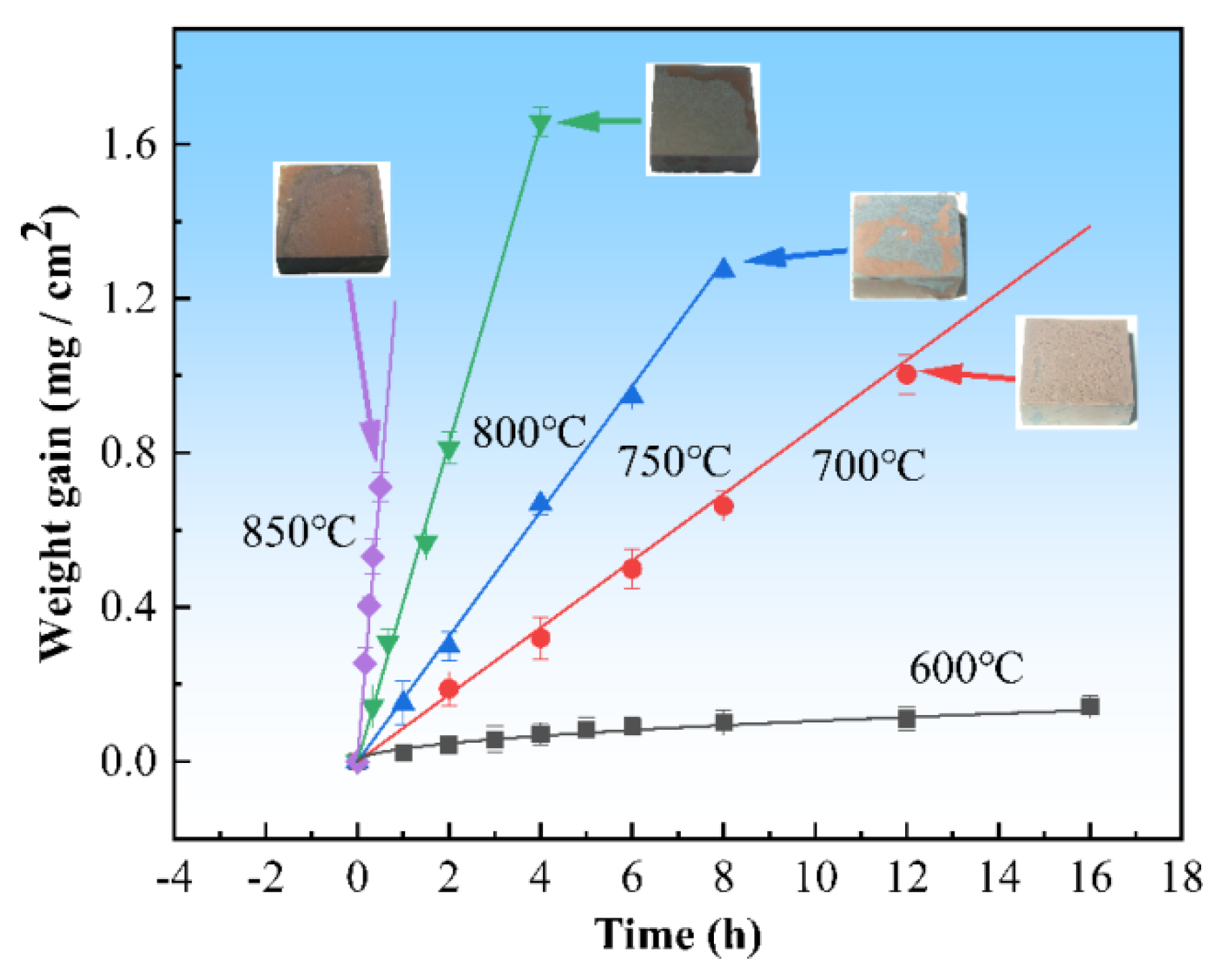
3.1. Effect of Temperature on the OBD Treatment Process
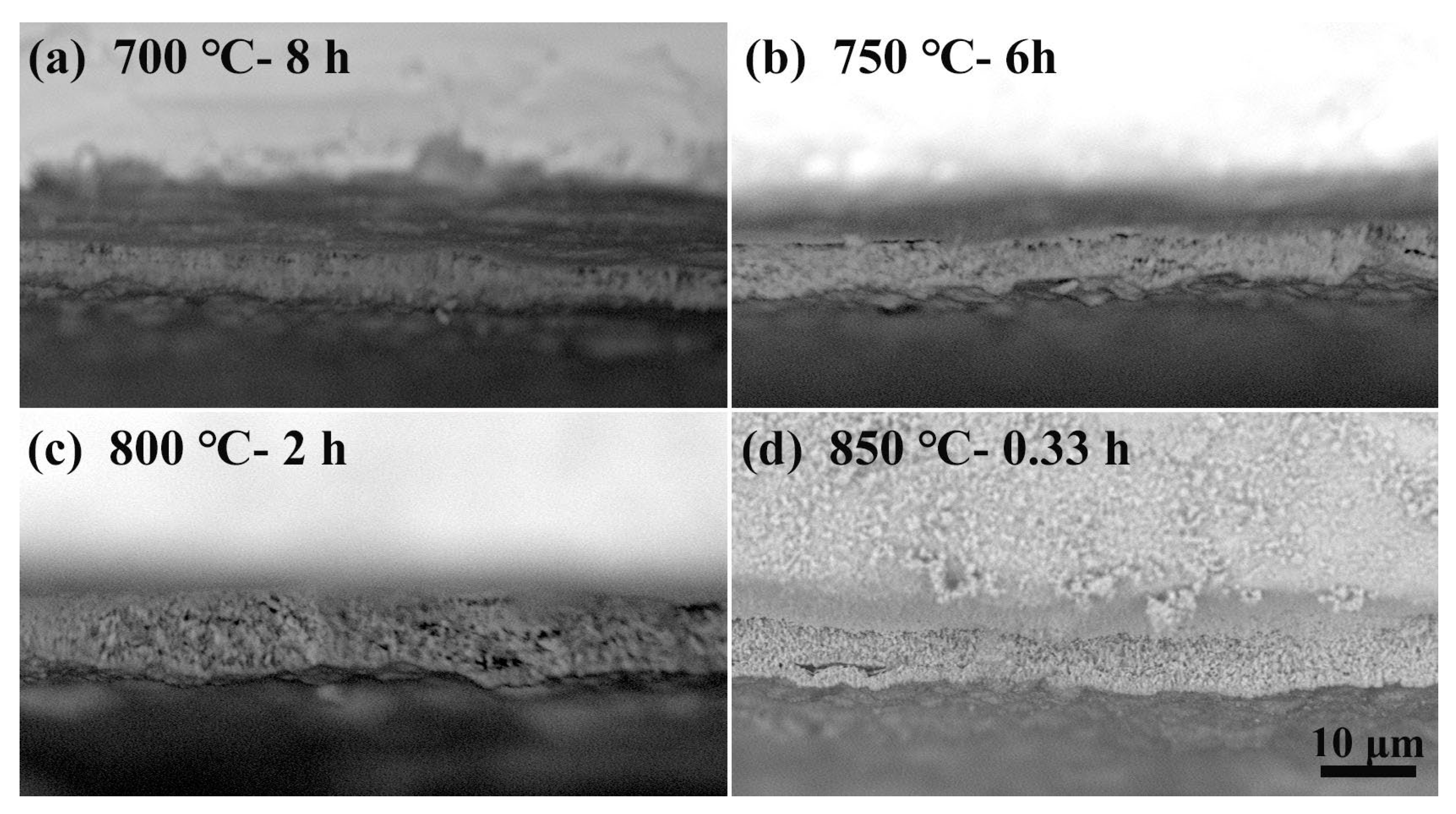
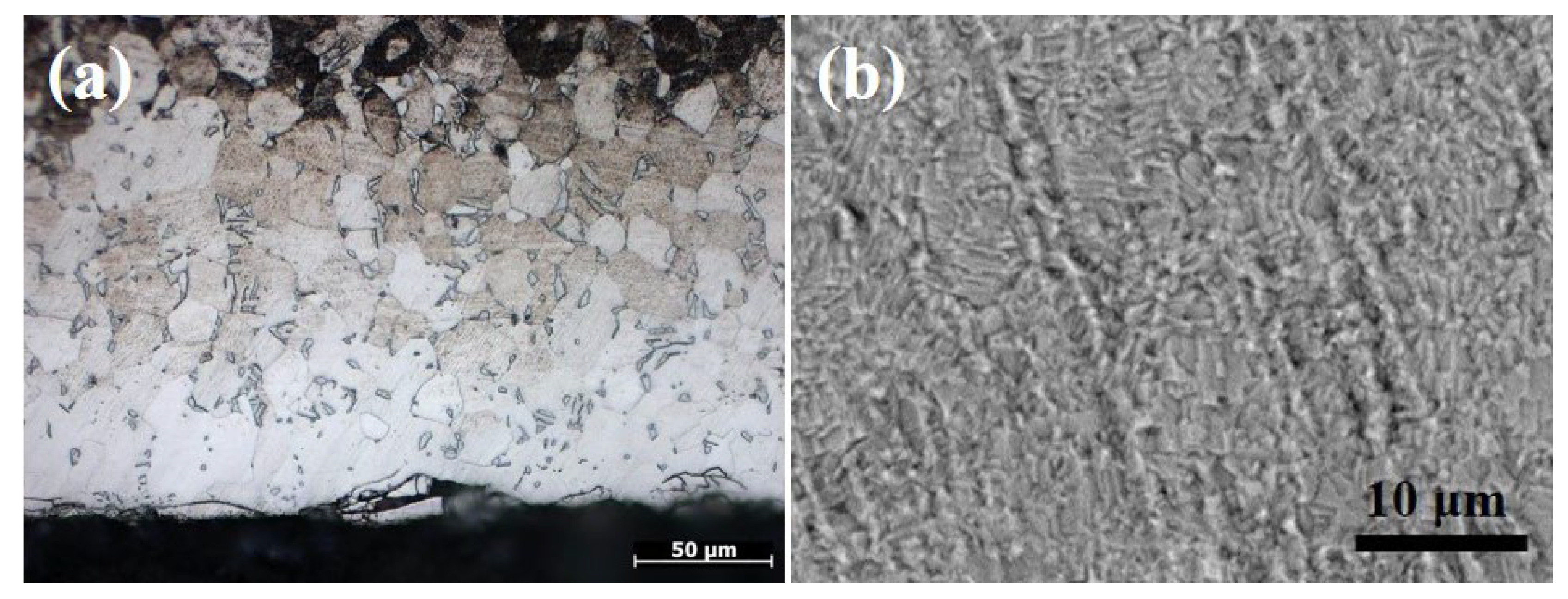
3.1.1. Effect of Temperature on the Oxidation and Diffusion Progress
3.1.2. Effect of Temperature on Mechanical Properties of the Substrate
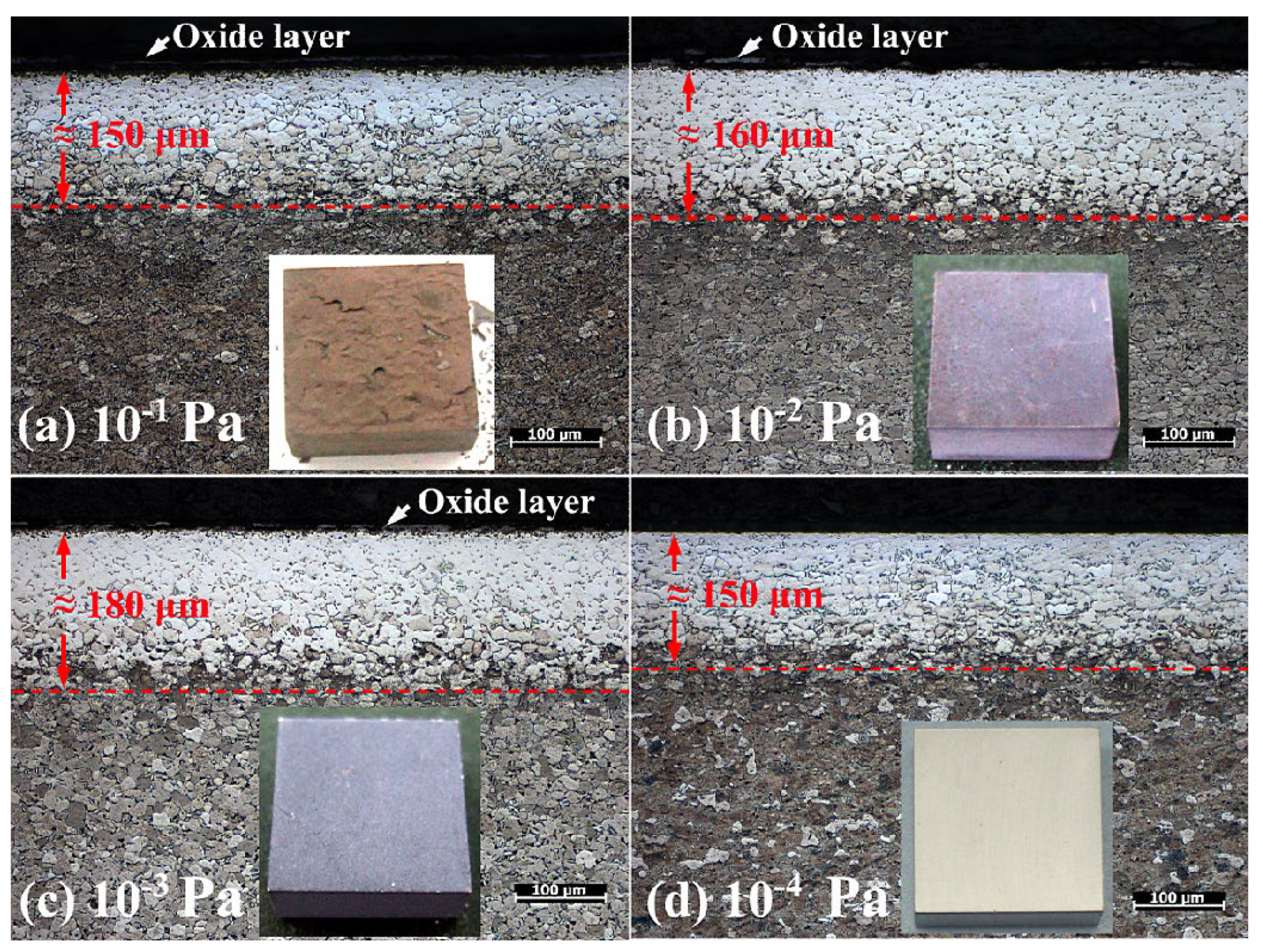
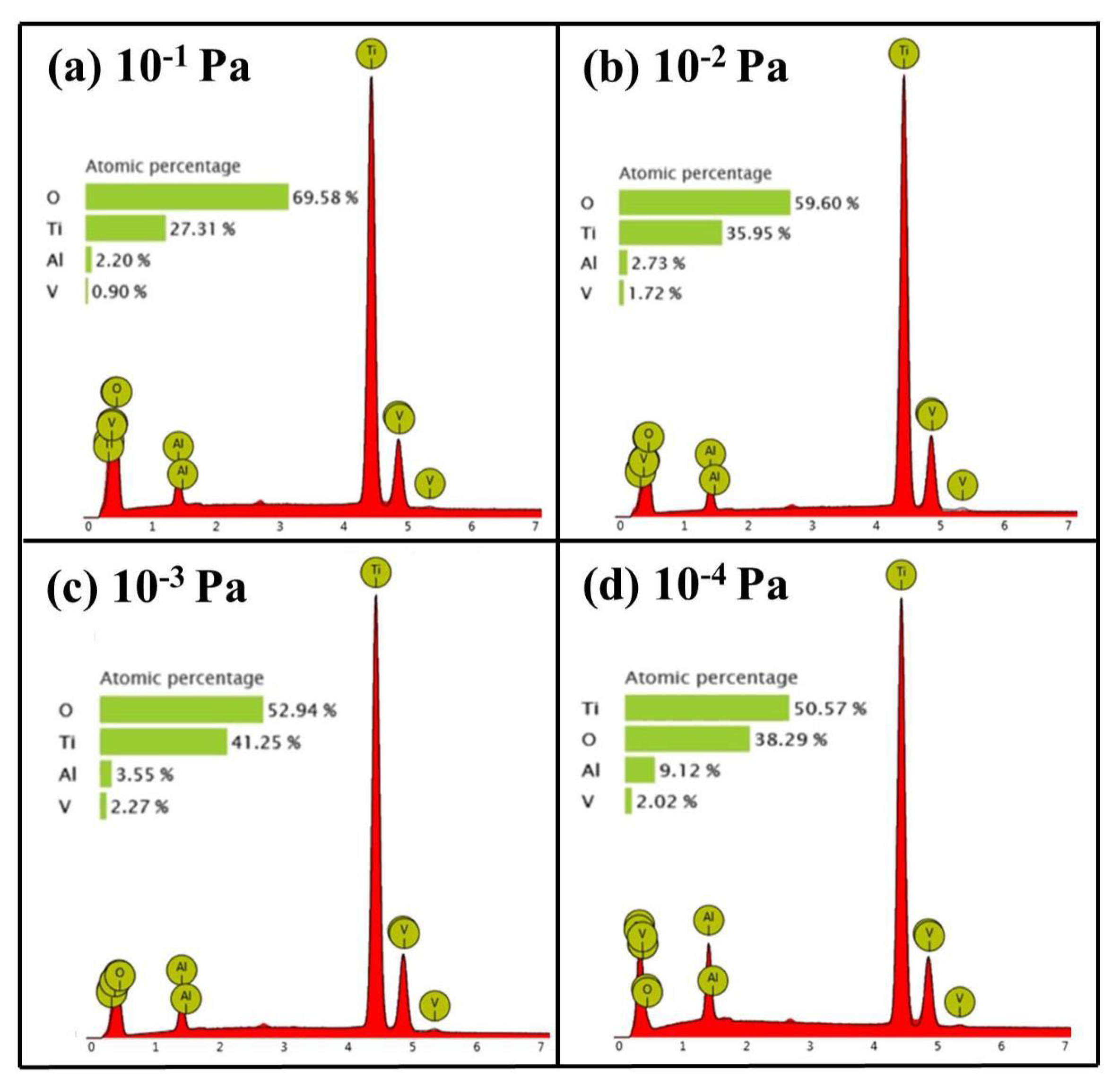
3.2. Effect of Vacuum Degree on OBD Treatment
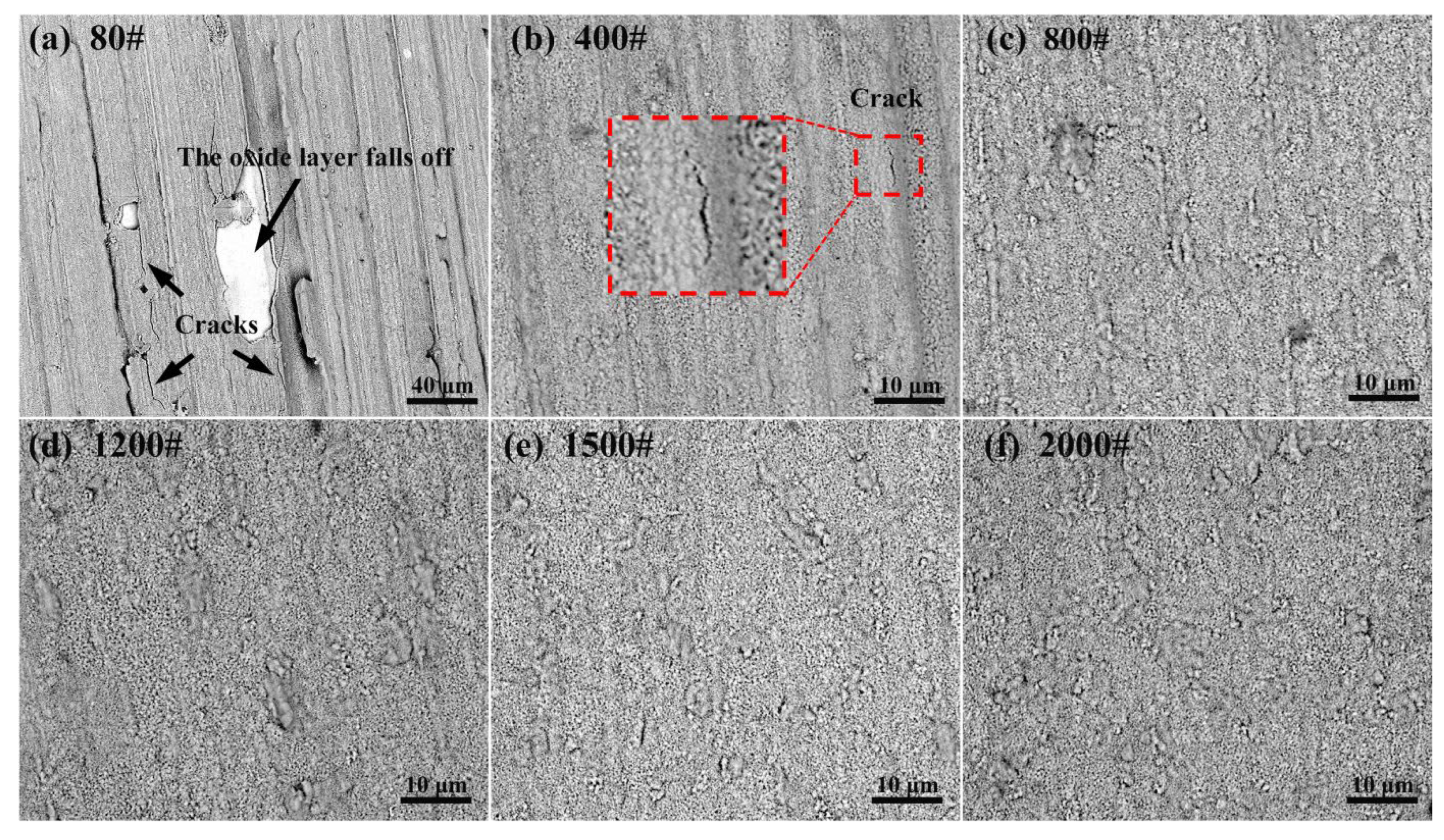
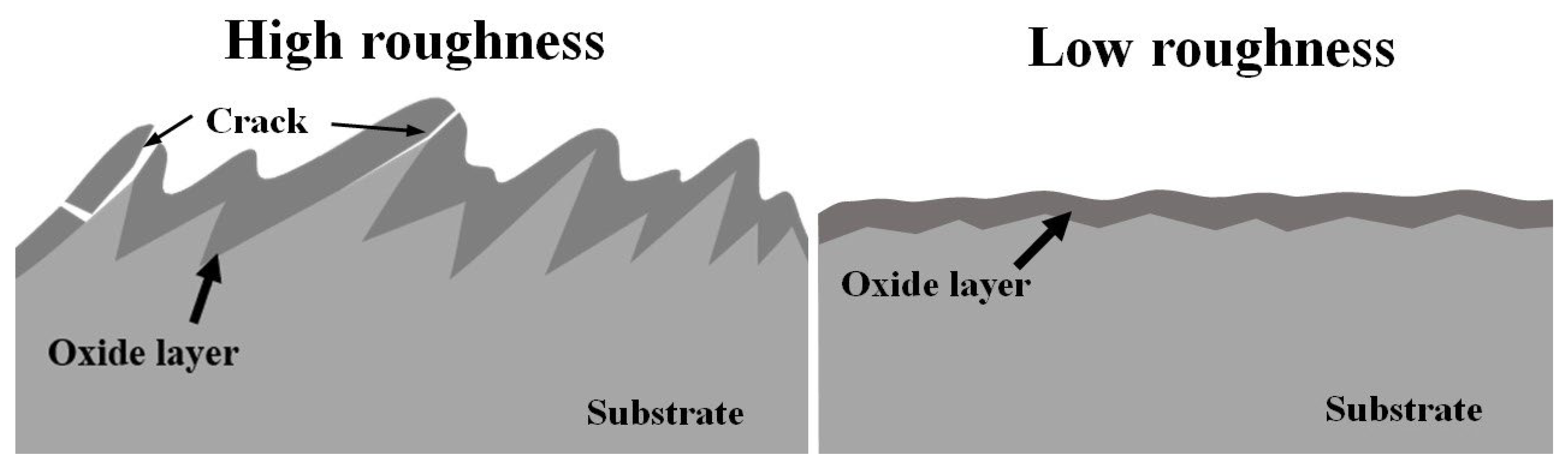
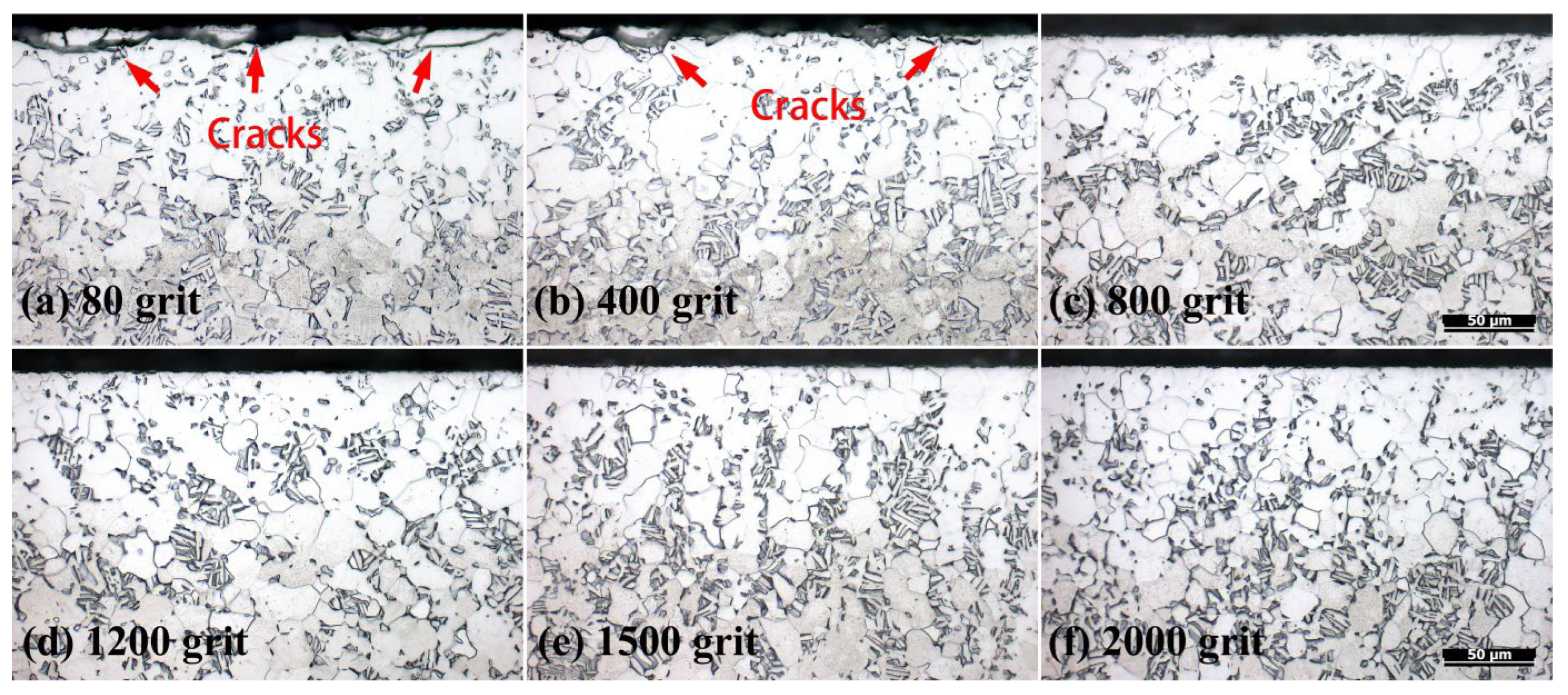
3.3. Effect of Surface Roughness on OBD Treatment
3.3.1. Surface Morphology
3.3.2. Effect of Surface Roughness on Thermal Oxidation
3.3.3. Effect of Surface Roughness on Oxygen Diffusion
4. Conclusions
- (1)
- Temperature not only affects the oxidation rate and diffusion rate but also affects the mechanical properties of the substrate. The relationship between weight gain and time follows the parabolic oxidation kinetics at low temperatures (600 °C). When the temperature is raised to 700–850 °C, the relationship becomes linear. This study also showed that if the surface oxide layer is too thick, it will crack or spall after the substrate is removed from the muffle furnace. The appropriate