Dual Microcapsules Encapsulating Liquid Diamine and Isocyanate for Application in Self-Healing Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
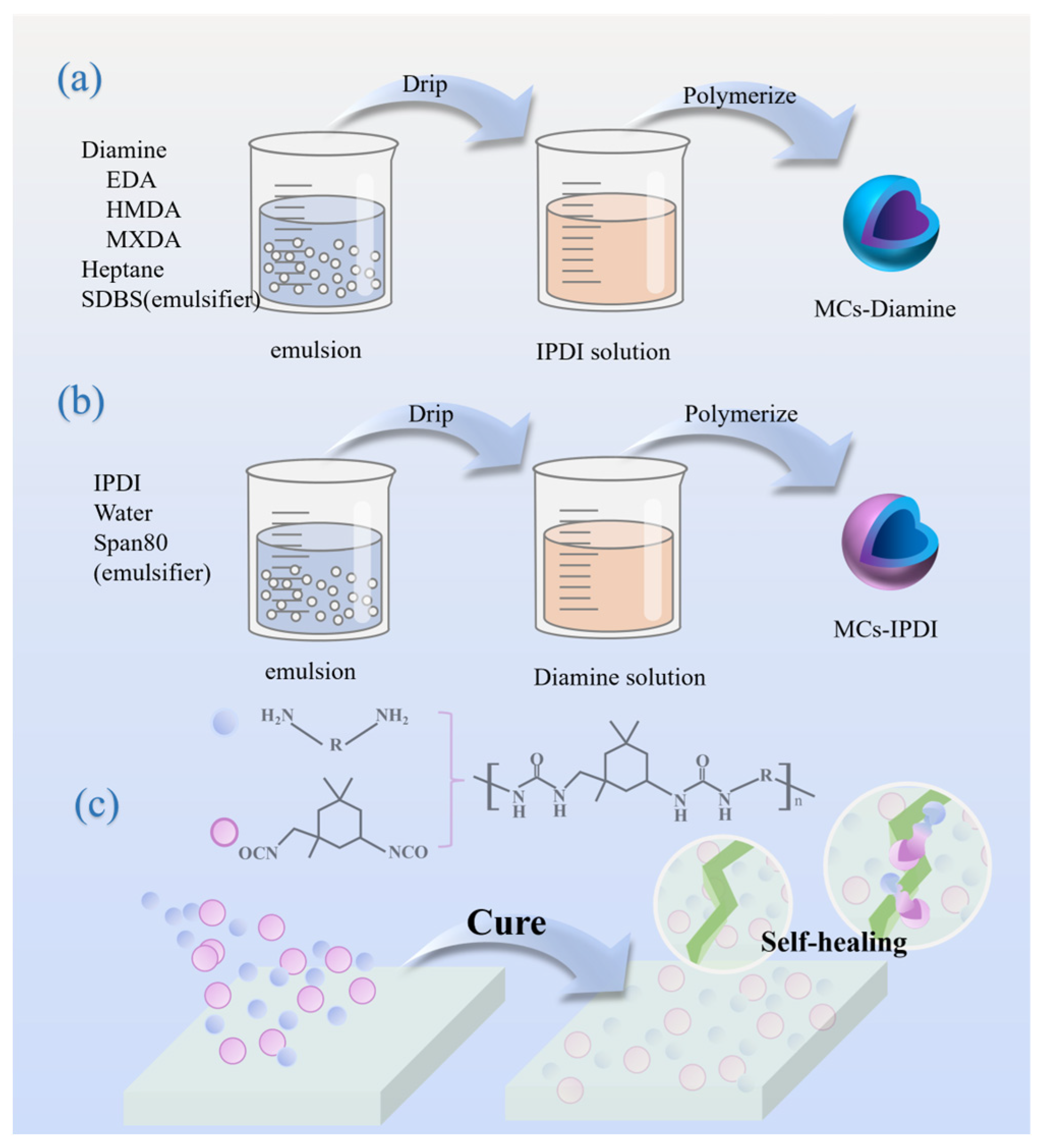
2.2. Synthesis of Diamine Microcapsules
2.3. Synthesis of IPDI Microcapsules
2.4. Preparation of Self-Healing Coatings
2.5. Characterization of Microcapsules
2.6. Characterization of Self-Healing Coatings
3. Result and Discussion
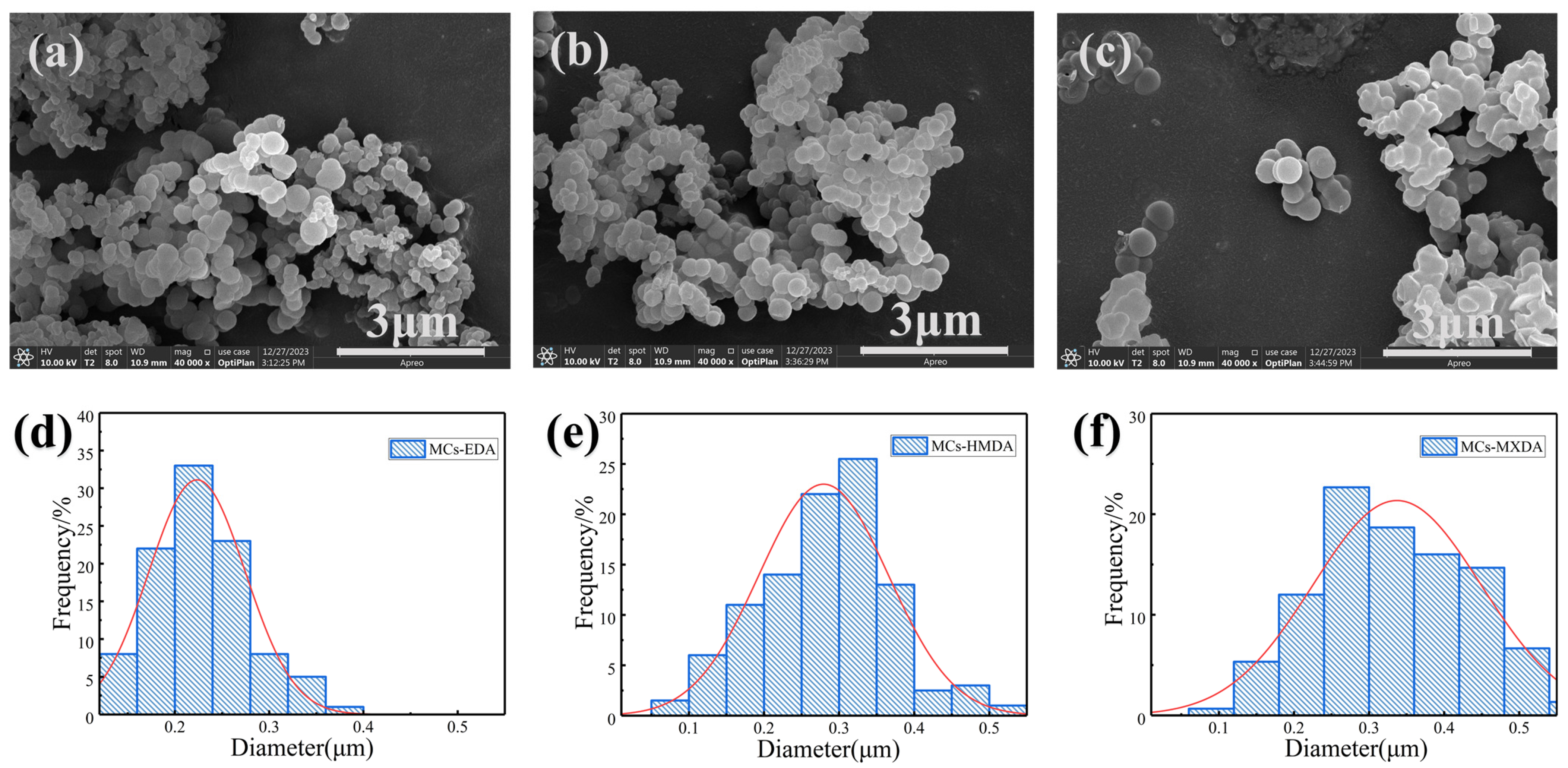
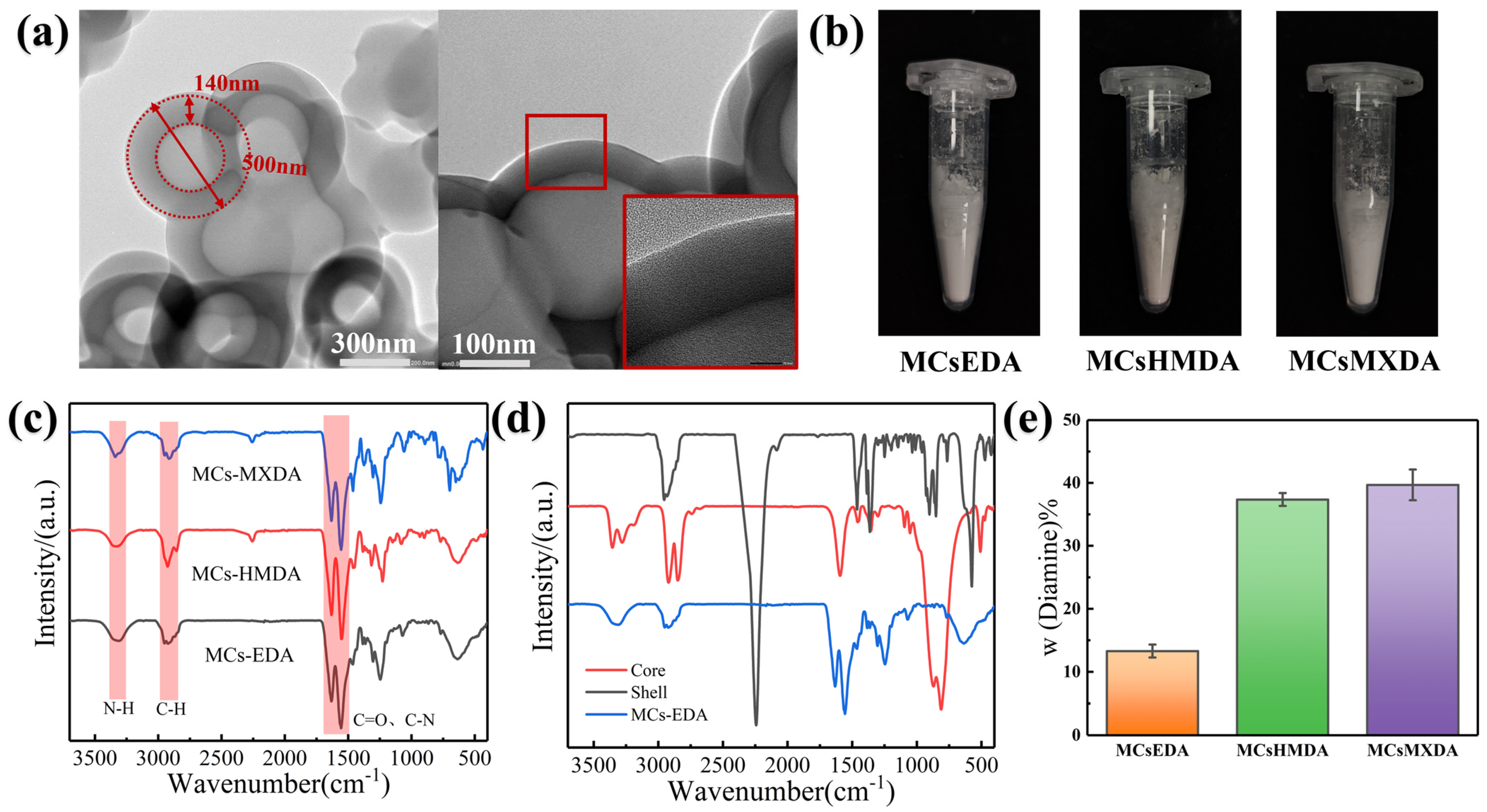
3.1. Synthesis of Diamine Microcapsules
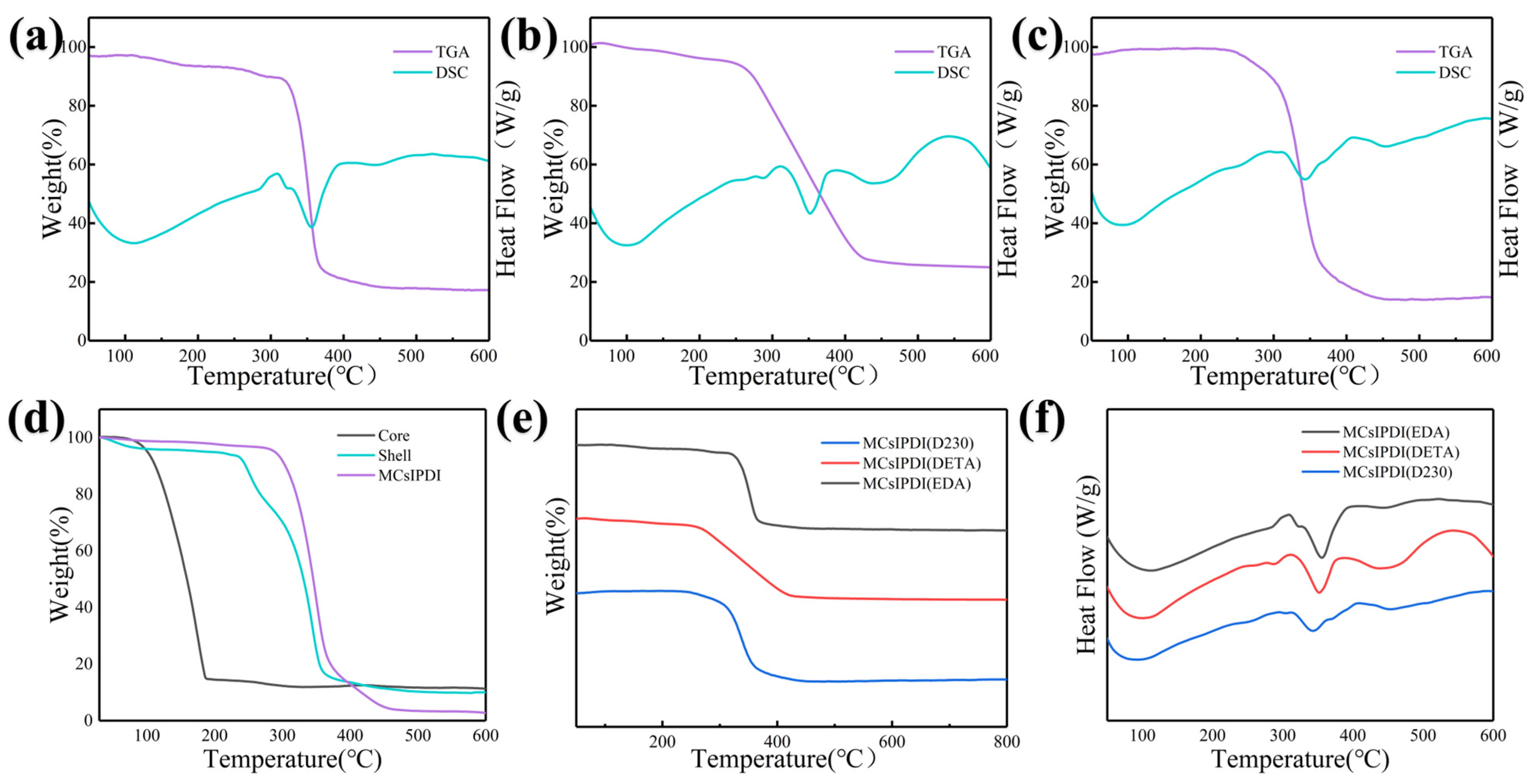
3.2. Synthesis of IPDI Microcapsules
3.3. Self-Healing Properties of Dual Microcapsules System
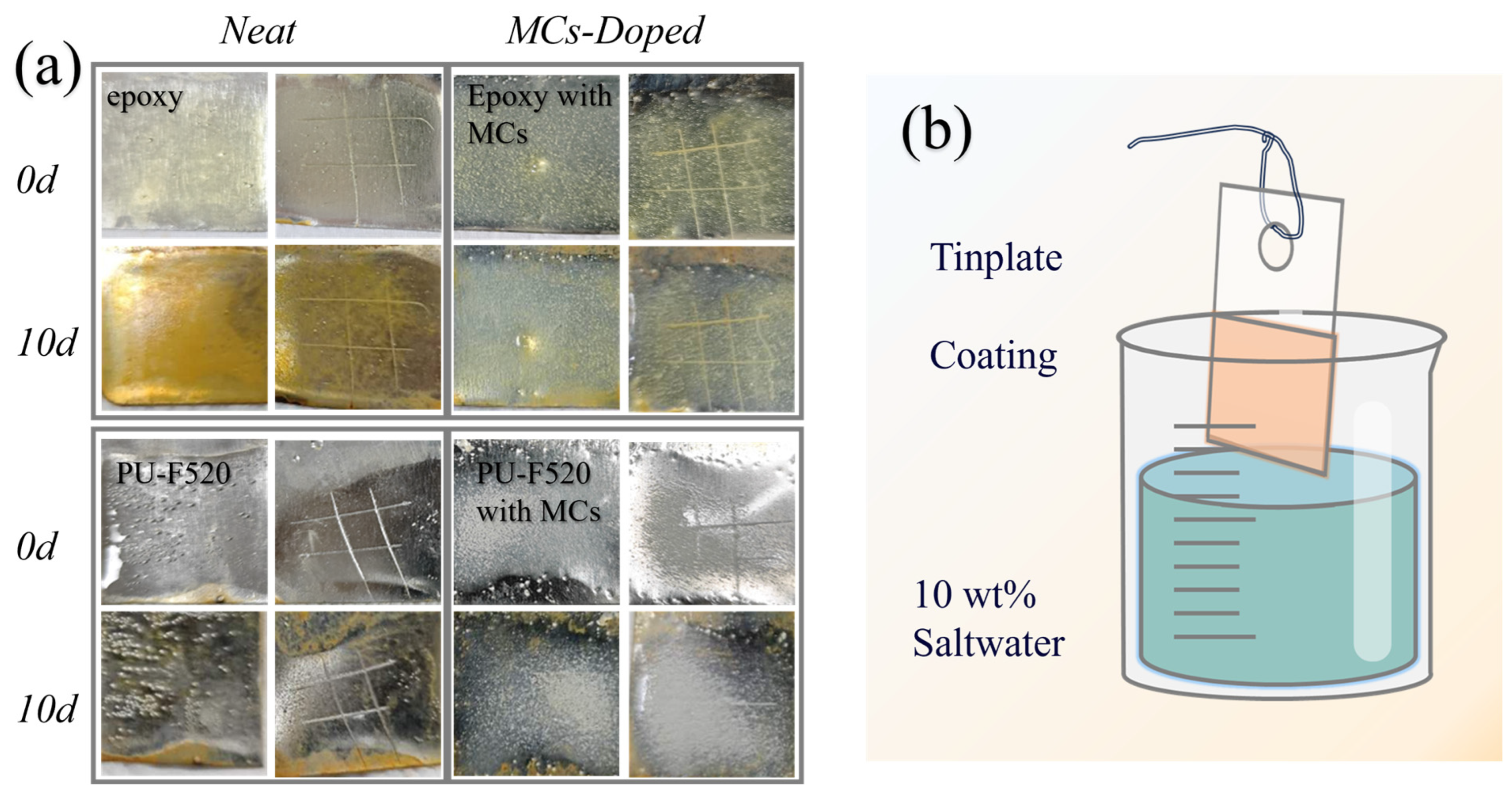
3.4. Anticorrosion Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Shi, H.; Gu, S.; Liu, F.; Han, E.-H. A Smart Anticorrosive Coating Based on pH-Sensitive Microspheres Fabricated via a Facile Method for Protection of AA2024-T3. Prog. Org. Coat. 2024, 189, 108259. [Google Scholar] [CrossRef]
- Panahi, P.; Khorasani, S.N.; Mensah, R.A.; Das, O.; Neisiany, R.E. A Review of the Characterization Methods for Self-Healing Assessment in Polymeric Coatings. Prog. Org. Coat. 2024, 186, 108055. [Google Scholar] [CrossRef]
- Huang, Y.; Shan, Y.; Liang, S.; Zhao, X.; Jiang, G.; Hu, C.; Yang, J.; Liu, L. Coordinated Silicon Elastomer Coating@fabrics with Oil/Water Separation Capabilities, Outstanding Durability and Ultra-Fast Room-Temperature Self-Healing Ability. J. Mater. Chem. A 2018, 6, 17156–17163. [Google Scholar] [CrossRef]
- Chen, Z.; Scharnagl, N.; Zheludkevich, M.L.; Ying, H.; Yang, W. Micro/Nanocontainer-Based Intelligent Coatings: Synthesis, Performance and Applications—A Review. Chem. Eng. J. 2023, 451, 138582. [Google Scholar] [CrossRef]
