Electrolyte Influence on Properties of Ultra-Thin Anodic Memristors on Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Ti Anodic Memristors
2.2. Electrical Characterization
2.3. XPS and TEM Analysis
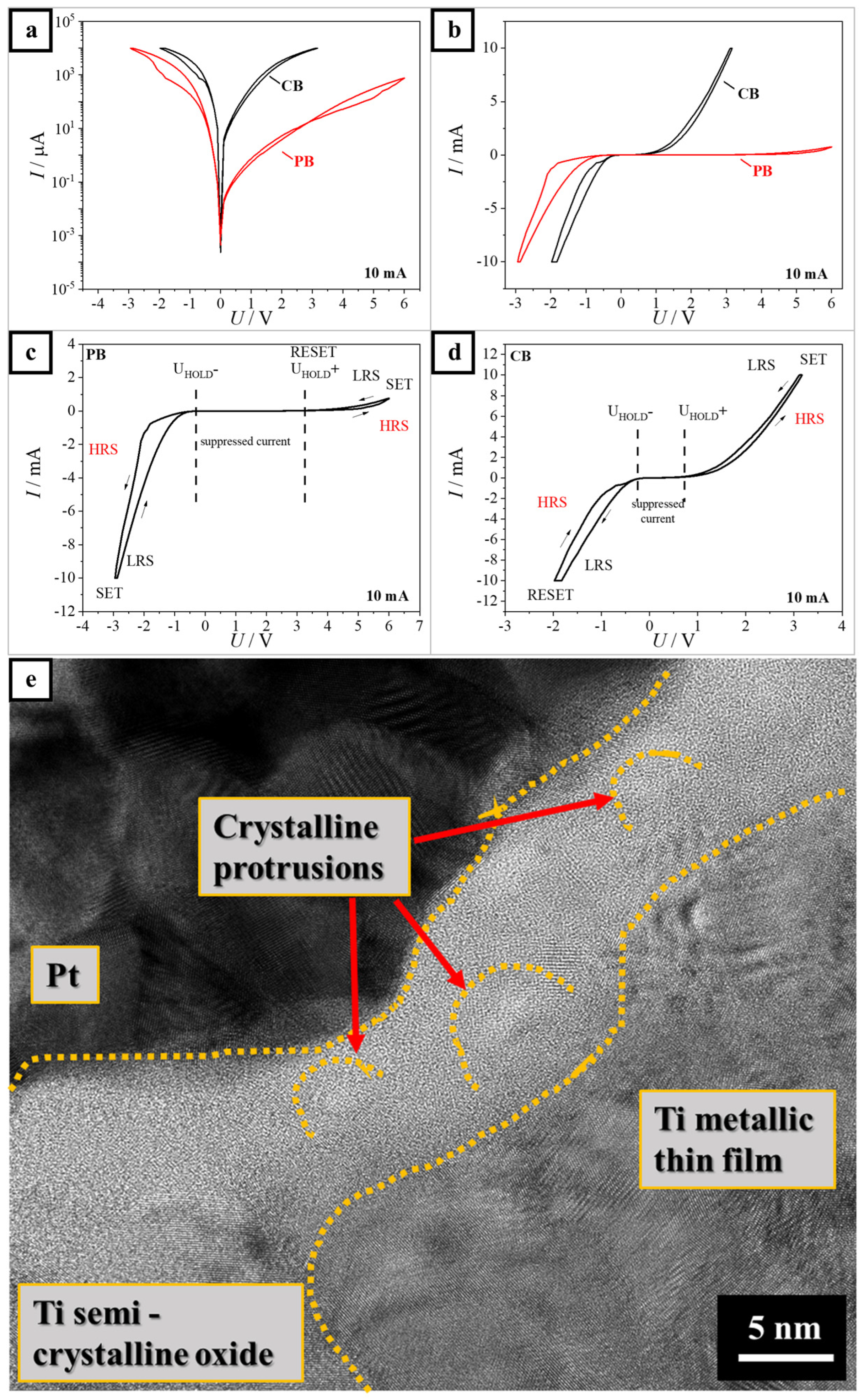
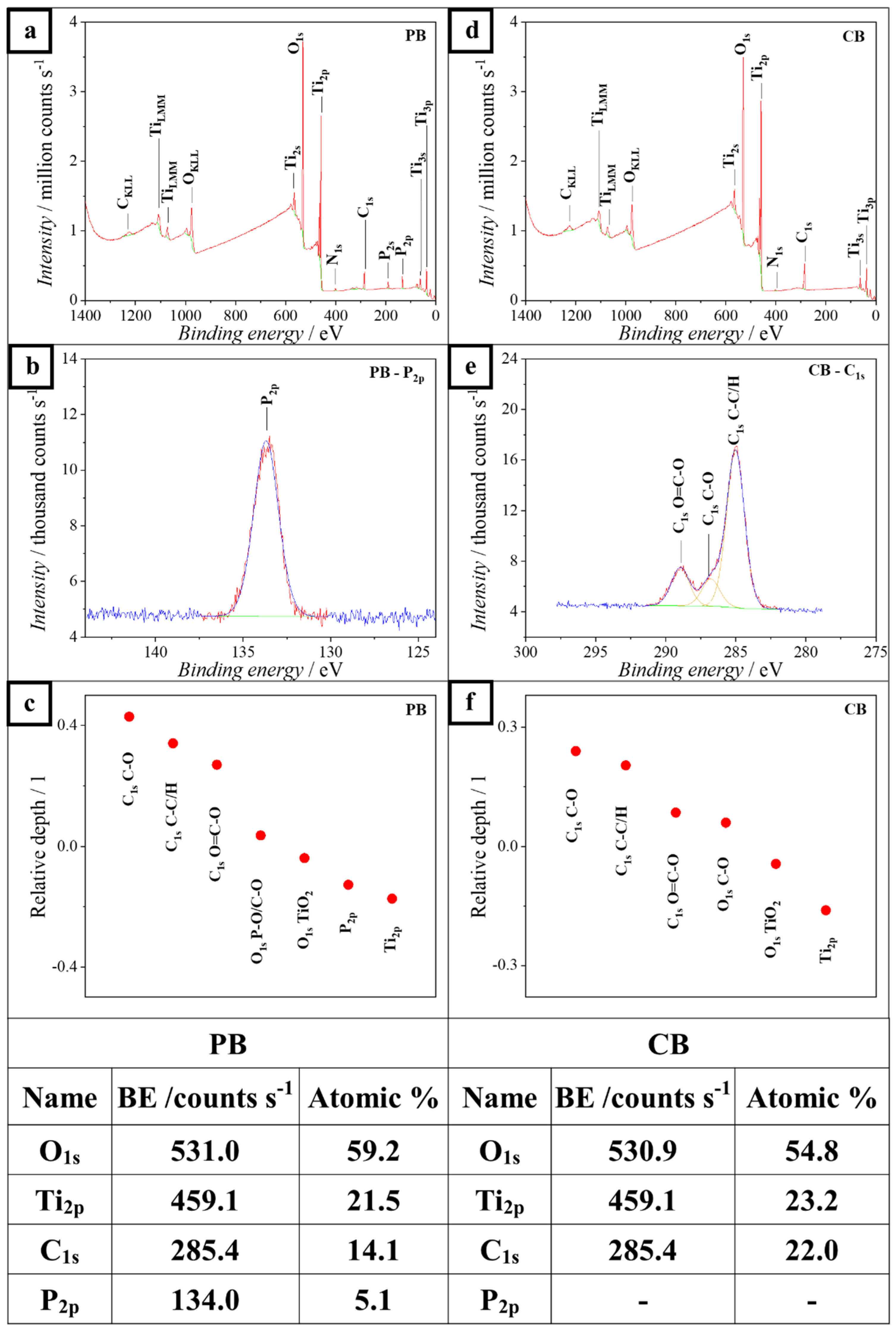
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.-Q.; Mao, J.-Y.; Wang, Z.-P.; Wu, S.; Zhou, M.; Chen, T.; Zhou, Y.; Han, S.-T. Recent Advances of Volatile Memristors: Devices, Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000055. [Google Scholar] [CrossRef]
- Aglieri, V.; Zaffora, A.; Lullo, G.; Santamaria, M.; Di Franco, F.; Lo Cicero, U.; Mosca, M.; Macaluso, R. Resistive switching in microscale anodic titanium dioxide-based memristors. Superlattices Microstruct. 2018, 113, 135–142. [Google Scholar] [CrossRef]
- Zrinski, I.; Zavašnik, J.; Duchoslav, J.; Hassel, A.W.; Mardare, A.I. Threshold Switching in Forming-Free Anodic Memristors Grown on Hf-Nb Combinatorial Thin-Film Alloys. Nanomaterials 2022, 12, 3944. [Google Scholar] [CrossRef]
- Kundale, S.S.; Kamble, G.U.; Patil, P.P.; Patil, S.L.; Rokade, K.A.; Khot, A.C.; Nirmal, K.A.; Kamat, R.K.; Kim, K.H.; An, H.-M.; et al. Review of Electrochemically Synthesized Resistive Switching Devices: Memory Storage, Neuromorphic Computing, and Sensing Applications. Nanomaterials 2023, 13, 1879. [Google Scholar] [CrossRef]
- Zrinski, I.; Minenkov, A.; Mardare, C.C.; Kollender, J.P.; Lone, S.A.; Hassel, A.W.; Mardare, A.I. Influence of electrolyte selection on performance of tantalum anodic oxide memristors. Appl. Surf. Sci. 2021, 565, 150608. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chou, T.-S.; Huang, J.-S.; Lin, S.-M.; Chueh, Y.-L. Self-Selecting Resistive Switching Scheme Using TiO2 Nanorod Arrays. Sci. Rep. 2017, 7, 2066. [Google Scholar] [CrossRef]
- Zrinski, I.; Mardare, C.C.; Jinga, L.-I.; Kollender, J.P.; Socol, G.; Minenkov, A.; Hassel, A.W.; Mardare, A.I. Electrolyte-Dependent Modification of Resistive Switching in Anodic Hafnia. Nanomaterials 2021, 11, 666. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, P.; Fu, L.; Li, R.; Gao, X.; Tao, C. Coexistence of diode-like volatile and multilevel nonvolatile resistive switching in a ZrO2/TiO2 stack structure. Nanotechnology 2015, 26, 391001. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Shi, T.; Wang, Y.; Wang, R.; Lu, J.; Wei, J.; Zhang, P.; Liu, Q. Convertible Volatile and non-Volatile Resistive Switching in a Self-Rectifying Pt/TiOx/Ti Memristor. In Proceedings of the 2021 5th IEEE Electron Devices Technology & Manufacturing Conference (EDTM), Chengdu, China, 8–11 April 2021; pp. 1–3. [Google Scholar] [CrossRef]
