Crystalline Structure and Optical Properties of Cobalt Nickel Oxide Thin Films Deposited with a Pulsed Hollow-Cathode Discharge in an Ar+O2 Gas Mixture
Abstract
1. Introduction
2. Experiment
3. Results and Discussion
3.1. Film Composition
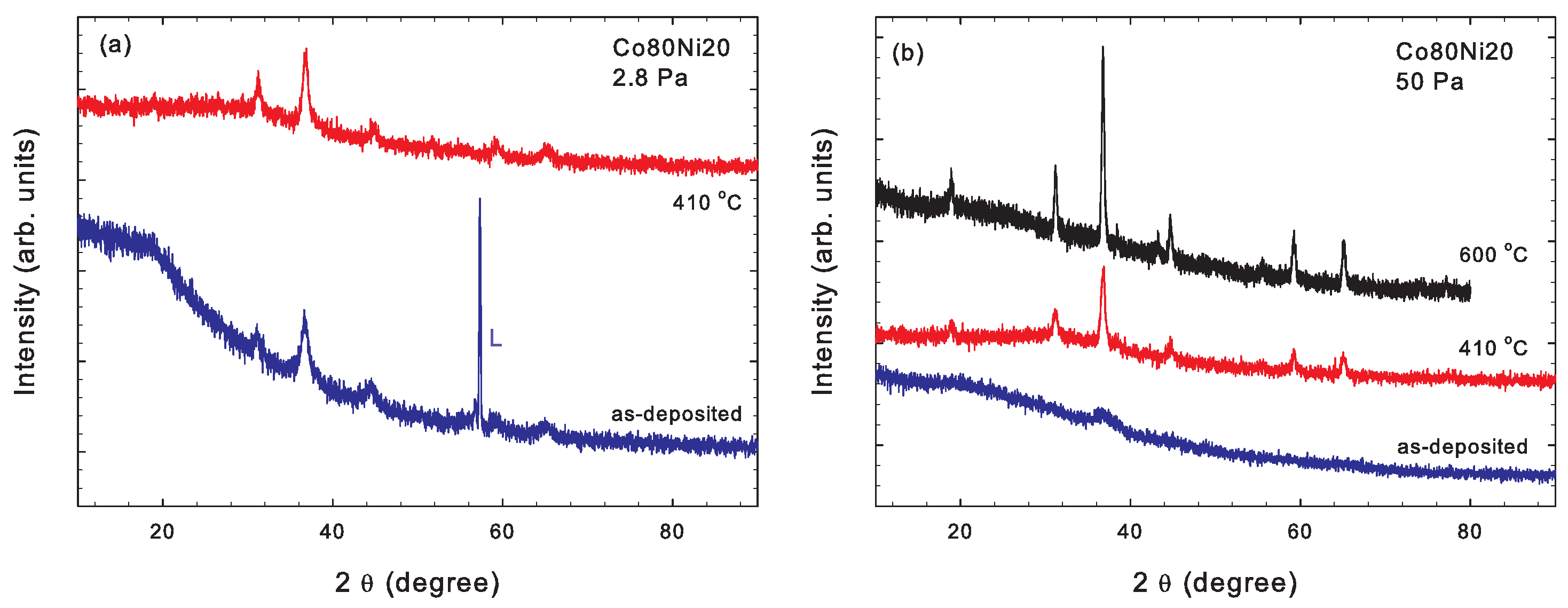
3.2. Crystal Structure
3.2.1. Co50Ni50 Cathode
3.2.2. Co20Ni80 Cathode
| Film | Crystalline Phase | Lattice Parameter (nm) | f (%) | D (nm) |
|---|---|---|---|---|
| (a1) Co50Ni50—2.8 Pa | ||||
| as-deposited | c-Co3O4/c-NiO | n/e | n/e | S/N |
| 410 °C | c-NiO | 0.4191 | 100 | 12 |
| 600 °C | c-NiO | 0.418 | 50 | 13 |
| c-Co3O4 | 0.810 | 50 | 13 | |
| (a2) Co50Ni50—50 Pa | ||||
| as-deposited | c-Co3O4/c-NiO | n/e | n/e | S/N |
| 410 °C | c-NiCo2O4 | 0.8165 | 100 | 9 |
| 600 °C | c-NiO | 0.4176 | 50 | 320 |
| c-Co3O4 | 0.8099 | 50 | 200 | |
| (b1) Co20Ni80—2.8 Pa | ||||
| as-deposited | c-NiO † | 0.4185 | 100 | 12 |
| 410 °C | c-NiO † | 0.4170 | 100 | 11 |
| 600 °C | c-NiO † | 0.4172 | 87 | 26 |
| c-Co3O4 | 0.8104 | 13 | 15 | |
| (b2) Co20Ni80—50 Pa | ||||
| as-deposited | c-NiO | 0.415 | 100 | 14 |
| 410 °C | c-NiO | 0.415 | 100 | 17 |
| 600 °C | c-NiO | 0.4182 | 83 | 34 |
| c-Co3O4 | 0.8078 | 17 | 15 | |
| (c1) Co80Ni20—2.8 Pa | ||||
| as-deposited | c-Co3O4 ‡ | 0.8139 | 100 | 22 |
| 410 °C | c-Co3O4 ‡ | 0.8098 | 100 | 56 |
| (c2) Co80Ni20—50 Pa | ||||
| as-deposited | c-Co3O4/c-NiO | n/e | n/e | S/N |
| 410 °C | c-Co3O4 ‡ | 0.8078 | 100 | 25 |
| 600 °C | c-NiO | 0.4205 | 25 | 200 |
| c-Co3O4 | 0.8098 | 75 | 200 | |
3.2.3. Co80Ni20 Cathode
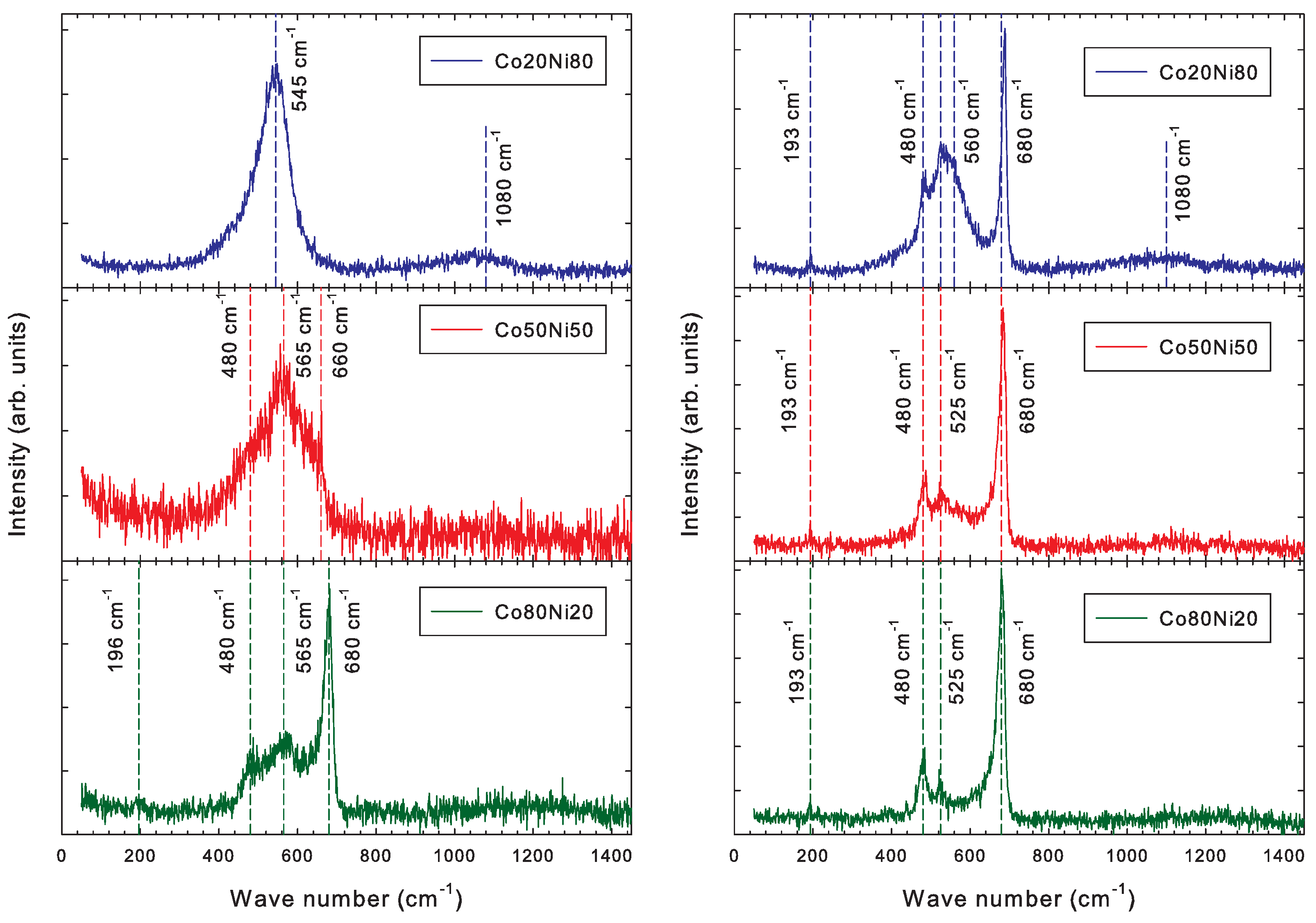
