Abstract
Polyaniline (PANI) doped with hydrochloric acid and phytic acid are prepared as benzotriazole (BTA) inhibitor carriers, and their anticorrosion properties are studied on epoxy resin-coated Q235 steel. The structure and morphology of the prepared PANI materials are investigated by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The thermal stability of PANI and its release rate of corrosion inhibitor are measured, and the test results indicate that hydrochloric acid-doped PANI can accommodate a larger amount of corrosion inhibitor. The results of contact angle and water absorption tests show that the PANI loaded with BTA can improve the hydrophobicity and reduce the water absorption of the coating. The anticorrosion performances of epoxy coatings with 0.6 wt% PANI are investigated in 3.5 wt% NaCl solution using electrochemical tests. The experimental results reveal that PANI loaded with BTA shows a good anticorrosion effect in the epoxy coating. Particularly, phytic acid-doped PANI loaded with BTA has a better inhibition efficiency (93.6%), which is superior to hydrochloric acid-doped PANI loaded with BTA (86.4%).
1. Introduction
As marine resources are developed, more metallic materials are used in constructing marine engineering facilities [1]. Nevertheless, the marine corrosion environment, particularly in the South China Sea, is exceptionally severe, marked by high temperatures, humidity, and salinity, alongside abundant marine microorganisms and macroorganisms in the seawater [2,3,4]. Equipment operating in such conditions is susceptible to metal corrosion, resulting in environmental pollution, economic losses, and potential safety hazards. To address the issues caused by corrosion damage, various approaches have been proposed, including the addition of corrosion inhibitors to concrete [5,6,7], the use of electrochemical protection methods [8], and the application of metal coating techniques [9,10]. The application of protective coatings stands out as an economically viable and effective anti-corrosion strategy. However, it is very common for coatings to suffer mechanical or chemical losses in the application process. Consequently, it is necessary to incorporate functional corrosion inhibitors into the coating, thereby enhancing its corrosion resistance and mechanical properties [11]. Research findings indicate that the direct addition of corrosion inhibitors to organic coatings may induce interactions with the resin, thereby compromising the shielding effectiveness of the coating and resulting in its failure [12,13]. Therefore, scholars encapsulate the corrosion inhibitor in an inert shell, such as polymer microcapsules [14], polyelectrolyte shell [15], or inorganic nano-particulate [16], to avoid direct contact between the corrosion inhibitor and the coating. Remarkably, introducing inhibitors into coatings this way can prevent coating failure and enhance the corrosion resistance of the coating, and certain coatings even exhibit self-healing capabilities in the presence of defects [17,18,19].
Polyaniline (PANI) is a chain polymer composed of oxidation units and reduction units. It can effectively reduce the corrosion of metal in acidic environments [20,21], and shows better physical properties and corrosion resistance after doping using protonic acid [22,23]. Processing stable corrosion inhibition performance, benzotriazole (BTA) is widely used in the anticorrosion research of metals [24,25,26]. Recently, an increasing number of studies have introduced PANI in various forms as the carriers for loading inhibitor. Huang [27] investigated PANI nanocapsules, which could release the BTA corrosion inhibitor in a controlled manner depending on the concentration change of NaCl. Vimalanandan [28] proposed a conducting polymer-based nanocapsule system, which comprises a redox-sensitive PANI shell decorated with gold nanoparticles and a self-healing agent encapsulated in the core. Liu [29] reported PANI-modified halloysite nanotubes to load the inhibitor BTA. Hao [30] obtained a mesoporous PANI hollow sphere and the corrosion inhibitor entered the cavity of hollow sphere through the large pores of the mesoporous PANI. These containers loaded with corrosion inhibitors have good corrosion inhibition effects, but the preparation methods are very complex. Hao [31] doped PANI with phytic acid to obtain fibrous PANI and selected the BTA inhibitor to infuse onto its rough surface, and it was found that the anticorrosion ability of PA-doped PANI was enhanced when it was used as a container loaded with BTA, and it had better anti-corrosion performance than other protonic acid-doped PANI. However, the anticorrosion effect of the other protonic acid-doped PANI loaded with BTA was not compared. In previous experiments [32], we compared the anticorrosion effects of organic acid and inorganic acid doped PANI and simultaneously found out that PANI synthesized by the chemical oxidation method has a rough surface onto which corrosion inhibitors can be adsorbed [33].