- Yang, S.; Sun, R.; Chen, K. Self-Healing Performance and Corrosion Resistance of Phytic Acid/Cerium Composite Coating on Microarc-Oxidized Magnesium Alloy. Chem. Eng. J. 2022, 428, 131198. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Shim, S.B.; Kim, M.S.; Shrimant, B.; Lee, J.H.; Nam, S.Y.; Kwon, D.-J.; Park, J.H. Influence of Milled and Acid-Treated Graphene Oxide on the Self-Healing Properties of Graphene Oxide Reinforced Polyurethane. Compos. Part B Eng. 2023, 259, 110702. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The Role of Graphene in Anti-Corrosion Coatings: A Review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, C.; Wang, X.; Szpunar, J. Layer-by-Layer Assembly of a Self-Healing Anticorrosion Coating on Magnesium Alloys. ACS Appl. Mater. Interfaces 2015, 7, 27271–27278. [Google Scholar] [CrossRef]
- Xiong, P.; Yan, J.; Wang, P.; Jia, Z.; Zhou, W.; Yuan, W.; Li, Y.; Liu, Y.; Cheng, Y.; Chen, D.; et al. A pH-Sensitive Self-Healing Coating for Biodegradable Magnesium Implants. Acta Biomater. 2019, 98, 160–173. [Google Scholar] [CrossRef]
- Sun, W.; Luo, N.; Liu, Y.; Li, H.; Wang, D. A New Self-Healing Triboelectric Nanogenerator Based on Polyurethane Coating and Its Application for Self-Powered Cathodic Protection. ACS Appl. Mater. Interfaces 2022, 14, 10498–10507. [Google Scholar] [CrossRef]
- Xu, J.H.; Ye, S.; Ding, C.D.; Tan, L.H.; Fu, J.J. Autonomous Self-Healing Supramolecular Elastomer Reinforced and Toughened by Graphitic Carbon Nitride Nanosheets Tailored for Smart Anticorrosion Coating Applications. J. Mater. Chem. A 2018, 6, 5887–5898. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Brancart, J.; Terryn, S.; Bosman, A.W.; Norvez, S.; Assche, G.V.; Iida, F.; Vanderborght, B.; Clemens, F. Soft Self-Healing Resistive-Based Sensors Inspired by Sensory Transduction in Biological Systems. Appl. Mater. Today 2022, 29, 101638. [Google Scholar] [CrossRef]
