Realizing Dual Functions through Y2O3 Modification to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Electrochemical Measurement
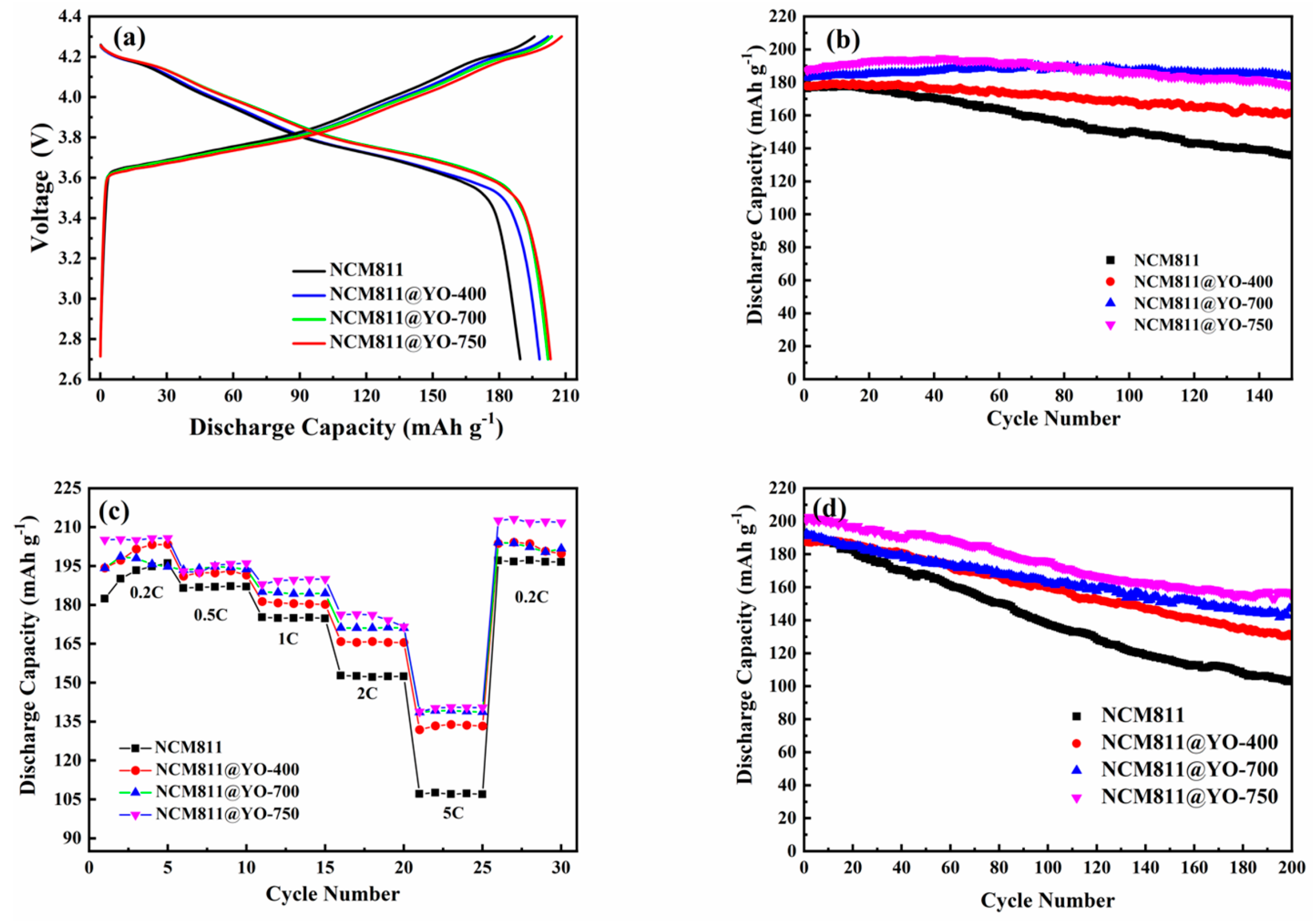
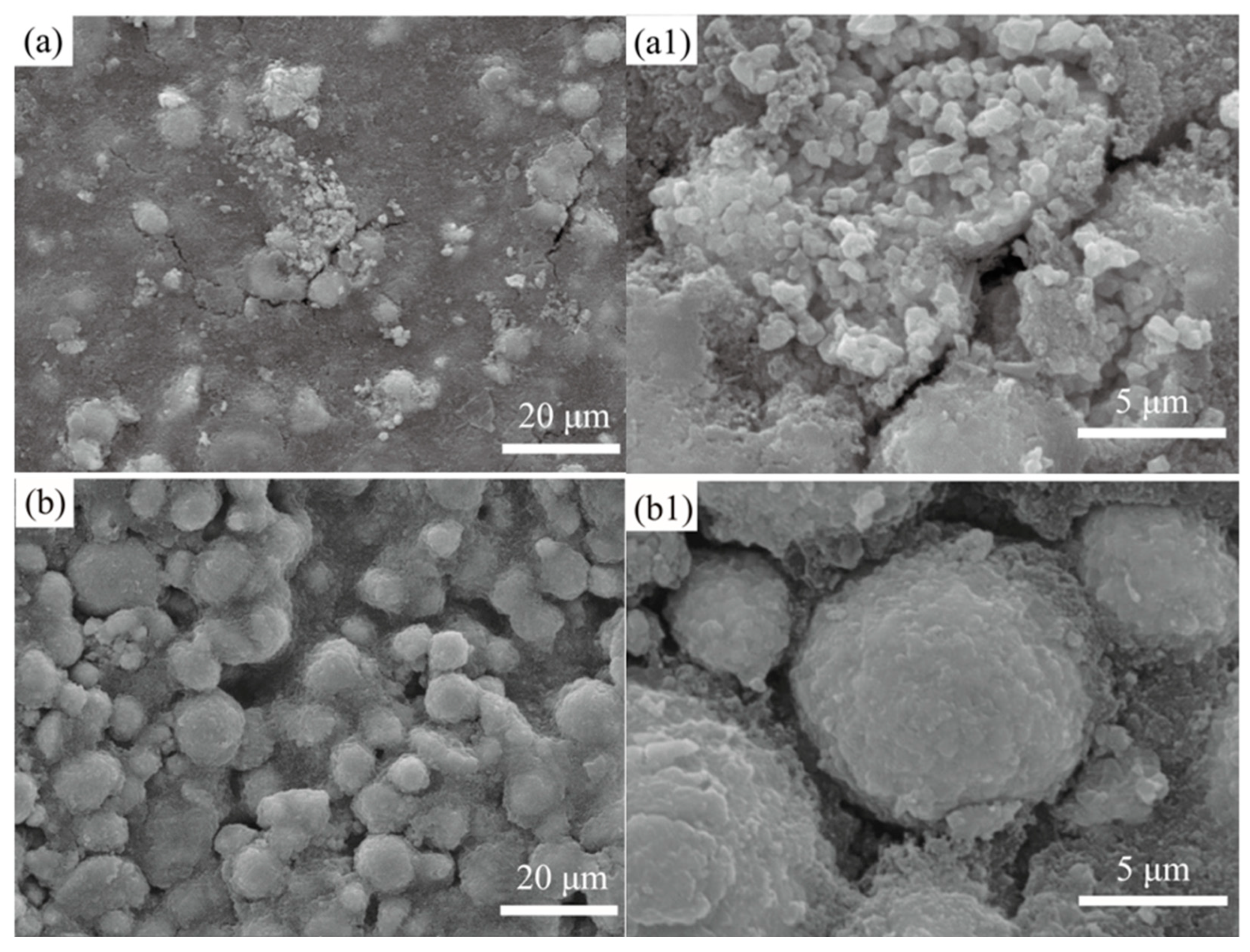
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Han, X.; Li, G.; Du, J.; Cao, Y.; Gong, H.; Wang, H.; Zhang, Y.; Liu, S.; Zhang, B.; et al. Nonsacrificial Nitrile Additive for Armoring High-Voltage LiNi(0.83)Co(0.07)Mn(0.1)O(2) Cathode with Reliable Electrode-Electrolyte Interface toward Durable Battery. Small 2022, 18, e2202989. [Google Scholar] [CrossRef]
- Yee, M.; An, K.; Nguyen, D.-T.; Yun, H.W.; Park, J.; Suk, J.; Song, S.-W. Confining nonflammable liquid in solid polymer electrolyte to enable nickel-rich cathode-based 4.2 V high-energy solid-state lithium-metal and lithium-ion batteries. Mater. Today Energy 2022, 24, 100950. [Google Scholar] [CrossRef]
- Xu, G.L.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and Strategies to Advance High-Energy Nickel-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, J.; Zhao, H.; Hu, Z.; Liu, X. Research progress of ternary layered oxide cathode materials for lithium ion batteries. J. Eng. Stud. 2017, 9, 523. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, J.; Huang, J.; Zeng, R.; Zhang, J.; Chen, H.; Shi, G. Improving the Ni-rich LiNi0.5Co0.2Mn0.3O2 cathode properties at high operating voltage by double coating layer of Al2O3 and AlPO4. J. Alloys Compd. 2017, 724, 1109–1116. [Google Scholar] [CrossRef]
- Li, J.; Xiang, J.; Yi, G.; Tang, Y.; Shao, H.; Liu, X.; Shan, B.; Chen, R. Reduction of Surface Residual Lithium Compounds for Single-Crystal LiNi0.6Mn0.2Co0.2O2 via Al2O3 Atomic Layer Deposition and Post-Annealing. Coatings 2022, 12, 84. [Google Scholar] [CrossRef]