In the present work, we investigated the effects of HCl and PA on the modified preparation of PANI as corrosion inhibitor carriers (recorded as HCl-PANI and PA-PANI, respectively). Simultaneously, BTA was loaded separately to explore their anticorrosion performance. Various physicochemical analytical methods were used to characterize HCl-PANI and PA-PANI with or without BTA loading. Epoxy coatings based on HCl-PANI and PA-PANI loaded with BTA were coated on the surfaces of Q235 carbon steel, and their anticorrosion performance was evaluated through electrochemical measurements while immersed in 3.5 wt% NaCl solution. The anticorrosion effects of HCl-PANI and PA-PANI with or without BTA loading were compared, and the corrosion inhibition mechanisms of PANI and BTA were discussed.
2. Materials and Experimental Methods
2.1. Materials
Aniline monomer (ANI, AR) and Nmethyl-2-pyrrolidone (NMP, AR) were purchased from Shanghai Aladdin Reagent Co., Ltd., Shanghai, China. Hydrochloric acid (HCl, AR), ammonium persulfate (APS, AR), sodium chloride (NaCl, AR), ammonium hydroxide, and absolute ethyl alcohol were acquired from the Guangzhou Chemical Reagent Factory, Guangzhou, China. Phytic acid (PA, AR) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China. N-butyl alcohol, xylene, epoxy resin (E44), and polyamide hardener (650) were provided by Zhenjiang Danbao Resin Co., Ltd., Zhenjiang, China. Benzotriazole (BTA, AR) was purchased from Tianjin Damao chemical reagent factory, Tianjin, China. Deionized water was laboratory prepared.
2.2. Synthesis of PANI Materials
Polyaniline was prepared using a chemical oxidation method. An amount of 0.1 mol of aniline monomer after secondary vacuum distillation was dissolved in 100 mL of 1 mol/L HCl solution, and then 100 mL of 1 mol/L APS solution was added dropwise as an oxidizing agent into the aniline and HCl mixture solution. After continuously stirring at 160 r/min for over 8 h, the PANI suspension was obtained, which was a kind of thick dark green mixture. The suspension was filtered using a vacuum pump, followed by washing with deionized water until the filtrate was colorless and the pH of the filtrate was 7. After vacuum drying at 60 °C and the subsequent grinding into powder, polyaniline doped with HCl was obtained, which was denoted as HCl-PANI.
Due to the inferior water solubility of some macromolecular organic acids, it is difficult to prepare polyaniline in organic acid system using direct mixing reaction, but it can be obtained by redropping the emeraldine base form of polyaniline (EB-PANI) with organic acids, which can introduce functional groups without damaging its morphology [34,35]. Adding the synthesized HCl-PANI powder into an appropriate amount of ammonia solution and stirring for 24 h and subsequently washing with deionized water and drying at 60 °C, the reddish-brown EB-PANI was obtained. An appropriate amount of EB-PANI powder was added into 0.1 mol/L PA solution and stirred for over 8 h. Then, the mixture was filtered using vacuum extraction and washed with deionized water repeatedly. When the pH of the filtrate reached 7, the residue was dried in a vacuum drying oven and then ground into powder. Finally, polyaniline doped with PA (PA-PANI) was obtained.
A total of 6.32 g of BTA was dissolved in 200 mL anhydrous ethanol, and then 0.5 g of HCl-PANI was added into the mixture. After continuous stirring, washing, filtration vacuum drying, and grinding as above, HCl-doped polyaniline loaded with BTA (HCl-BTA-PANI) was obtained. Replacing HCl-PANI with PA-PANI, PA-doped polyaniline loaded with BTA (PA-BTA-PANI) was obtained following the same steps.