- Kim, M.; Rehman, M.A.; Lee, D.; Wang, Y.; Lim, D.-H.; Khan, M.F.; Choi, H.; Shao, Q.Y.; Suh, J.; Lee, H.-S.; et al. Filamentary and Interface-Type Memristors Based on Tantalum Oxide for Energy-Efficient Neuromorphic Hardware. ACS Appl. Mater. Interfaces 2022, 14, 44561–44571. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, B.; Lee, Y.; Kim, D.; Cho, Y.; Kim, S. Prospects and applications of volatile memristors. Appl. Phys. Lett. 2022, 121, 010501. [Google Scholar] [CrossRef]
- Kunwar, S.; Somodi, C.B.; Lalk, R.A.; Rutherford, B.X.; Corey, Z.; Roy, P.; Zhang, D.; Hellenbrand, M.; Xiao, M.; MacManus-Driscoll, J.L.; et al. Protons: Critical Species for Resistive Switching in Interface-Type Memristors. Adv. Elect. Mater. 2023, 9, 2200816. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Li, Y.; Jiang, H.; Ge, N.; Montgomery, E.; Zhang, J.; Song, W.; Dávila, N.; Graves, C.E.; et al. Analogue signal and image processing with large memristor crossbars. Nat. Electron. 2018, 1, 52–59. [Google Scholar] [CrossRef]
- Ji, X.; Dong, Z.; Zhou, G.; Lai, C.S.; Yan, Y.; Qi, D. Memristive System Based Image Processing Technology: A Review and Perspective. Electronics 2021, 10, 3176. [Google Scholar] [CrossRef]
- Zrinski, I.; Knapic, D.; Hassel, A.W.; Mardare, A.I. Anodic HfO2 crossbar arrays for hydroxide-based memristive sensing in liquids. J. Electrochem. Sci. Eng. 2023, 13, 805–815. [Google Scholar] [CrossRef]
- Mohamad Hadis, N.S.; Abd Manaf, A.; Ngalim, S.H.; Herman, S.H. Fabrication and characterisation of fluidic based memristor sensor for liquid with hydroxyl group. Sens. Bio-Sens. Res. 2017, 14, 21–29. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Lei, M.; Zhao, Y.; Sun, B.; Elshekh, H.; Zheng, L.; Zhang, X.; Hou, W. The pH-controlled memristive effect in a sustainable bioelectronic device prepared using lotus root. Mater. Today Sustain. 2020, 7–8, 100029. [Google Scholar] [CrossRef]
- Čajko, K.O.; Sekulić, D.L.; Lukić-Petrović, S.R. Dielectric and bipolar resistive switching properties of Ag doped As–S–Se chalcogenide for non-volatile memory applications. Mater. Chem. Phys. 2023, 296, 127301. [Google Scholar] [CrossRef]