3.3. Raman Spectroscopy
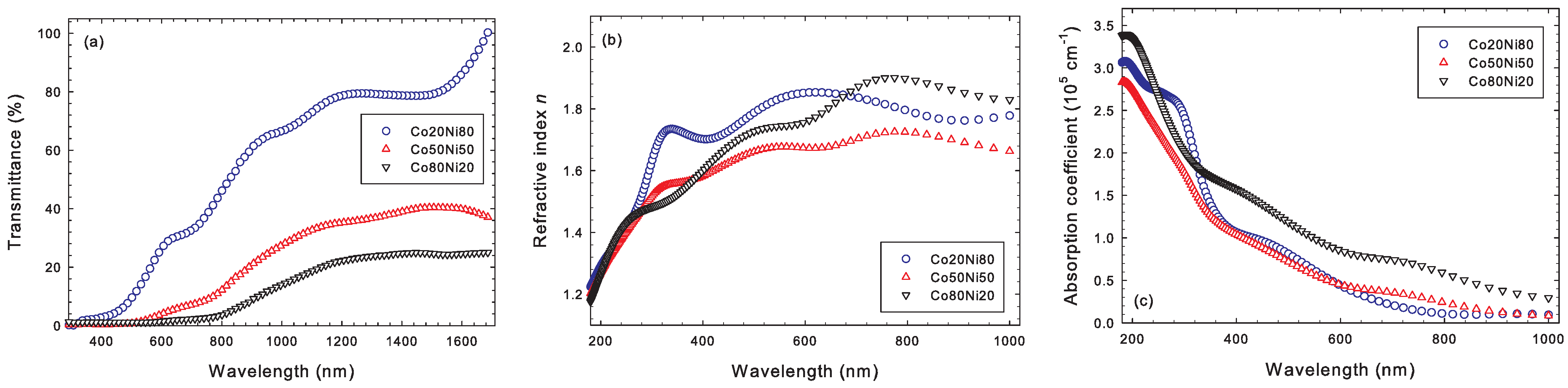
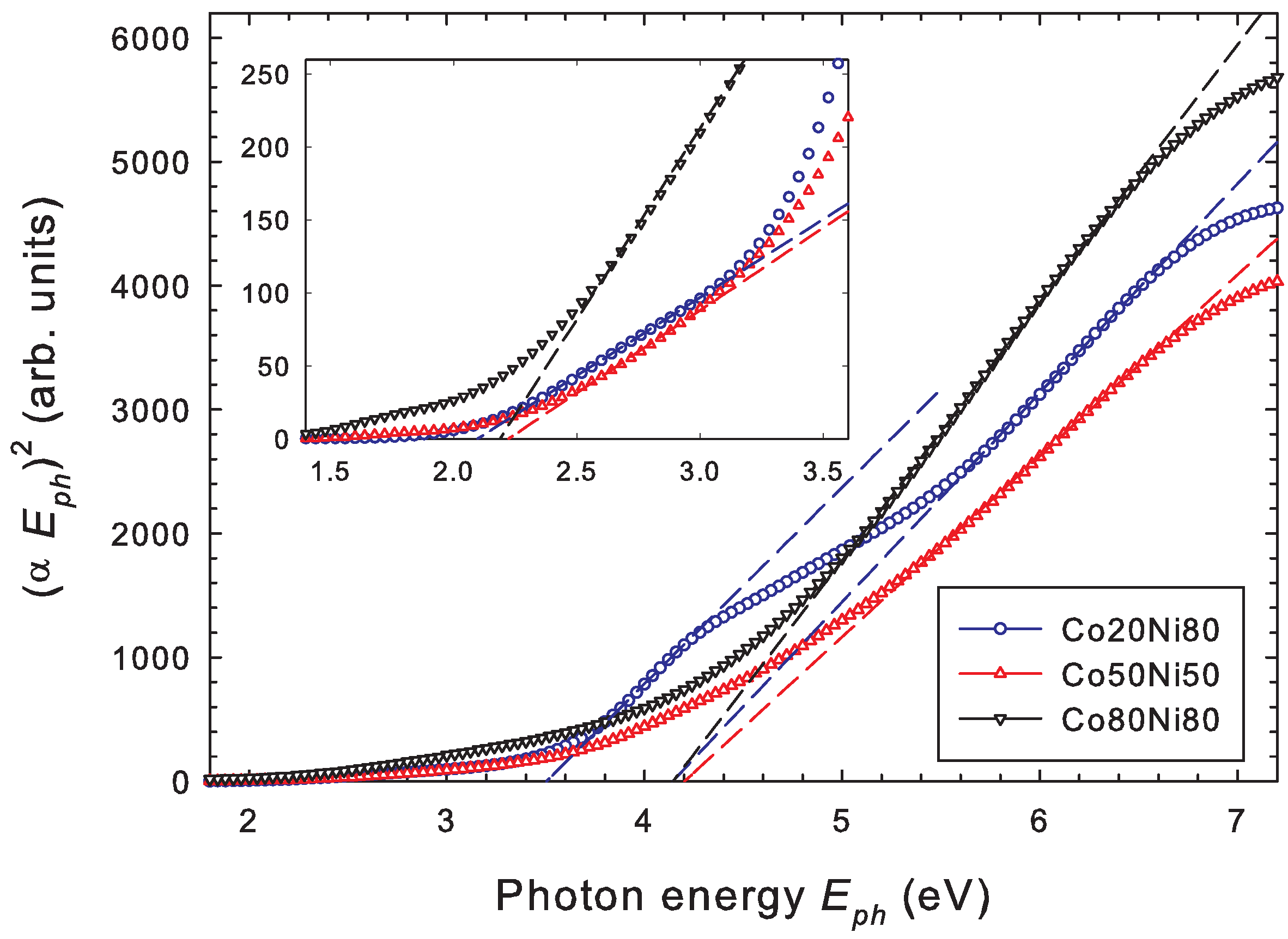
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poonam; Sharma, K.; Singh, N.; Tripathi, S.K. Characterization of nickel cobalt oxide: A potential material for supercapacitor. Mater. Res. Express 2018, 6, 025502. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Guo, R.; Lin, Y.; Zhang, P. Aligned nickel–cobalt oxide nanosheet arrays for lithium ion battery applications. Int. J. Hydrogen Energy 2014, 39, 21399–21404. [Google Scholar] [CrossRef]
- Masoud, E.M. Improved initial discharge capacity of nanostructured Ni-Co spinel ferrite as anode material in lithium ion batteries. Solid State Ionics 2013, 253, 247–252. [Google Scholar] [CrossRef]
- He, Q.; Gu, S.; Wu, T.; Zhang, S.; Ao, X.; Yang, J.; Wen, Z. Self-supported mesoporous FeCo2O4 nanosheets as high capacity anode material for sodium-ion battery. Chem. Eng. J. 2017, 330, 764–773. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Subbarao, G.; Chowdari, B. Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ionics 2008, 179, 587–597. [Google Scholar] [CrossRef]
- Julien, C.; Gendron, F.; Amdouni, A.; Massot, M. Lattice vibrations of materials for lithium rechargeable batteries. VI: Ordered spinels. Mater. Sci. Eng. B 2006, 130, 41–48. [Google Scholar] [CrossRef]
- Chavhan, M.P.; Sethi, S.R.; Ganguly, S. Mixed metal oxides in synergy at nanoscale: Electrospray induced porosity of in situ grown film electrode for use in electrochemical capacitor. Electrochim. Acta 2020, 347, 136277. [Google Scholar] [CrossRef]