- Bian, X.; Fu, Q.; Bie, X.; Yang, P.; Qiu, H.; Pang, Q.; Chen, G.; Du, F.; Wei, Y. Improved electrochemical performance and thermal stability of Li-excess Li1. 18Co0. 15Ni0. 15Mn0. 52O2 cathode material by Li3PO4 surface coating. Electrochim. Acta 2015, 174, 875–884. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Wei, G.; Qiu, S.; Qu, J. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO. J. Solid State Electrochem. 2019, 23, 2213–2224. [Google Scholar] [CrossRef]
- Yu, Z.; Tong, Q.; Zhao, G.; Zhu, G.; Tian, B.; Cheng, Y. Combining Surface Holistic Ge Coating and Subsurface Mg Doping to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathodes. ACS Appl. Mater. Interfaces 2022, 14, 25490–25500. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Lin, C.; Wang, Q.; Zhang, Y. Improving the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathodes using a simple Ce4+-doping and CeO2-coating technique. N. J. Chem. 2021, 45, 21617–21623. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and electrochemical performances of TiO2-coated Mg–Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. N. J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Tang, W.; Peng, Z.; Shi, Y.; Xu, S.; Shuai, H.; Zhou, S.; Kong, Y.; Yan, K.; Lu, T.; Wang, G. Enhanced cyclability and safety performance of LiNi0.6Co0.2Mn0.2O2 at elevated temperature by AlPO4 modification. J. Alloys Compd. 2019, 810, 151834. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Zhang, X.; Xiao, J.; Xiong, H.; Li, W.; Guo, P.; Yang, Z.; Xie, M. Enhanced cyclic stability of LiNi0.8Co0.1Mn0.1O2 (NCM811) by AlF3 coating via atomic layer deposition. Ionics 2022, 28, 4547–4554. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, L.; Zhang, F.; He, D. The combined effect of CaF2 and graphite two-layer coatings on improving the electrochemical performance of Li-rich layer oxide material. J. Solid State Chem. 2019, 272, 38–46. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Zhang, Z.; Liu, S.; Wang, R. Improving the Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 Cathode Material by LiF Modification. Coatings 2023, 13, 727. [Google Scholar] [CrossRef]
- Dai, S.; Yan, G.; Wang, L.; Luo, L.; Li, Y.; Yang, Y.; Liu, H.; Liu, Y.; Yuan, M. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0. 8Co0. 1Mn0. 1O2 cathode material via CaF2 coating. J. Electroanal. Chem. 2019, 847, 113197. [Google Scholar] [CrossRef]
- Dong, H.; Xie, M.; Cai, M.; Liu, H.; Zhang, Z.; Ye, B.; Zhao, P.; Dong, W.; Huang, F. Amorphous fluorine glaze for crack-free nickel-rich layered cathode grains under electrochemical cycling. Chem. Eng. J. 2022, 436, 135227. [Google Scholar] [CrossRef]
- Breddemann, U.; Krossing, I. Review on Synthesis, Characterization, and Electrochemical Properties of Fluorinated Nickel-Cobalt-Manganese Cathode Active Materials for Lithium-Ion Batteries. ChemElectroChem 2020, 7, 1389–1430. [Google Scholar] [CrossRef]
- Yang, S.; Meng, T.; Wang, Z.; Hu, X. Unveiling decaying mechanism of non-flammable all-fluorinated carbonate electrolytes in lithium metal batteries with 4.6-V LiCoO2 cathodes at elevated temperatures. Energy Storage Mater. 2024, 65, 103177. [Google Scholar] [CrossRef]
- Ren, Q.; Yuan, Y.; Wang, S. Interfacial Strategies for Suppression of Mn Dissolution in Rechargeable Battery Cathode Materials. ACS Appl. Mater. Interfaces 2021, 14, 23022–23032. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, X.; Xiao, L.; Xiao, Z. Influence of Al3+ and PO43− co-doping on structure and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials. J. Solid State Electrochem. 2023, 27, 1185–1194. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.