- Yang, P.; Li, Z.; Fang, B.; Liu, L. Self-Healing Hydrogels Based on Biological Macromolecules in Wound Healing: A Review. Int. J. Biol. Macromol. 2023, 253, 127612. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, H.; Peng, H.; Zhang, J. Biological Self-Healing Strategies from Mechanically Robust Heterophasic Liquid Metals. Matter 2023, 6, 226–238. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Evolution of Self-Healing Elastomers, from Extrinsic to Combined Intrinsic Mechanisms: A Review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Li, W.; Dong, B.; Yang, Z.; Xu, J.; Chen, Q.; Li, H.; Xing, F.; Jiang, Z. Recent Advances in Intrinsic Self-Healing Cementitious Materials. Adv. Mater. 2018, 30, 1705679. [Google Scholar] [CrossRef]
- Wan, P.; Wu, S.; Liu, Q.; Wang, H.; Gong, X.; Zhao, Z.; Xu, S.; Jiang, J.; Fan, L.; Tu, L. Extrinsic Self-Healing Asphalt Materials: A Mini Review. J. Clean. Prod. 2023, 425, 138910. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-Healing Polymeric Materials Based on Microencapsulated Healing Agents: From Design to Preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Luo, J.; Liu, R.; Sun, G.; Liu, X. Robust Damage-Reporting Strategy Enabled by Dual-Compartment Microcapsules. ACS Appl. Mater. Interfaces 2021, 13, 14518–14529. [Google Scholar] [CrossRef]
- Lu, T.; Li, B.; Sun, D.; Hu, M.; Ma, J.; Sun, G. Advances in Controlled Release of Microcapsules and Promising Applications in Self-Healing of Asphalt Materials. J. Clean. Prod. 2021, 294, 126270. [Google Scholar] [CrossRef]
- Barbaz-Isfahani, R.; Saber-Samandari, S.; Salehi, M. Novel Electrosprayed Enhanced Microcapsules with Different Nanoparticles Containing Healing Agents in a Single Multicore Microcapsule. Int. J. Biol. Macromol. 