- Pradeepa, S.S.; Rajkumar, P.; Diwakar, K.; Sutharthani, K.; Subadevi, R.; Sivakumar, M. A Facile One-Pot Hydrothermal Synthesis of Zn, Mn Co-Doped NiCo2O4 as an Efficient Electrode for Supercapacitor Applications. ChemistrySelect 2021, 6, 6851–6862. [Google Scholar] [CrossRef]
- Hong, W.-L.; Lin, L.-Y. Design of nickel cobalt oxide and nickel cobalt oxide@nickel molybdenum oxide battery-type materials for flexible solid-state battery supercapacitor hybrids. J. Power Sources 2019, 435, 226797. [Google Scholar] [CrossRef]
- Banno, S.; Imanaka, N.; Adachi, G. Selective nitrogen dioxide sensor based on nickel copper oxide mixed with rare earths. Sens. Actuators B Chem. 1995, 25, 619–622. [Google Scholar] [CrossRef]
- Lee, J.H.; Noh, Y.W.; Jin, I.S.; Park, S.H.; Jung, J.W. Efficient perovskite solar cells with negligible hysteresis achieved by sol–gel-driven spinel nickel cobalt oxide thin films as the hole transport layer. J. Mater. Chem. C 2019, 7, 7288–7298. [Google Scholar] [CrossRef]
- Vidales, A.G.; Choi, K.; Omanovic, S. Nickel-cobalt-oxide cathodes for hydrogen production by water electrolysis in acidic and alkaline media. Int. J. Hydrogen Energy 2018, 43, 12917–12928. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Kongsawatvoragul, K.; Kalasina, S.; Phattharasupakun, N.; Sawangphruk, M. A single energy conversion and storage cell of nickel-doped cobalt oxide under UV and visible light illumination. Electrochim. Acta 2019, 328, 135120. [Google Scholar] [CrossRef]