-T.; Liu, G.; Wang, J.; Wang, J. Boosting the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials with Zn3(PO4)2 surface coating. Adv. Powder Technol. 2021, 32, 4651–4657. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Lin, R.; Zhu, X.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Gui, W.; Zeng, Q. First-principles study of structure, mechanical and optical properties of La- and Sc-doped Y2O3. J. Rare Earths 2019, 37, 879–885. [Google Scholar] [CrossRef]
- Liu, X.-H.; Kou, L.-Q.; Shi, T.; Liu, K.; Chen, L. Excellent high rate capability and high voltage cycling stability of Y2O3-coated LiNi0.5Co0.2Mn0.3O2. J. Power Sources 2014, 267, 874–880. [Google Scholar] [CrossRef]
- Zou, P.; Lin, Z.; Fan, M.; Wang, F.; Liu, Y.; Xiong, X. Facile and efficient fabrication of Li3PO4-coated Ni-rich cathode for high-performance lithium-ion battery. Appl. Surf. Sci. 2020, 504, 144506. [Google Scholar] [CrossRef]
- Jo, C.-H.; Cho, D.-H.; Noh, H.-J.; Yashiro, H.; Sun, Y.-K.; Myung, S.T. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 2014, 8, 1464–1479. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Ma, Y.; Goonetilleke, D.; Sann, J.; Walther, F.; Bianchini, M.; Janek, J.; Brezesinski, T. High Performance All-Solid-State Batteries with a Ni-Rich NCM Cathode Coated by Atomic Layer Deposition and Lithium Thiophosphate Solid Electrolyte. ACS Appl. Energy Mater. 2021, 4, 7338–7345. [Google Scholar] [CrossRef]
- Wang, X.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Nisar, U.; Muralidharan, N.; Essehli, R.; Amin, R.; Belharouak, I. Valuation of Surface Coatings in High-Energy Density Lithium-ion Battery Cathode Materials. Energy Storage Mater. 2021, 38, 309–328. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Liu, P.; Balogun, M.-S.; Deng, J.; Wang, Z. Improved Cycling Performance and High Rate Capacity of LiNi0.8Co0.1Mn0.1O2 Cathode Achieved by Al(PO3)3 Modification via Dry Coating Ball Milling. Coatings 2022, 12, 319. [Google Scholar] [CrossRef]