2022, 200, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Zhou, Q. Preparation and Characterization of Microcapsules Based Self-Healing Coatings Containing Epoxy Ester as Healing Agent. Prog. Org. Coat. 2018, 125, 403–410. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Luo, J.; Liu, R.; Sun, G.; Liu, X. Preparation of Dual-Chamber Microcapsule by Pickering Emulsion for Self-Healing Application with Ultra-High Healing Efficiency. J. Colloid Interface Sci. 2021, 600, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lim, T.-W.; Sottos, N.R.; White, S.R. Manufacture of Carbon-Fiber Prepreg with Thermoplastic/Epoxy Resin Blends and Microencapsulated Solvent Healing Agents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 365–375. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Gu, G.; Wang, F.; Ren, J.; Zhang, H.; Zhao, Y. Microfluidic Electrospray Niacin Metal-Organic Frameworks Encapsulated Microcapsules for Wound Healing. Research 2019, 2019, 6175398. [Google Scholar] [CrossRef] [PubMed]
- Ahangaran, F.; Hayaty, M.; Navarchian, A.H. Morphological Study of Polymethyl Methacrylate Microcapsules Filled with Self-Healing Agents. Appl. Surf. Sci. 2017, 339, 721–731. [Google Scholar] [CrossRef]
- Kardar, P. Preparation of Polyurethane Microcapsules with Different Polyols Component for Encapsulation of Isophorone Diisocyanate Healing Agent. Prog. Org. Coat. 2015, 89, 271–276. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as Wall Material for Microcapsules: A Review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Choi, D.; de Hoyos-Vega, J.M.; Baskaran, H.; Gonzalez-Suarez, A.M.; Lee, S.; Hong, H.J.; Nguyen, K.M.; Dharmesh, E.; Sugahara, G.; et al. Function of Hepatocyte Spheroids in Bioactive Microcapsules Is Enhanced by Endogenous and Exogenous Hepatocyte Growth Factor. Bioact. Mater. 2023, 28, 183–195. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Z.; Wang, C.; Chen, K. Fabrication of Dual Self-Healing Multifunctional Coating Based on Multicompartment Microcapsules. ACS Appl. Mater. Interfaces 2021, 13, 59298–59309. [Google Scholar] [CrossRef]