- Jirátová, K.; Čada, M.; Naiko, I.; Ostapenko, A.; Balabánová, J.; Koštejn, M.; Maixner, J.; Babii, T.; Topka, P.; Soukup, K.; et al. Plasma Jet Sputtering as an Efficient Method for the Deposition of Nickel and Cobalt Mixed Oxides on Stainless-Steel Meshes: Application to VOC Oxidation. Catalysts 2022, 13, 79. [Google Scholar] [CrossRef]
- Jirátová, K.; Soukal, P.; Kapran, A.; Babii, T.; Balabánová, J.; Koštejn, M.; Čada, M.; Maixner, J.; Topka, P.; Hubička, Z.; et al. Nickel-Copper Oxide Catalysts Deposited on Stainless Steel Meshes by Plasma Jet Sputtering: Comparison with Granular Analogues and Synergistic Effect in VOC Oxidation. Catalysts 2023, 13, 595. [Google Scholar] [CrossRef]
- Peng, T.; Xiao, X.; Han, X.; Zhou, X.; Wu, W.; Ren, F.; Jiang, C. Characterization of DC reactive magnetron sputtered NiO films using spectroscopic ellipsometry. Appl. Surf. Sci. 2011, 257, 5908–5912. [Google Scholar] [CrossRef]
- Duraisamy, N.; Kandiah, K.; Rajendran, R.; S, P.; R, R.; Dhanaraj, G. Electrochemical and photocatalytic investigation of nickel oxide for energy storage and wastewater treatment. Res. Chem. Intermed. 2018, 44, 5653–5667. [Google Scholar] [CrossRef]
- Awais, M.; Rahman, M.; MacElroy, J.D.; Coburn, N.; Dini, D.; Vos, J.G.; Dowling, D.P. Deposition and characterization of NiOx coatings by magnetron sputtering for application in dye-sensitized solar cells. Surf. Coat. Technol. 2010, 204, 2729–2736. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Takata, S.; Yamada, T. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Films 1993, 236, 27–31. [Google Scholar] [CrossRef]