thickness that has no cracking is ≈5–8 μm. Mechanical property test results indicate that low temperatures require longer diffusion times, which is uneconomical and leads to a greater reduction in the mechanical properties of the substrate compared to short-term treatment at high temperatures. Based on the test results, 850 °C is the preferred temperature for OBD treatment.
- (2)
- The influence of vacuum conditions on oxygen diffusion is significant. Oxygen contained in the vacuum diffusion environment can promote oxygen potential and accelerate the occurrence of diffusion to obtain a thicker OBD layer. However, if the oxygen content is too high (such as under a vacuum condition of 1 × 10−1 Pa), oxidation will also be accelerated and the formed OBD layer will be consumed due to oxidation, resulting in a final OBD layer that is ≈150 μm thick and has an outermost cracked layer and a significant oxide layer on the surface. Except for an increase in the thickness to ≈160 μm under moderate vacuum conditions (such as 1 × 10−2 Pa), both the cracked layer and the oxide layer still exist. The OBD layer obtained under the 1 × 10−3 Pa vacuum condition has the highest thickness (≈180 μm), hardness and no cracked surface layer; however, it also has a remaining oxide layer. Though the OBD layer obtained under the 1 × 10−4 Pa vacuum condition is slightly thinner, it has no remaining oxide layer. These results indicate that during the diffusion process, the vacuum condition should not be lower than 1 × 10−4 Pa.
- (3)
- Surface roughness does not affect the thickness of the OBD layer, but it does affect the surface quality of the OBD layer. When the surface roughness is too high, mutual compression of the oxide layer at sharp locations will induce cracking of the oxide layer during the oxidation process; the outermost surface of the OBD layer will also crack during the diffusion process. Based on the experimental results, the appropriate surface roughness is Ra ≤ 0.132 (equivalent to polishing with sandpaper that is no coarser than 800#).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Poondla, N.; Srivatsan, T.S.; Patnaik, A.; Petraroli, M. A study of the microstructure and hardness of two titanium alloys: Commercially pure and Ti–6Al–4V. J. Alloys Comp. 2009, 486, 162–167. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Bloyce, A.; Morton, P.H.; Bell, T. Surface Engineering; ASM: Metals Park, OH, USA, 1994; Volume 5, pp. 835–851. [Google Scholar]