- Ran, A.; Chen, S.; Cheng, M.; Liang, Z.; Li, B.; Zhou, G.; Kang, F.; Zhang, X.; Wei, G. A single-crystal nickel-rich material as a highly stable cathode for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 19680–19689. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, M.; Zhang, D.; Yan, Y.; Wang, Y.; Li, J.; Li, Z. Enhanced electrochemical properties of NCM811 cathode material due to synergistic modification with Sm as doping and coating agent. J. Alloys Compd. 2022, 909, 164712. [Google Scholar] [CrossRef]
- Xu, Y.-D.; Xiang, W.; Wu, Z.-G.; Xu, C.-L.; Li, Y.-C.; Guo, X.-D.; Lv, G.-P.; Peng, X.; Zhong, B.-H. Improving cycling performance and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials by Li4Ti5O12 coating. Electrochim. Acta 2018, 268, 358–365. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, Y.; Qian, Y.; Wang, N.; Zhao, M.; Yao, J.; Xu, Y. Annealing effects of TiO2 coating on cycling performance of Ni-rich cathode material LiNi0.8Co0.1Mn0.1O2 for lithium-ion battery. Mater. Lett. 2020, 265, 127418. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Su, Y.; Lu, Y.; Bao, L.; Chen, L.; Zhang, Q.; Wang, J.; Chen, R.; Wu, F. Ni-Rich LiNi(0.8)Co(0.1)Mn(0.1)O(2) Oxide Coated by Dual-Conductive Layers as High Performance Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Su, J.; Chen, C.; Liu, M.; Chen, X.; Wu, J.; Huang, T.; Yu, A. Revealing the role of NH(4)VO(3) treatment in Ni-rich cathode materials with improved electrochemical performance for rechargeable lithium-ion batteries. Nanoscale 2018, 10, 8820–8831. [Google Scholar] [CrossRef]