- Hong, T.; Liu, Z.; Zheng, X.; Zhang, J.; Yan, L. Efficient photoelectrochemical water splitting over Co3O4 and Co3O4/Ag composite structure. Appl. Catal. B Environ. 2017, 202, 454–459. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Salehmin, M.N.I.; Arifin, K.; Minggu, L.J.; Kassim, M.B. Synthesis of Cobalt Oxide on FTO by Hydrothermal Method for Photoelectrochemical Water Splitting Application. Appl. Sci. 2021, 11, 3031. [Google Scholar] [CrossRef]
- Jung, S.; Nandi, D.K.; Yeo, S.; Kim, H.; Jang, Y.; Bae, J.-S.; Hong, T.E.; Kim, S.-H. Phase-controlled growth of cobalt oxide thin films by atomic layer deposition. Surf. Coat. Technol. 2018, 337, 404–410. [Google Scholar] [CrossRef]
- Al-Tuwirqi, R.M.; Al-Hazmi, F.; Alnowaiser, F.; Al-Ghamdi, A.A.; Aal, N.A.; El-Tantawy, F. Synthesis and physical properties of mixed Co3O4/CoO nanorods by microwave hydrothermal technique. Superlattices Microstruct. 2011, 50, 437–448. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Khil, M.S.; Sheikh, F.A.; Kim, H.Y. Synthesis and Optical Properties of Two Cobalt Oxides (CoO and Co3O4) Nanofibers Produced by Electrospinning Process. J. Phys. Chem. C 2008, 112, 12225–12233. [Google Scholar] [CrossRef]
- Prieto, P.; Marco, J.; Serrano, A.; Manso, M.; de la Figuera, J. Highly oriented (111) CoO and Co3O4 thin films grown by ion beam sputtering. J. Alloys Compd. 2019, 810, 151912. [Google Scholar] [CrossRef]
- Kapran, A.; Hippler, R.; Wulff, H.; Olejnicek, J.; Pisarikova, A.; Cada, M.; Hubicka, Z. Characteristics of a pulsed hollow cathode discharge operated in an Ar+O2 gas mixture and deposition of copper nickel oxide thin films. Vacuum 2023, 215, 112272. [Google Scholar] [CrossRef]