- Bell, T.; Dong, H. Tribological enhancement of titanium alloys. In Proceedings of the First Asia International Conference on Tribology; Tsinghua University Press: Beijing, China, 1998; pp. 421–427. [Google Scholar]
- Hutson, A.L.; Niinomi, M.; Nicholas, T.; Eylon, D. Effect of various surface conditions on fretting fatigue behavior of Ti–6Al–4V. Int. J. Fatigue 2002, 24, 1223–1234. [Google Scholar] [CrossRef]
- Taheridoustabad, I.; Khosravi, M.; Yaghoubinezhad, Y. Fabrication of GO/RGO/TiC/TiB2 nanocomposite coating on Ti–6Al–4V alloy using electrical discharge coating and exploring its tribological properties. Tribol Int. 2021, 156, 106860. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, M.; Fan, Z.; Huang, S.; Huang, H. Laser deposition of wear-resistant titanium oxynitride/titanium composite coatings on Ti-6Al-4V alloy. Appl. Surf. Sci. 2020, 531, 147212. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, W.L.; Niu, Z.Q.; Zheng, X.B.; Wang, Q.; Wang, C.L.; Cao, Z.W.; Fu, X.S.; Chen, G.Q.; Li, Z.Q. Effect of different surface asperities and surface hardness induced by shot-peening on the fretting wear behavior of Ti-6Al-4V. Surf. Coat. Technol. 2018, 349, 1098–1106. [Google Scholar] [CrossRef]