- Lee, W.; Iqbal, S.; Kim, J.; Lee, S.; Lee, J.; Kumar, M.; Seo, H. Vanadium oxide thin film deposited on Si by atomic layer deposition for non-volatile resistive switching memory devices. Appl. Surf. Sci. 2023, 639, 158240. [Google Scholar] [CrossRef]
- Patil, A.R.; Dongale, T.D.; Namade, L.D.; Mohite, S.V.; Kim, Y.; Sutar, S.S.; Kamat, R.K.; Rajpure, K.Y. Sprayed FeWO4 thin film-based memristive device with negative differential resistance effect for non-volatile memory and synaptic learning applications. J. Colloid Interface Sci. 2023, 642, 540–553. [Google Scholar] [CrossRef]
- Miller, K.; Nalwa, K.S.; Bergerud, A.; Neihart, N.M.; Chaudhary, S. Memristive Behavior in Thin Anodic Titania. IEEE Electron Device Lett. 2010, 31, 737–739. [Google Scholar] [CrossRef]
- Moon, K.; Fumarola, A.; Sidler, S.; Jang, J.; Narayanan, P.; Shelby, R.M.; Burr, G.W.; Hwang, H. Bidirectional Non-Filamentary RRAM as an Analog Neuromorphic Synapse, Part I: Al/Mo/Pr 0.7 Ca 0.3 MnO 3 Material Improvements and Device Measurements. IEEE J. Electron Devices Soc. 2018, 6, 146–155. [Google Scholar] [CrossRef]
- Gutsche, A.; Siegel, S.; Zhang, J.; Hambsch, S.; Dittmann, R. Exploring Area-Dependent Pr0.7Ca0.3MnO3-Based Memristive Devices as Synapses in Spiking and Artificial Neural Networks. Front. Neurosci. 2021, 15, 661261. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, S.; Michalas, L.; Khiat, A.; Serb, A.; Prodromakis, T. An Electrical Characterisation Methodology for Benchmarking Memristive Device Technologies. Sci. Rep. 2019, 9, 19412. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Chang, T.; Ebong, I.; Bhadviya, B.B.; Mazumder, P.; Lu, W. Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett. 2010, 10, 1297–1301. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, W.; Li, C.; Huang, J. Forgetting memristors and memristor bridge synapses with long- and short-term memories. Neurocomputing 2021, 456, 126–135. [Google Scholar] [CrossRef]
- Yan, B.; Mahmoud, A.M.; Yang, J.J.; Wu, Q.; Chen, Y.; Li, H.H. A neuromorphic ASIC design using one-selector-one-memristor crossbar. In Proceedings of the 2016 IEEE International Symposium on Circuits and Systems (ISCAS), Montréal, QC, Canada, 22–25 May 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1390–1393, ISBN 978-1-4799-5341-7. [Google Scholar]