- Ding, Y.; Deng, B.; Wang, H.; Li, X.; Chen, T.; Yan, X.; Wan, Q.; Qu, M.; Peng, G. Improved electrochemical performances of LiNi0. 6Co0. 2Mn0. 2O2 cathode material by reducing lithium residues with the coating of Prussian blue. J. Alloys Compd. 2019, 774, 451–460. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Fu, S.; Liu, Z. F127-assisted synthesis of LiNi0. 5Co0. 2Mn0. 3O1. 99F0. 01 as a high rate and long lifespan cathode material for lithium-ion batteries. Appl. Surf. Sci. 2019, 476, 1061–1071. [Google Scholar] [CrossRef]
- House, R.A.; Marie, J.-J.; Pérez-Osorio, M.A.; Rees, G.J.; Boivin, E.; Bruce, P.G. The role of O2 in O-redox cathodes for Li-ion batteries. Nat. Energy 2021, 6, 781–789. [Google Scholar] [CrossRef]
- He, R.; Bai, X.; Wei, A.; Zhang, L.; Liu, P.; Liu, Z. Y2O3 modification on nickel-rich LiNi0.8Co0.1Mn0.1O2 with improved electrochemical performance in lithium-ion batteries. J. Rare Earths 2022, 40, 309–317. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yang, P.; He, W.; Bie, S.; Zhao, H.; Yin, J.; Zou, Z.; Liu, J. Enhanced high-voltage cycling stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode coated with Li2O–2B2O3. J. Alloys Compd. 2019, 805, 991–998. [Google Scholar] [CrossRef]
- Peng, Z.; Li, T.; Zhang, Z.; Du, K.; Hu, G.; Cao, Y. Surface architecture decoration on enhancing properties of LiNi0.8Co0.1Mn0.1O2 with building bi-phase Li3PO4 and AlPO4 by Al(H2PO4)3 treatment. Electrochim. Acta 2020, 338, 135870. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, Z.; Dou, A.; Pan, J.; Su, M. Investigation the electrochemical performance of layered cathode material Li1. 2Ni0. 2Mn0. 6O2 coated with Li4Ti5O12. Adv. Powder Technol. 2016, 27, 1481–1487. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, M.; Zhang, D.; Yan, Y.; Wang, Y.; Li, Z. Inhibited intracrystalline cracks and enhanced electrochemical properties of NCM811 cathode materials coated by EPS. Ceram. Int. 2021, 47, 32710–32719. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Li, Y.; Chen, C.; Zeng, W.; Yuan, M.; Wang, R.; Xiao, S. Synergistic Modification of Magnesium Fluoride/Sodium for Improving the Electrochemical Performances of High-Nickel Ternary (NCM811) Cathode Materials. J. Electrochem. Soc. 2019, 166, A3480–A3486. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Q. Improving the electrochemical performance of LiNi0. 7Mn0. 3O2 cathode material by CeO2 coating via Ce doping. Solid State Ion. 2023, 399, 116296. [Google Scholar] [CrossRef]
- Wu, Y.S.; Pham, Q.-T.; Yang, C.-C.; Chern, C.-S.; Babulal, L.M.; Seenivasan, M.; Jeyakumar, J.; Mengesha, T.H.; Placke, T.; Brunklaus, G.; et al. Coating of a Novel Lithium-Containing Hybrid Oligomer Additive on Nickel-Rich LiNi0.8Co0.1Mn0.1O2 Cathode Materials for High-Stability and High-Safety Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 7394–7408. [Google Scholar] [CrossRef]
- Cheng, W.; Li, L.; Hao, S.; Liu, L.; Wu, Y.; Huo, J.; Ji, Y.; Liu, X. Face-lifting the surface of LiNi0.8Co0.15Al0.05O2 cathode via Y(PO3)3 to form an in situ triple composite Li-ion conductor coating layer with the enhanced electrochemical performance. Nanotechnology 2022, 33, 375701. [Google Scholar] [CrossRef]
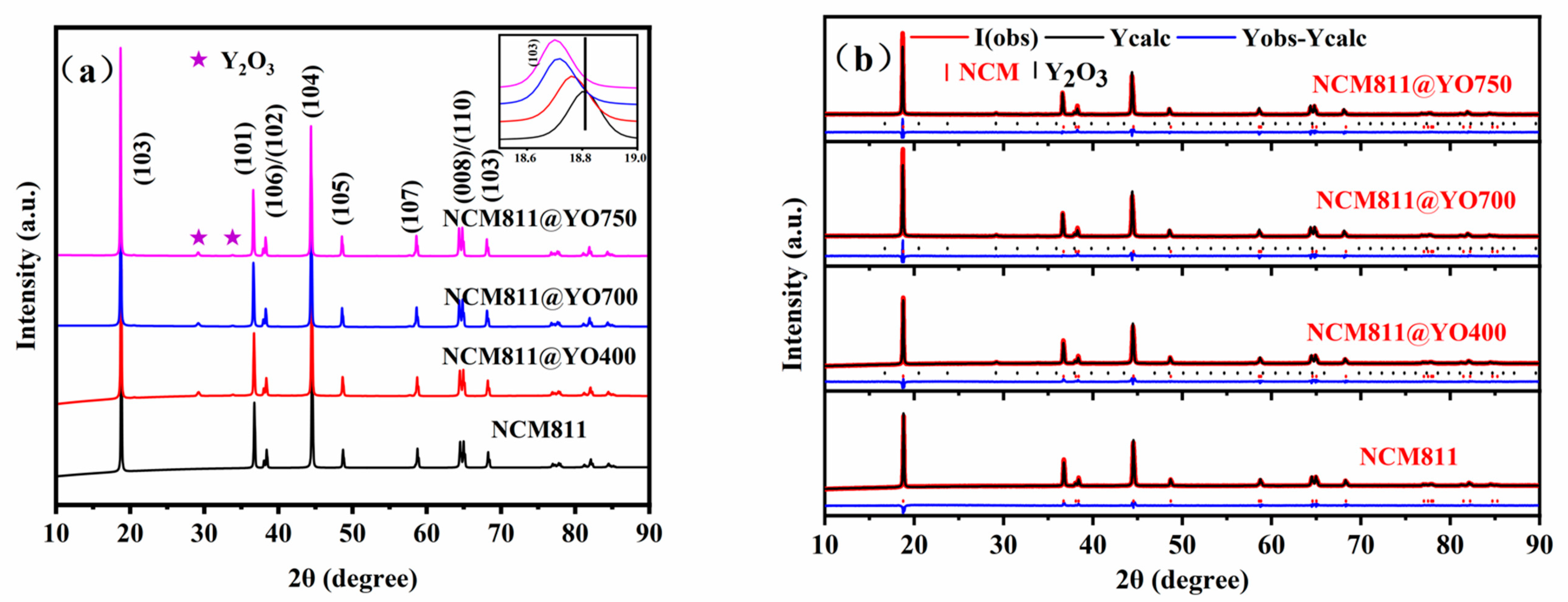
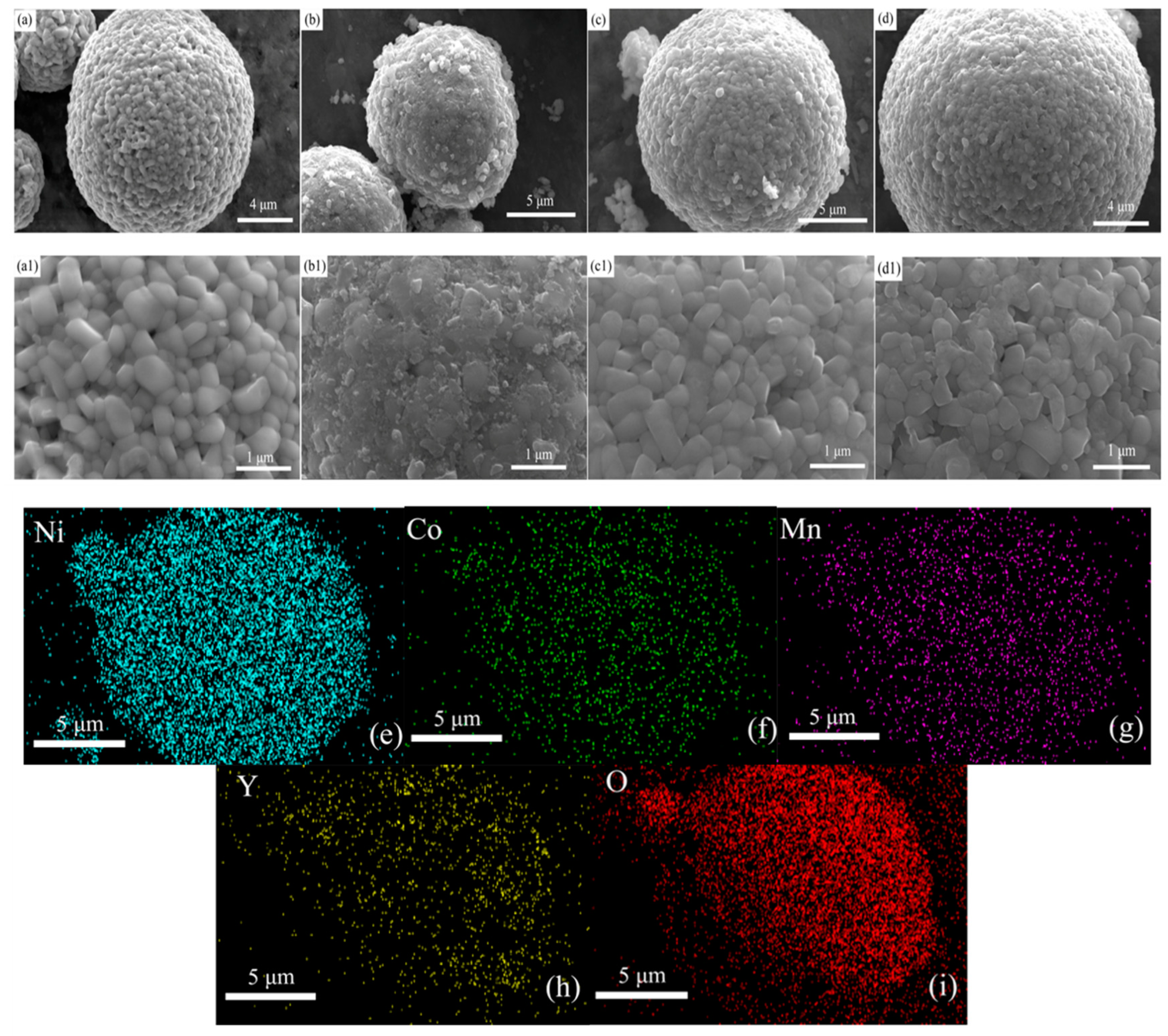
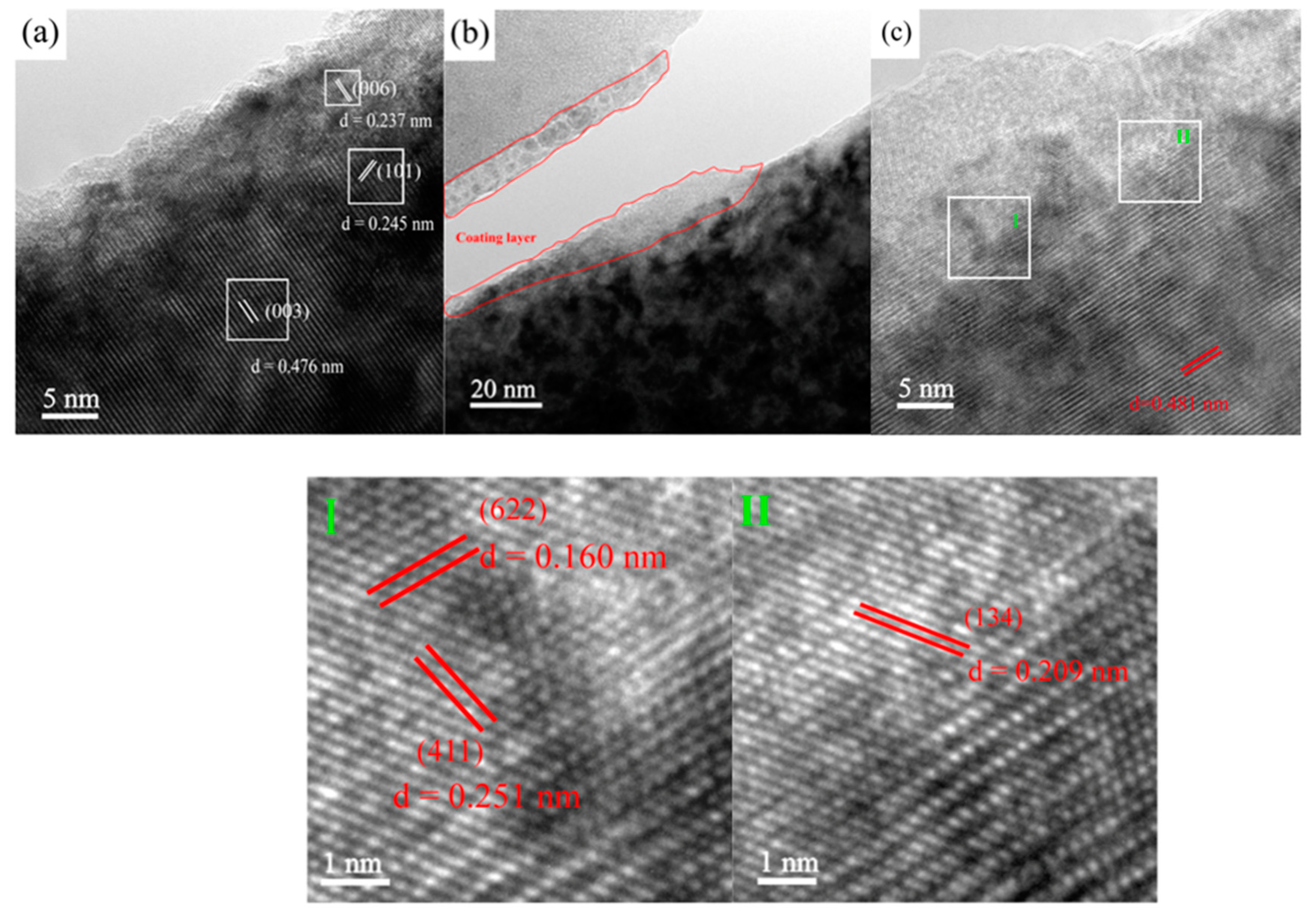
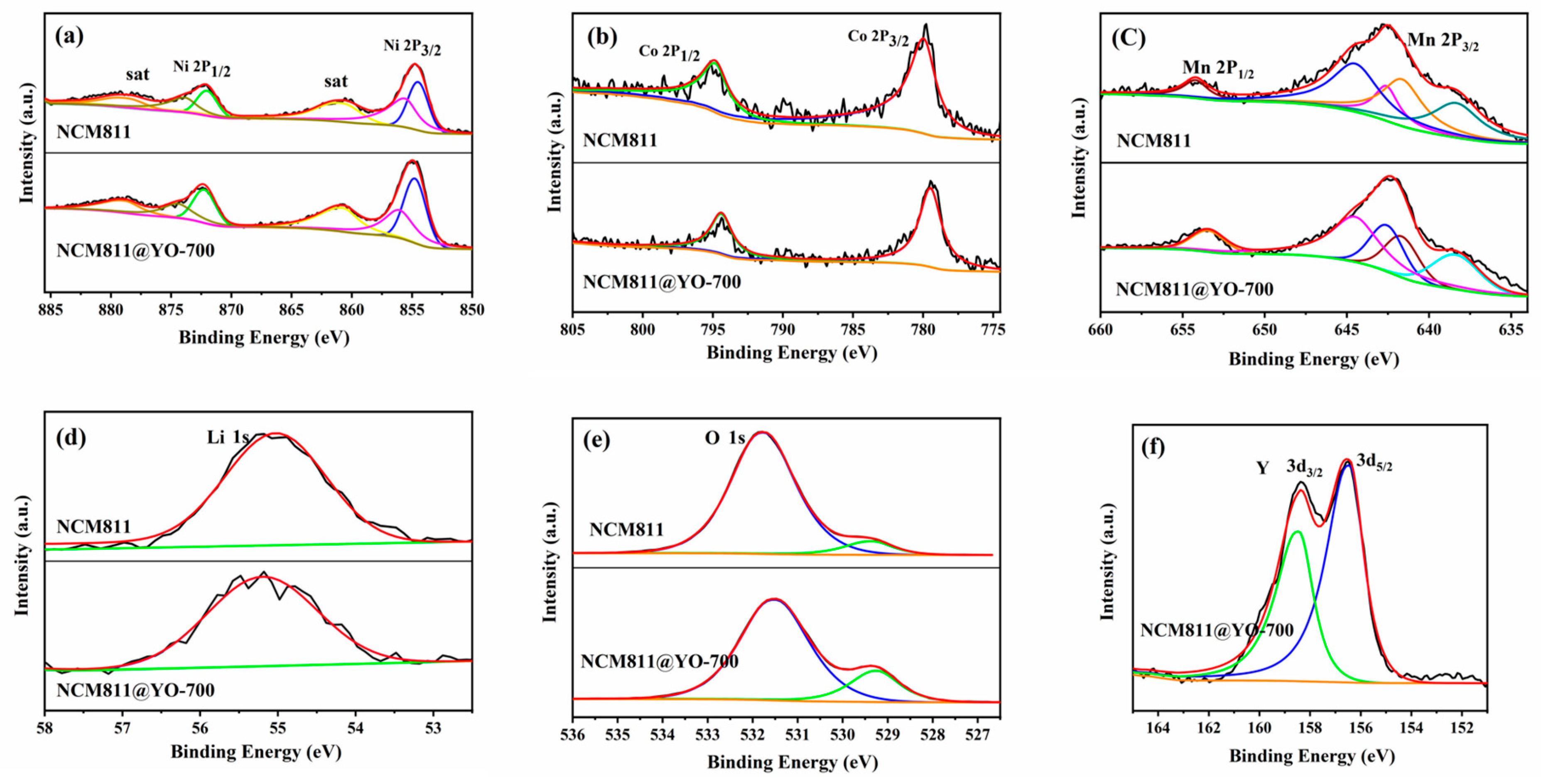

| Sample | a (Å) | C (Å) | c/a | V (Å3) | Rp (%) | I (003)/I (104) |
|---|---|---|---|---|---|---|
| NCM811 | 2.8709 | 14.2044 | 4.9477 | 101.4 | 3.95 | 1.5066 |
| NCM811@YO400 | 2.8715 | 14.2042 | 4.9466 | 101.4 | 4.45 | 1.5506 |