- Tsuji, N.; Tanaka, S.; Takasugi, T. Effects of combined plasma-carburizing and shot-peening on fatigue and wear properties of Ti–6Al–4V alloy. Surf. Coat. Technol. 2009, 203, 1400–1405. [Google Scholar] [CrossRef]
- Dalili, N.; Edrisy, A.; Farokhzadeh, K.; Li, J.; Riahi, A.R. Improving the wear resistance of Ti–6Al–4V/TiC composites through thermal oxidation (TO). Wear 2010, 269, 590–601. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.Y. Oxygen boost diffusion for the deep-case hardening of titanium alloys. Mater. Sci. Eng. A 2000, 280, 303–310. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Liu, H.; Li, S.; Wang, X. In vitro bio-tribological behaviour of textured nitride coating on selective laser melted Ti-6Al-4V alloy. Surf. Coat. Technol. 2021, 409, 126904. [Google Scholar] [CrossRef]
- Belkin, P.N.; Kusmanov, S.A.; Dyakov, I.G.; Komissarova, M.R.; Parfenyuk, V.I. Anode plasma electrolytic carburizing of commercial pure titanium. Surf. Coat. Technol. 2016, 307, 1303–1309. [Google Scholar] [CrossRef]
- Streicher, R.M.; Weber, H.; Schon, R.; Semlitsch, M. New surface modification for Ti-6Al-7Nb alloy: Oxygen diffusion hardening (0DH). Biomaterials 1991, 12, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Bloyce, A.; Morton, P.H.; Bell, T. Surface Oxidation of a Titanium or Titanium Alloy Article. U.S. Patent 6,210,807, 3 April 2001. [Google Scholar]