- Xia, Q.; Yang, J.J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 2019, 18, 309–323. [Google Scholar] [CrossRef]
- Pyo, J.; Kim, S. Non-volatile and volatile switching behaviors determined by first reset in Ag/TaO /TiN device for neuromorphic system. J. Alloys Compd. 2022, 896, 163075. [Google Scholar] [CrossRef]
- Knapic, D.; Muck, M.; Heitz, J.; Baumgartner, W.; Mardare, A.I.; Kleber, C.; Hassel, A.W. Electrochemical and surface characterization of anodized and fs-laser treated Ti6Al4V for osseo-repellent bone screws and dental implants. Electrochim. Acta 2023, 466, 142965. [Google Scholar] [CrossRef]
- Zrinski, I.; Löfler, M.; Zavašnik, J.; Cancellieri, C.; Jeurgens, L.P.H.; Hassel, A.W.; Mardare, A.I. Impact of Electrolyte Incorporation in Anodized Niobium on Its Resistive Switching. Nanomaterials 2022, 12, 813. [Google Scholar] [CrossRef]
- Citrate Buffer (0.01 m, pH 5.6–6). Cold Spring Harb. Protoc. 2014, 2014, pdb.rec085159. [CrossRef]
- Sodium phosphate. Cold Spring Harb. Protoc 2006, 2006, pdb.rec8303. [CrossRef]
- Kwon, S.; Kim, T.-W.; Jang, S.; Lee, J.-H.; Kim, N.D.; Ji, Y.; Lee, C.-H.; Tour, J.M.; Wang, G. Structurally Engineered Nanoporous Ta2O5-x Selector-Less Memristor for High Uniformity and Low Power Consumption. ACS Appl. Mater. Interfaces 2017, 9, 34015–34023. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, B.; Tang, J.; Li, X.; Wu, W.; Qian, H.; Wu, H. Analog-Type Resistive Switching Devices for Neuromorphic Computing. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1900204. [Google Scholar] [CrossRef]
- Aglieri, V.; Lullo, G.; Mosca, M.; Macaluso, R.; Zaffora, A.; Di Franco, F.; Santamaria, M.; Lo Cicero, U.; Razzari, L. Forming-Free and Self-Rectifying Resistive Switching Effect in Anodic Titanium Dioxide-Based Memristors. In Proceedings of the 2018 IEEE 4th International Forum on Research and Technology for Society and Industry (RTSI), Palermo, Italy, 10–13 September 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Huang, J.-J.; Kuo, C.-W.; Chang, W.-C.; Hou, T.-H. Transition of stable rectification to resistive-switching in Ti/TiO2/Pt oxide diode. Appl. Phys. Lett. 2010, 96, 262901. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, G.; Tian, B.; Dkhil, B.; Duan, C. Research progress on solutions to the sneak path issue in memristor crossbar arrays. Nanoscale Adv. 2020, 2, 1811–1827. [Google Scholar] [CrossRef]