- Xiang, H.; Xia, H.; Chen, Y.; Wu, Y.; Chen, H.; Yan, M. Pavement Anti-Icing Coating Based on a Functional Composite of NaCl Microcapsules. Constr. Build. Mater. 2021, 307, 125010. [Google Scholar] [CrossRef]
- Philippe, E.; Ramos, J.A.T.; Miguel, A.C.; Vicente, A.A. Physiological Protection of Probiotic Microcapsules by Coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, K.; Chen, M.; Wu, L. Synthesis of UV-Responsive Self-Healing Microcapsules and Their Potential Application in Aerospace Coatings. ACS Appl. Mater. Interfaces 2019, 11, 33314–33322. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Application of Starch Microcapsules Containing Essential Oil in Food Preservation. Crit. Rev. Food Sci. Nutr. 2020, 60, 2825–2836. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Li, L.; Deng, W.; Liu, M.; Hu, J. Biodegradable Starch-Based Packaging Films Incorporated with Polyurethane-Encapsulated Essential-Oil Microcapsules for Sustained Food Preservation. Int. J. Biol. Macromol. 2023, 235, 123889. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Shao, J.; Shu, X.; Ren, X.; Wu, B.; Yan, Z. Preservative Effects of Allicin Microcapsules on Daily Foods. LWT 2018, 98, 225–230. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of Microcapsules Derived from Coated Fertilizer in Paddy Fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- Cesari, A.; Loureiro, M.V.; Vale, M.; Yslas, E.I.; Dardanelli, M.; Marques, A.C. Polycaprolactone Microcapsules Containing Citric Acid and Naringin for Plant Growth and Sustainable Agriculture: Physico-Chemical Properties and Release Behavior. Sci. Total Environ. 2020, 703, 135548. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zheng, M.; Liu, Y.; Zhang, J.; Ma, S.; Jin, J. Analysis of Behavior and Mechanism of Repairing Agent of Microcapsule in Asphalt Micro Crack Based on Molecular Dynamics Simulation. Constr. Build. Mater. 2021, 305, 124791. [Google Scholar] [CrossRef]