- Hippler, R.; Cada, M.; Hubicka, Z. A positively biased external anode for energy control of plasma ions: Hollow cathode and magnetron sputtering discharge. Plasma Sources Sci. Technol. 2021, 30, 045003. [Google Scholar] [CrossRef]
- Olejníček, J.; Šmíd, J.; Perekrestov, R.; Ksirova, P.; Rathouský, J.; Kohout, M.; Dvořáková, M.; Kment, Š.; Jurek, K.; Čada, M.; et al. Co3O4 thin films prepared by hollow cathode discharge. Surf. Coat. Technol. 2019, 366, 303–310. [Google Scholar] [CrossRef]
- Olejníček, J.; Šmíd, J.; Čada, M.; Kšírová, P.; Kohout, M.; Perekrestov, R.; Tvarog, D.; Kment, Š.; Kmentová, H.; Hubička, Z. High rate deposition of photoactive TiO2 films by hot hollow cathode. Surf. Coat. Technol. 2020, 383, 125256. [Google Scholar] [CrossRef]
- Olejníček, J.; Hrubantová, A.; Volfová, L.; Dvořáková, M.; Kohout, M.; Tvarog, D.; Gedeon, O.; Wulff, H.; Hippler, R.; Hubička, Z. WO3 and WO3-x thin films prepared by DC hollow cathode discharge. Vacuum 2021, 195, 110679. [Google Scholar] [CrossRef]
- Wulff, H.; Steffen, H. Characterization of Thin Films. In Low Temperature Plasmas; Hippler, R., Kersten, H., Schmidt, M., Schoenbach, K.-H., Eds.; Wiley-VCH: Berlin, Germany, 2008; pp. 329–362. [Google Scholar]
- ICSD, Inorganic Crystal Structure Database, FIZ Karlsruhe—Leibniz-Institut für Informationsinfrastruktur GmbH. Eggenstein-Leopoldshafen, Germany. Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 25 February 2024).