- Dong, H.; Bloyce, A.; Morton, P.H.; Bell, T. Surface engineering to improve tribological performance of Ti–6Al–4V. Surf. Eng. 1997, 13, 402–406. [Google Scholar] [CrossRef]
- Yazdi, R.; Ghasemi, H.M.; Abedini, M.; Wang, C.; Neville, A. OBD layer on Ti–6Al–4V alloy: Scratch and dry wear resistance. Tribol Lett. 2019, 67, 101. [Google Scholar] [CrossRef]
- Ebrahimi, A.R.; Zarei, F.; Khosroshahi, R.A. Effect of thermal oxidation process on fatigue behavior of Ti-4Al-2V alloy. Surf. Coat. Technol. 2008, 203, 199–203. [Google Scholar] [CrossRef]
- Takahashi, K.; Sato, E. Influence of surface treatments on fatigue strength of Ti6Al4V alloy. Mater. Trans. 2010, 51, 694–698. [Google Scholar] [CrossRef]
- Omidbakhsh, F.; Ebrahimi, A.R.; Mousavi, S.H.; Khosroshahi, R.A.; Nazarpour, S. Effect of oxygen boost diffusion treatment on fatigue behavior of Ti–4Al–2V alloy. Surf. Coat. Technol. 2011, 205, 2954–2963. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dong, H.; Bell, T.; Xu, B. The effect of treatment condition on boost diffusion of thermally oxidised titanium alloy. J. Alloys Compd. 2007, 431, 93–99. [Google Scholar] [CrossRef]
- Hurlen, T. Oxidation of titanium. J. Inst. Met. 1960, 89, 128–136. [Google Scholar]