- Li, Z.; Tang, W.; Zhang, B.; Yang, R.; Miao, X. Emerging memristive neurons for neuromorphic computing and sensing. Sci. Technol. Adv. Mater. 2023, 24, 2188878. [Google Scholar] [CrossRef]
- Yang, R.; Terabe, K.; Yao, Y.; Tsuruoka, T.; Hasegawa, T.; Gimzewski, J.K.; Aono, M. Synaptic plasticity and memory functions achieved in a WO3-x-based nanoionics device by using the principle of atomic switch operation. Nanotechnology 2013, 24, 384003. [Google Scholar] [CrossRef]
- Hellenbrand, M.; MacManus-Driscoll, J. Multi-level resistive switching in hafnium-oxide-based devices for neuromorphic computing. Nano Converg. 2023, 10, 44. [Google Scholar] [CrossRef]
- Hellenbrand, M.; Bakhit, B.; Dou, H.; Xiao, M.; Hill, M.O.; Sun, Z.; Mehonic, A.; Chen, A.; Jia, Q.; Wang, H.; et al. Thin-film design of amorphous hafnium oxide nanocomposites enabling strong interfacial resistive switching uniformity. Sci. Adv. 2023, 9, eadg1946. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Tang, W.-W.; Tang, X.-G.; Liu, Q.-X.; Jiang, Y.-P.; Li, W.-H.; Tang, Z.-H.; Guo, X.-B.; Tang, Z.-F. Analog-type resistive switching behavior of Au/HfO2/ZnO memristor fabricated on flexible Mica substrate. Phys. E Low-Dimens. Syst. Nanostructures 2020, 120, 114047. [Google Scholar] [CrossRef]
- Chiu, F.-C. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Sci. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef]
- Lim, E.; Ismail, R. Conduction Mechanism of Valence Change Resistive Switching Memory: A Survey. Electronics 2015, 4, 586–613. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Deng, N.; Shih, C.-C.; Tseng, Y.-T.; Chang, T.-C.; Chang, K.-C.; Wang, M.-H.; Chen, W.-C.; Zheng, H.-X.; Wu, H.; et al. Conduction Mechanism and Improved Endurance in HfO2-Based RRAM with Nitridation Treatment. Nanoscale Res. Lett. 2017, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Das, M.; Mukherjee, S. Oxide Based Memristors: Fabrication, Mechanism, and Application; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Im, H.; Kim, H.; Park, B. Leakage Transport in the High-resistance State of a Resistive-switching NbOx Thin Film Prepared by Pulsed Laser Deposition. J. Korean Phy. Soc. 2011, 59, 2778–2781. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, S.; Hong, K.; Park, J.; Hwang, Y.; Park, B.-G.; Kim, H. Multilevel switching memristor by compliance current adjustment for off-chip training of neuromorphic system. Chaos Solitons Fractals 2021, 153, 111587. [Google Scholar] [CrossRef]