- De Faria, L.; Prestat, M.; Koenig, J.-F.; Chartier, P.; Trasatti, S. Surface properties of Ni+Co mixed oxides: A study by X-rays, XPS, BET and PZC. Electrochim. Acta 1998, 44, 1481–1489. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Javadian, A.; Fadavieslam, M.R. Impact of copper doping in NiO thin films deposited by spray pyrolysis on their physical properties. J. Mater. Sci. Mater. Electron. 2022, 33, 23362–23374. [Google Scholar] [CrossRef]
- Mahala, C.; Basu, M. Nanosheets of NiCo2O4/NiO as Efficient and Stable Electrocatalyst for Oxygen Evolution Reaction. ACS Omega 2017, 2, 7559–7567. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y.S. Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Puust, L.; Grabis, J. Magnon and Phonon Excitations in Nanosized NiO. Latv. J. Phys. Tech. Sci. 2019, 56, 61–72. [Google Scholar] [CrossRef]
- Hippler, R.; Cada, M.; Ksirova, P.; Olejnicek, J.; Jiricek, P.; Houdkova, J.; Wulff, H.; Kruth, A.; Helm, C.; Hubicka, Z. Deposition of cobalt oxide films by reactive pulsed magnetron sputtering. Surf. Coat. Technol. 2021, 405, 126590. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Roffi, T.M.; Uchida, K.; Nozaki, S. Structural, electrical, and optical properties of CoxNi1-xO films grown by metalorganic chemical vapor deposition. J. Cryst. Growth 2015, 414, 123–129. [Google Scholar] [CrossRef]
- Fernández, C.d.J.; Mattei, G.; Sada, C.; Battaglin, C.; Mazzoldi, P. Nanostructural and optical properties of cobalt and nickel–oxide/silica nanocomposites. Mater. Sci. Eng. C 2006, 26, 987–991. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Liu, Y.-Z.; Li, J.-B.; Sun, P.; Zhao, X.-C.; Liu, C.-J. Photophysical and Photocatalytic Properties of Core-Ring Structured NiCo2O4 Nanoplatelets. J. Phys. Chem. C 2009, 113, 14083–14087. [Google Scholar] [CrossRef]
- Bitla, Y.; Chin, Y.-Y.; Lin, J.-C.; Van, C.N.; Liu, R.; Zhu, Y.; Liu, H.-J.; Zhan, Q.; Lin, H.-J.; Chen, C.-T.; et al. Origin of metallic behavior in NiCo2O4 ferrimagnet. Sci. Rep. 2015, 5, 15201. [Google Scholar] [CrossRef] [PubMed]
- Nashaat, A.M.; Abu El-Fadl, A.; Nakamura, H.; Kassem, M.A. Glassy magnetic freezing and exchange bias effect in NiCo2O4/(Co-Ni)O nanoparticles composite. J. Magn. Magn. Mater. 2023, 567, 170366. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Mane, A.U.; Shivashankar, S. MOCVD of cobalt oxide thin films: Dependence of growth, microstructure, and optical properties on the source of oxidation. J. Cryst. Growth 2003, 254, 368–377. [Google Scholar] [CrossRef]
- Pandey, V.; Adiba, A.; Munjal, S.; Ahmad, T. Optical bandgap tuning of cubic spinel Co3O4 by annealing temperature. Materialia 2022, 26, 101554. [Google Scholar] [CrossRef]
- Manjunatha, K.N.; Paul, S. Investigation of optical properties of nickel oxide thin films deposited on different substrates. Appl. Surf. Sci. 2015, 352, 10–15. [Google Scholar] [CrossRef]