- Robertson, J.; Manning, M.I. Limits to adherence of oxide scales. Mater. Sci. Technol. 1990, 6, 81–92. [Google Scholar] [CrossRef]
- Deadmore, D.L.; Lowell, C.E. The effect of ΔT (oxidizing temperature minus cooling temperature) on oxide spallation. Oxid. Met. 1977, 11, 91–106. [Google Scholar] [CrossRef]
- Jia, Y.F.; Pan, R.J.; Zhang, P.Y.; Sun, Z.T.; Chen, X.R.; Zhang, X.C.; Wu, X.J. Enhanced surface strengthening of titanium treated by combined surface deep-rolling and oxygen boost diffusion technique. Corros. Sci. 2019, 157, 256–267. [Google Scholar] [CrossRef]
- “Titanium Dioxide—Titania (TiO2)”. Azo Materials. 11 January 2002. Available online: www.azom.com/properties.aspx?ArticleID=1179 (accessed on 3 November 2023).
- “GRADE 5 (R56400) Ti-6Al-4V”. Usa-Titanium. 26 September 2016. Available online: www.usa-titanium.com/grade-5-r56400-ti-6al-4v (accessed on 3 November 2023).
- Coddet, C.; Craze, A.M.; Beranger, G. Measurements of the adhesion of thermal oxide films: Application to the oxidation of titanium. J. Mater. Sci. 1987, 22, 2969–2974. [Google Scholar] [CrossRef]
- ASTM E915-2010; Standard Test Method for Verifying the Alignment of X-ray Diffraction Instrumentation for Residual Stress Measurement. ASTM: West Conshohocken, PA, USA, 2010.