- Safaei, F.; Khorasani, S.N.; Rahnama, H.; Neisiany, R.E.; Koochaki, M.S. Single Microcapsules Containing Epoxy Healing Agent Used for Development in the Fabrication of Cost Efficient Self-Healing Epoxy Coating. Prog. Org. Coat. 2018, 114, 40–46. [Google Scholar] [CrossRef]
- Zhi, Y.; Che, J.; Zhu, H.; Liu, R.; Zhao, Y. Glycyrrhetinic Acid Liposomes Encapsulated Microcapsules from Microfluidic Electrospray for Inflammatory Wound Healing. Adv. Funct. Mater. 2023, 33, 2304353. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Wang, X.; Zhang, M.; Zhang, M.; Gao, Y.; Wang, H. Melamine-Formaldehyde Microcapsules Encapsulating HEDP for Sustained Scale inhibition. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127361. [Google Scholar] [CrossRef]
- Gan, J.; Sun, L.; Chen, G.; Ma, W.; Zhao, Y.; Sun, L. Mesenchymal Stem Cell Exosomes Encapsulated Oral Microcapsules for Acute Colitis Treatment. Adv. Healthc. Mater. 2022, 11, 2201105. [Google Scholar] [CrossRef] [PubMed]
- Mamusa, M.; Mastrangelo, R.; Glen, T.; Murgia, S.; Palazzo, G.; Smets, J.; Baglioni, P. Rational Design of Sustainable Liquid Microcapsules for Spontaneous Fragrance Encapsulation. Angew. Chem. Int. Ed. 2021, 60, 23849–23857. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhong, S.; Wang, J.; Gao, Y.; Cui, X. 10-Hydroxycamptothecin-Loaded Starch-Based Microcapsules with the Stepwise Responsive Release Strategy for Targeted Controlled Release. Int. J. Biol. Macromol. 2023, 252, 126424. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Arjona, J.; das Graças Silva-Valenzuela, M.; Wang, S.