- Rajesh, M.; Vengatesan, K.; Aly, M.H.; Sitharthan, R.; Dhanabalan, S.S.; Karthikeyan, M. Electrical and optical properties of sol–gel-deposited NiO films and corresponding response to annealing temperature. Opt. Quantum Electron. 2023, 55, 1167. [Google Scholar] [CrossRef]
- Lahiji, F.A.F.; Bairagi, S.; Magnusson, R.; Sortica, M.A.; Primetzhofer, D.; Ekström, E.; Paul, B.; le Febvrier, A.; Eklund, P. Growth and optical properties of NiO thin films deposited by pulsed dc reactive magnetron sputtering. J. Vac. Sci. Technol. A 2023, 41, 063402. [Google Scholar] [CrossRef]
- Powell, R.J.; Spicer, W.E. Optical Properties of NiO and CoO. Phys. Rev. B 1970, 2, 2182–2193. [Google Scholar] [CrossRef]
- Silambarasan, M.; Ramesh, P.S.; Geetha, D. Facile one-step synthesis, structural, optical and electrochemical properties of NiCo2O4 nanostructures. J. Mater. Sci. Mater. Electron. 2016, 28, 323–336. [Google Scholar] [CrossRef]
- Louardi, A.; Rmili, A.; Chtouki, T.; Elidrissi, B.; Erguig, H.; El Bachiri, A.; Ammous, K.; Mejbri, H. Effect of annealing treatment on Co3O4 thin films properties prepared by spray pyrolysis. J. Mater. Environ. Sci. 2017, 8, 485. [Google Scholar]


| Cathode | Co (at %) | Ni (at %) | O (at %) | Co/(Co+Ni) Ratio |
|---|---|---|---|---|
| Co20Ni80 | 14.1 | 30.7 | 55.1 | 0.32 |
| Co50Ni50 | 24.7 | 26.9 | 48.4 | 0.48 |
| Co80Ni20 | 54.1 | 11.9 | 35.5 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapran, A.; Hippler, R.; Wulff, H.; Olejnicek, J.; Volfova, L.; Pisarikova, A.; Nepomniashchaia, N.; Cada, M.; Hubicka, Z. Crystalline Structure and Optical Properties of Cobalt Nickel Oxide Thin Films Deposited with a Pulsed Hollow-Cathode Discharge in an Ar+O2 Gas Mixture. Coatings 2024, 14, 319. https://doi.org/10.3390/coatings14030319
Kapran A, Hippler R, Wulff H, Olejnicek J, Volfova L, Pisarikova A, Nepomniashchaia N, Cada M, Hubicka Z. Crystalline Structure and Optical Properties of Cobalt Nickel Oxide Thin Films Deposited with a Pulsed Hollow-Cathode Discharge in an Ar+O2 Gas Mixture. Coatings. 2024; 14(3):319. https://doi.org/10.3390/coatings14030319
Chicago/Turabian StyleKapran, Anna, Rainer Hippler, Harm Wulff, Jiri Olejnicek, Lenka Volfova, Aneta Pisarikova, Natalia Nepomniashchaia, Martin Cada, and Zdenek Hubicka. 2024. "Crystalline Structure and Optical Properties of Cobalt Nickel Oxide Thin Films Deposited with a Pulsed Hollow-Cathode Discharge in an Ar+O2 Gas Mixture" Coatings 14, no. 3: 319. https://doi.org/10.3390/coatings14030319
APA StyleKapran, A., Hippler, R., Wulff, H., Olejnicek, J., Volfova, L., Pisarikova, A., Nepomniashchaia, N., Cada, M., & Hubicka, Z. (2024). Crystalline Structure and Optical Properties of Cobalt Nickel Oxide Thin Films Deposited with a Pulsed Hollow-Cathode Discharge in an Ar+O2 Gas Mixture. Coatings, 14(3), 319. https://doi.org/10.3390/coatings14030319