2.3. Preparation of PANI/Epoxy Resin Coatings
In this study, Q235 carbon steel was used in the corrosion tests. Before preparing the coating, the steel specimen was abraded with metallographic papers (180#, 360#, 500#, 800#, and 1000# grade) in turn, followed by the subsequent rinsing with anhydrous ethanol and deionized water, and then dried for use.
After grinding with mortar and pestle and sifting through a 200-mesh filter, 0.078 g of the prepared PANI powder was added into 3 g diluent (xylene:n-butanol= 7:3 w/w) for ultrasonic dispersion for 30 min. Subsequently, 5 g of epoxy resin and 5 g of polyamide hardener was added to the dispersion and stirred for 30 min at 800 r/min to process polyaniline-epoxy resin paint with 0.6%wt PANI. Then, the paint was applied on steel samples and dried for 7 days; the thickness of dry coatings was about 120 ± 20 μm. Coatings with different PANI materials were prepared, which were epoxy coatings with HCl-PANI (HCl-PANI/EP), PA-PANI (PA-PANI/EP), HCl-BTA-PANI (HCl-BTA-PANI/EP), and PA-BTA-PANI (PA-BTA-PANI/EP).
2.4. Characterization Methods
2.4.1. PANI Materials
The Fourier transform infrared spectra (FTIR, VERTX 70, Brucker, Karlsruhe, Germany) was used to analyze the structure and functional groups of the synthesized polyaniline materials in KBr pellets by employing wavelength range scanning from 4000 to 400 cm−1. The effects of BTA on the structure of polyaniline and the structure of different polyaniline materials were analyzed by Raman spectroscopy (Raman, LabRAM Aramis, Jobin Yvon, Paris, France).
The scanning electron microscopy (SEM, S-3700N, Hitachi, Tokyo, Japan) was used to observe the morphology of PANI materials. Before putting the samples into the sample chamber, a small amount of PANI powder was dispersed in anhydrous ethanol for over 30 min and then a drop of it was dropped onto a flat copper sheet, and then gilded after the powder had dried. In addition, the surface morphologies of the coating and the corrosion morphologies of the metals were presented by SEM.
The thermogravimetric analysis (TGA, TG-209F1, NETZSCH, Selb, Germany) was employed to characterize thermal stability of the polyaniline samples performed in nitrogen atmosphere from 30 °C to 900 °C with a heating rate of 20 °C/min.
The UV-Vis spectra (UV-Vis, UV755B, YOKE INSTRUMENT, Shanghai, China) was used to measure the released BTA concentration from polyaniline loaded with inhibitor in 3.5 wt% NaCl solution, which was performed in the wavelength range of 220–600 nm. The absorption peak at 258 nm belongs to BTA.
2.4.2. PANI Coatings
The water contact angle estimation (WCA, OCA40 Micro, Data Physics, Filderstadt, Germany) was carried out to analyze the wettability of the coating.
Water absorption tests were used to characterize the anticorrosive ability of the coatings. In order to avoid the impact of metal corrosion, glass was used as the template for the water absorption test of the coatings. The prepared paint was coated onto glasses of the same size and dried at room temperature. Then, the weight of each coated samples wiped with filtrate paper was recorded before and after being immersed in deionized water, which were, respectively, denoted as m0 and mt. The water absorption Qt was calculated by Equation (1) [36].
Qt = (mt − m0)/m0
The electrochemical impedance spectroscopy (EIS) of the coatings was tested with a Princeton 263A workstation (Princeton Applied Research Corporation, Princeton, NJ, USA) performing with the scanning frequency ranging from 10−2 to approximately 105 Hz and a voltage disturbance value (AC) of 20 mV. In addition, the potentiodynamic polarization curve was tested with the Princeton 263A workstation performing with a scanning range of −500 mV~500 mV with a speed of 0.5 mV/s. The electrochemical test was employed with a three-electrode electrochemical test system in 3.5 wt% NaCl solution, which consisted of a coated steel of 1 cm2 as the working electrode, a saturated calomel electrode (SCE) as the reference electrode and a platinum sheet as the counter electrode. The results were fitted with the Zsimp Win software (Version 3.30).