- Ingo, G.M.; Dirè, S.; Babonneau, F. XPS studies of SiO2-TiO2 powders prepared by sol-gel process. Appl. Surf. Sci. 1993, 70–71, 230–234. [Google Scholar] [CrossRef]
- Bender, H.; Chen, W.D.; Portillo, J.; van den Hove, L.; Vandervorst, W. AES and XPS analysis of the interaction of Ti with Si and SiO2 during RTA. Appl. Surf. Sci. 1989, 38, 37–47. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Uosaki, K. Preparation of Tantalum Anodic Oxide Film in Citric Acid Solution—Evidence and Effects of Citrate Anion Incorporation. J. Electrochem. Sci. Technol. 2013, 4, 163–170. [Google Scholar] [CrossRef]
- Arndt, M.; Duchoslav, J.; Itani, H.; Hesser, G.; Riener, C.K.; Angeli, G.; Preis, K.; Stifter, D.; Hingerl, K. Nanoscale analysis of surface oxides on ZnMgAl hot-dip-coated steel sheets. Anal. Bioanal. Chem. 2012, 403, 651–661. [Google Scholar] [CrossRef]
- Duchoslav, J.; Arndt, M.; Steinberger, R.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Riener, C.K.; Angeli, G.; Stifter, D. Nanoscopic view on the initial stages of corrosion of hot dip galvanized Zn–Mg–Al coatings. Corros. Sci. 2014, 83, 327–334. [Google Scholar] [CrossRef]
- Marino, C.E.; Nascente, P.A.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. XPS characterization of anodic titanium oxide films grown in phosphate buffer solutions. Thin Solid Films 2004, 468, 109–112. [Google Scholar] [CrossRef]
- Zrinski, I.; Mardare, C.C.; Jinga, L.-I.; Kollender, J.P.; Socol, G.; Hassel, A.W.; Mardare, A.I. Phosphate incorporation in anodic hafnium oxide memristors. Appl. Surf. Sci. 2021, 548, 149093. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapic, D.; Atanasova, E.; Zrinski, I.; Hassel, A.W.; Mardare, A.I. Electrolyte Influence on Properties of Ultra-Thin Anodic Memristors on Titanium. Coatings 2024, 14, 446. https://doi.org/10.3390/coatings14040446
Knapic D, Atanasova E, Zrinski I, Hassel AW, Mardare AI. Electrolyte Influence on Properties of Ultra-Thin Anodic Memristors on Titanium. Coatings. 2024; 14(4):446. https://doi.org/10.3390/coatings14040446
Chicago/Turabian StyleKnapic, Dominik, Elena Atanasova, Ivana Zrinski, Achim Walter Hassel, and Andrei Ionut Mardare. 2024. "Electrolyte Influence on Properties of Ultra-Thin Anodic Memristors on Titanium" Coatings 14, no. 4: 446. https://doi.org/10.3390/coatings14040446