-H.; Valenzuela-Diaz, F.R. Biodegradable Nanocomposite Microcapsules for Controlled Release of Urea. Polymers 2021, 13, 722. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.-T.; Ju, X.-J.; Zhang, L.; Huang, X.-B.; Faraj, Y.; Liu, Z.; Wang, W.; Xie, R.; Deng, Y.; Chu, L.-Y. Chitosan Microcapsule Membranes with Nanoscale Thickness for Controlled Release of Drugs. J. Membr. Sci. 2019, 590, 117275. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Shi, Z.; Zeng, R.; Ren, T.; Zhang, B. Glutathione-Responsive Pyraclostrobin-Loaded Polyurea Microcapsules for Their Intelligent Controlled Release. J. Agric. Food Chem. 2022, 70, 5310–5318. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Yu, R.; Yuan, L.; Yang, Y.; He, X.; Wang, J.; Li, Z. Microencapsulation of Ethylenediamine and Its Application in Binary Self-Healing System Using Dual-Microcapsule. Mater. Des. 2020, 189, 108535. [Google Scholar] [CrossRef]
- Ma, B.; Xiong, F.; Wang, H.; Qing, Y.; Chu, F.; Wu, Y. Tailorable and Scalable Production of Eco-Friendly Lignin Micro-Nanospheres and Their Application in Functional Superhydrophobic Coating. Chem. Eng. J. 2023, 457, 141309. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, Y.; Tan, H.; Zhang, Y.; Gu, J. A Rapid and Efficient Route to Preparation of Isocyanate Microcapsules. Polymers 2017, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, S.-R.; Lee, D.-S. Self-Healing of Cross-Linked PU via Dual-Dynamic Covalent Bonds of a Schiff Base from Cystine and Vanillin. Mater. Des. 2019, 172, 107774. [Google Scholar] [CrossRef]
- Liu, W.; He, Y.; Leng, J. Shape Memory Supramolecular Polyurea with Adjustable Toughness and Ultrahigh Energy Density. ACS Appl. Polym. Mater. 2022, 4, 6092–6102. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of Microcapsules Containing Dual-Functional Tung Oil and Properties Suitable for Self-Healing and Self-Lubricating Coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, J.; Sun, Y.; Ge, Y.; Yan, X.; Wu, J. Preparation and Characterization of Ethylenediamine-Polyurea Microcapsule Epoxy Self-Healing Coating. Materials 2020, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Wang, J.; Wang, Z.; Wang, S. Preparation of Epoxy Microcapsule Based Self-Healing Coatings and Their Behavior. Surf. Coat. Technol. 2012, 206, 4976–4980. [Google Scholar] [CrossRef]
- He, Z.; Jiang, S.; Li, Q.; Wang, J.; Zhao, Y.; Kang, M. Facile and Cost-Effective Synthesis of Isocyanate Microcapsules via Polyvinyl Alcohol-Mediated Interfacial Polymerization and Their Application in Self-Healing Materials. Compos. Sci. Technol. 2017, 138, 15–23. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of Graphene and Its Derivatives on the Anticorrosive Properties of Waterborne Polyurethane Coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile Preparation of Water-Dispersible Graphene Sheets Stabilized by Carboxylated Oligoanilines and Their Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17648. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, H.; Deng, Y.; Zou, W.; Yang, X.; Zhao, Q. Dual Microcapsules Encapsulating Liquid Diamine and Isocyanate for Application in Self-Healing Coatings. Coatings 2024, 14, 410. https://doi.org/10.3390/coatings14040410
Mu H, Deng Y, Zou W, Yang X, Zhao Q. Dual Microcapsules Encapsulating Liquid Diamine and Isocyanate for Application in Self-Healing Coatings. Coatings. 2024; 14(4):410. https://doi.org/10.3390/coatings14040410
Chicago/Turabian StyleMu, Huaixuan, Yiqing Deng, Wangcai Zou, Xiandi Yang, and Qiang Zhao. 2024. "Dual Microcapsules Encapsulating Liquid Diamine and Isocyanate for Application in Self-Healing Coatings" Coatings 14, no. 4: 410. https://doi.org/10.3390/coatings14040410