3. Results and Discussion
3.1. Characterization of PANI Materials
3.1.1. FTIR Spectroscopy
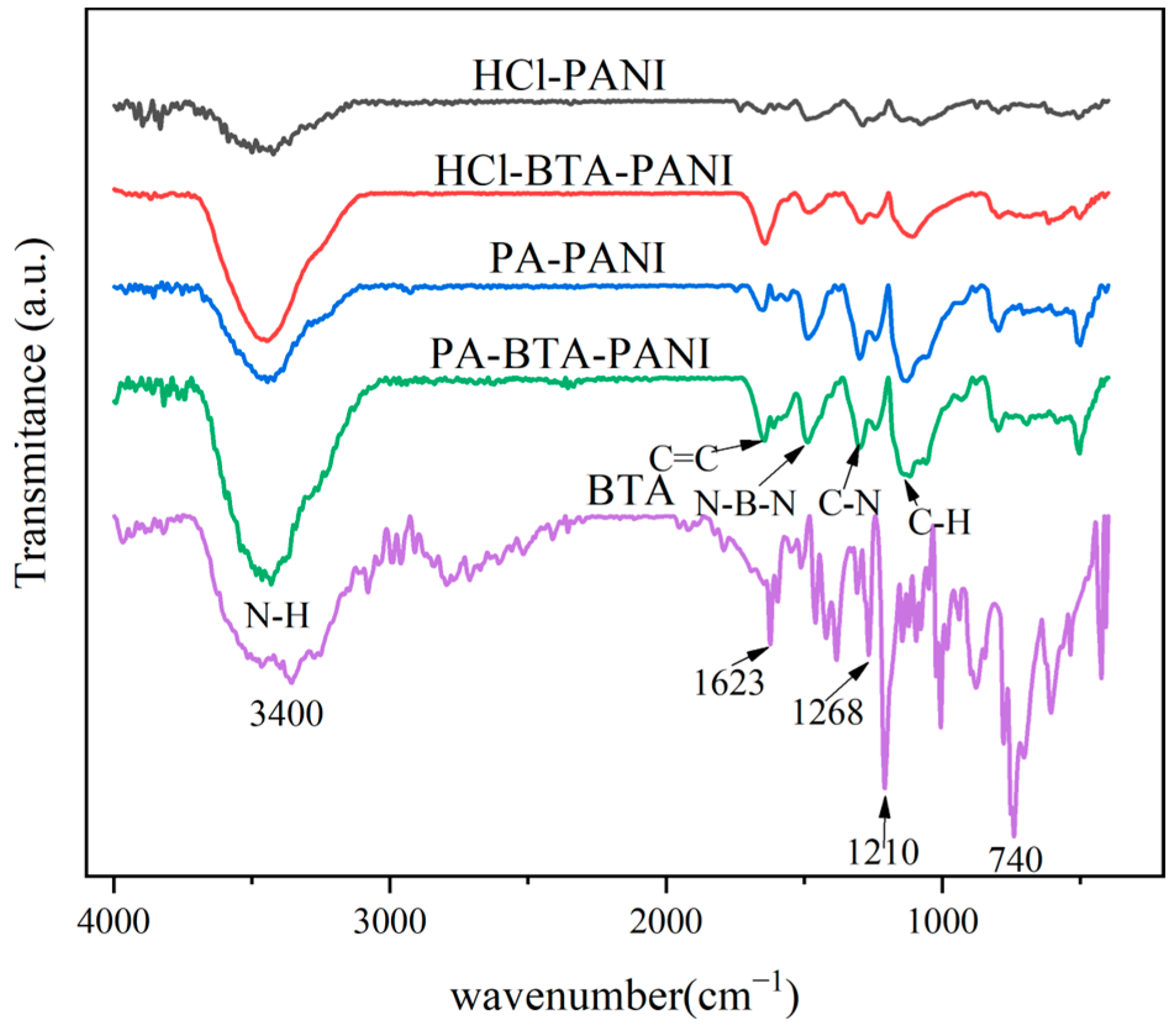
The infrared spectra for HCl-PANI, HCl-BTA-PANI, PA-PANI, PA-BTA-PANI, and BTA are presented in Figure 1. As shown in Figure 1, the modified PANI materials have typical PANI characteristic peaks. The peak at 3450 cm−1 corresponds to N-H stretching vibration, and the peak located at 1640 cm−1 is attributed to C-C stretching vibration. The peaks observed at 1596 cm−1 and 1490 cm−1 correspond to the stretching of the N=Q=N and N-B-N, among which Q is quinone and B is benzene ring. The peak located at 1290 cm−1 is assigned to the absorption vibration peak of aromatic amine C-N [37]. The peaks observed at 1161 cm−1 and 822 cm−1 are assigned to C-H stretching vibration in and outside the benzene torus [38]. The infrared spectrum test results of modified polyaniline materials are consistent with relevant research results [37,38,39]. The absorption peaks of PA-PANI and PA-BTA-PANI at 1122 cm−1 are wider than that of HCl-PANI and HCl-BTA-PANI, possibly due to the overlap of the P=O stretching vibration peak at 1044 cm−1 with the phosphate group at 960 cm−1 [40].