| NCM811@YO700 | 2.8740 | 14.2116 | 4.9449 | 101.7 | 4.01 | 1.5123 |
| NCM811@YO750 | 2.8748 | 14.2136 | 4.9442 | 101.7 | 4.23 | 1.5263 |
| Systems | 1st Discharge Capacity (mAh g−1) | Maximum Discharge Capacity (mAh g−1) | 150 st Discharge Capacity (mAh g−1) | Capacity Retention (%) |
|---|---|---|---|---|
| Original | 176.3 | 177.9 | 135.7 | 76.28 |
| NCM811@YO400 | 177.7 | 179.3 | 161.5 | 90.07 |
| NCM811@YO700 | 182.9 | 190.1 | 183.6 | 96.58 |
| NCM811@YO750 | 187.3 | 194.7 | 177.8 | 91.32 |
| Electrode | Rs (Ω) | Rsf (Ω) | Rct (Ω) | DLi+ (cm2 s−1) |
|---|---|---|---|---|
| NCM811 | 14.5 | 179.6 | 535.1 | 1.12 × 10−14 |
| NCM811@YO700 | 9.5 | 85.3 | 322.8 | 2.28 × 10−14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, F.; Zheng, M.; Rong, M.; Wang, J.; Deng, J.; Liu, P.; Liu, D. Realizing Dual Functions through Y2O3 Modification to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Material. Coatings 2024, 14, 443. https://doi.org/10.3390/coatings14040443
Wang X, Wang F, Zheng M, Rong M, Wang J, Deng J, Liu P, Liu D. Realizing Dual Functions through Y2O3 Modification to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Material. Coatings. 2024; 14(4):443. https://doi.org/10.3390/coatings14040443
Chicago/Turabian StyleWang, Xintao, Feng Wang, Meiqi Zheng, Maohua Rong, Jiang Wang, Jianqiu Deng, Peng Liu, and Daosheng Liu. 2024. "Realizing Dual Functions through Y2O3 Modification to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Material" Coatings 14, no. 4: 443. https://doi.org/10.3390/coatings14040443
APA StyleWang, X., Wang, F., Zheng, M., Rong, M., Wang, J., Deng, J., Liu, P., & Liu, D. (2024). Realizing Dual Functions through Y2O3 Modification to Enhance the Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Material. Coatings, 14(4), 443. https://doi.org/10.3390/coatings14040443