- Jiang, Y.; Mao, H.R.; Wang, X.W.; Lu, Y.L.; Gong, J.M. Effect of oxygen boost diffusion treatment on the mechanical properties of Ti-6Al-4V alloy. Surf. Interfaces 2021, 25, 101248. [Google Scholar] [CrossRef]
- Li, Z.; Qian, S.; Wang, W. Influence of superalloy substrate roughness on adhesion and oxidation behavior of magnetron-sputtered NiCoCrAlY coatings. Appl. Surf. Sci. 2011, 257, 10414–10420. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Hou, P.Y.; Gesmundo, F.; Niu, Y. Effect of surface roughness on the development of protective Al2O3 on Fe–10Al (at.%) alloys containing 0–10 at.% Cr. Appl. Surf. Sci. 2006, 253, 881–888. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B. Effect of surface treatment on high-temperature oxidation behavior of IN 713C. J. Mater. Eng. Perform. 2018, 27, 5280–5290. [Google Scholar] [CrossRef]





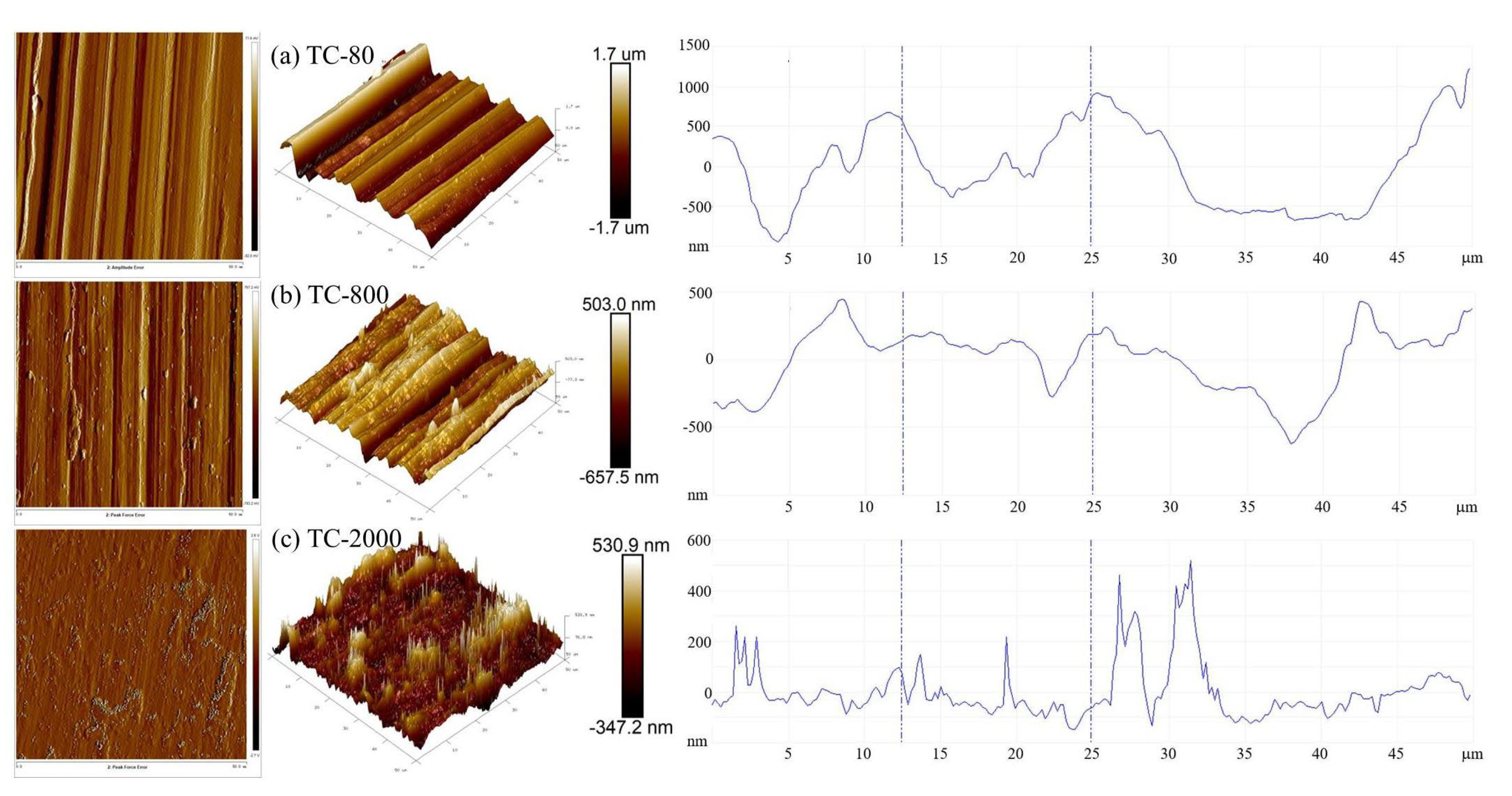


| Sandpaper | Ra/μm | Areal/μm2 | Ain/% |
|---|---|---|---|
| 80 grit | 0.463 | 2627 | 5.08 |
| 400 grit | 0.267 | 2576 | 3.04 |
| 800 grit | 0.132 | 2549 | 1.96 |
| 1200 grit | 0.102 | 2518 | 0.72 |
| 1500 grit | 0.083 | 2509 | 0.36 |
| 2000 grit | 0.076 | 2506 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jiang, Y.; Xie, J.; Xu, Q.; Fei, H.; Lu, Y.; Gong, J. Effect of Temperature, Vacuum Condition and Surface Roughness on Oxygen Boost Diffusion of Ti–6Al–4V Alloy. Coatings 2024, 14, 314. https://doi.org/10.3390/coatings14030314
Xu Y, Jiang Y, Xie J, Xu Q, Fei H, Lu Y, Gong J. Effect of Temperature, Vacuum Condition and Surface Roughness on Oxygen Boost Diffusion of Ti–6Al–4V Alloy. Coatings. 2024; 14(3):314. https://doi.org/10.3390/coatings14030314
Chicago/Turabian StyleXu, Yujie, Yong Jiang, Jinyang Xie, Qingchen Xu, Hao Fei, Yilan Lu, and Jianming Gong. 2024. "Effect of Temperature, Vacuum Condition and Surface Roughness on Oxygen Boost Diffusion of Ti–6Al–4V Alloy" Coatings 14, no. 3: 314. https://doi.org/10.3390/coatings14030314