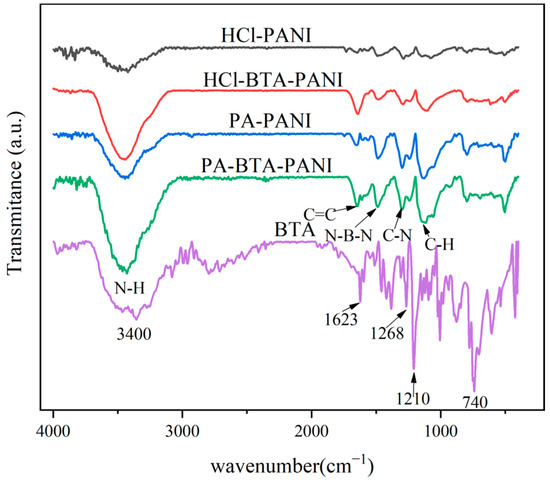
Figure 1.
FTIR spectra of polyaniline materials and BTA.
The characteristic peaks of BTA structure can be seen in the infrared spectrum. The characteristic peak at 3400 cm−1 is confirmed as N-H stretching vibration and the peak at 1623 cm−1 is ascribed to C=C vibration. The peak at 1268 cm−1 is relevant to the stretching vibration of C-H and the peak at 1210 cm−1 is the vibration of N=N. In addition, the peak at 740 cm−1 is the stretching vibration in the benzene ring [41].
By comparing the infrared spectra of PANI materials and BTA, it is clear that certain characteristic peaks of PANI are enhanced after loading with BTA. These include N-H stretching vibration peak, C=C stretching vibration peak, C-N absorption vibration peak, and C-H bending vibration peak. The presence of BTA enhanced the characteristic peaks of PANI, indicating successful adsorption of BTA by HCl-BTA-PANI and PA-BTA-PANI, both of which have a similar structure to BTA.
3.1.2. Raman Spectroscopy
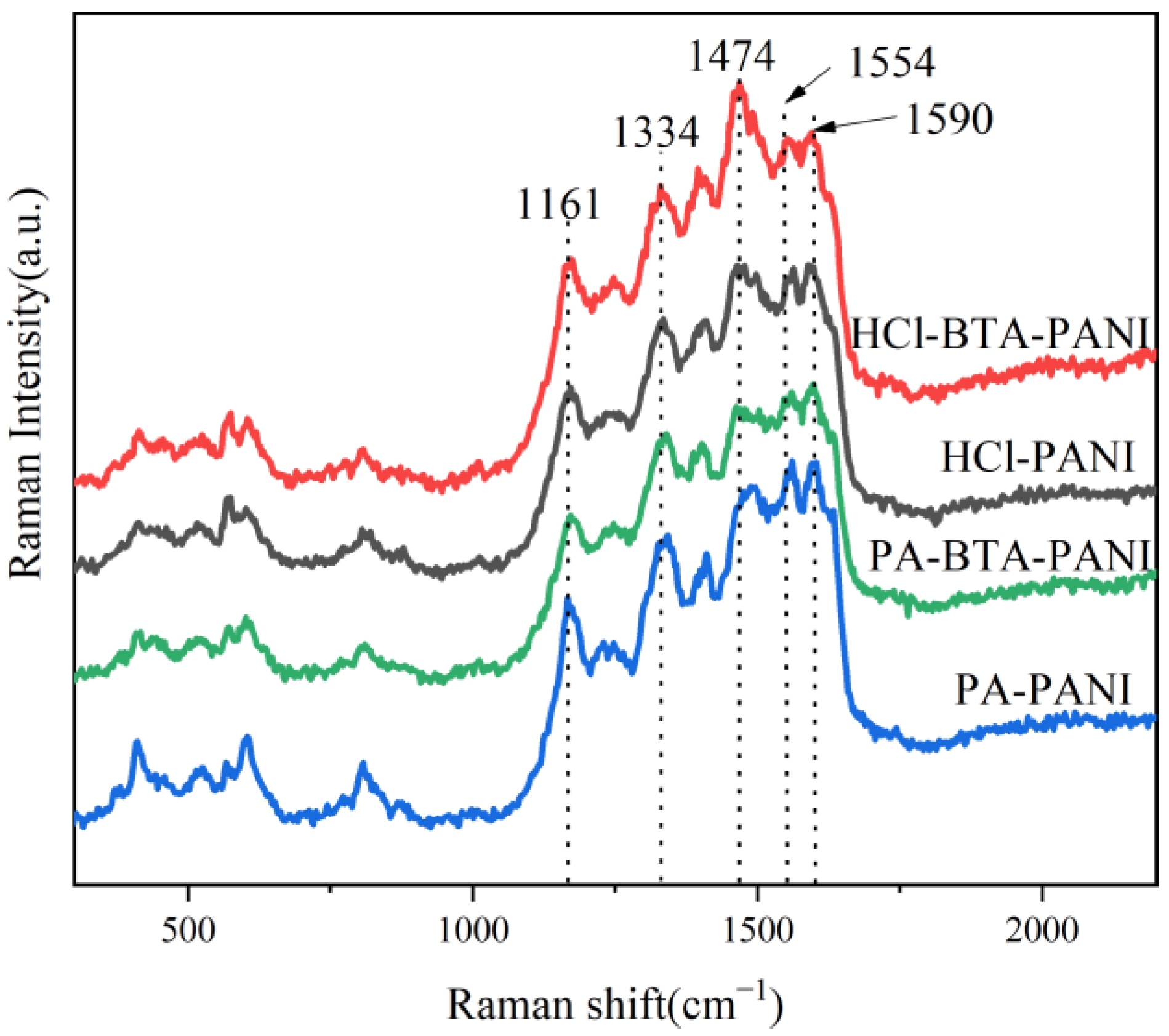
In Figure 2, the Raman spectra of HCl-PANI, HCl-BTA-PANI, PA-PANI, and PA-BTA-PANI are presented. HCl-BTA-PANI and PA-BTA-PANI maintain the typical PANI structure, evidenced by absorption peaks at 1161 cm−1, 1334 cm−1, 1554 cm−1, and 1590 cm−1. These peaks correspond to the C-H bond bending vibration in the benzene ring and quinone ring, the C-N+ stretching vibration in the semi-quinone ring, and the C-C stretching vibration on the benzene ring and quinone ring, respectively [42]. The intensity and width of these characteristic peaks remain relatively unchanged, indicating that BTA exerts no discernible impact on these structural aspects of PANI. Furthermore, the characteristic peaks at 1474 cm−1 of HCl-BTA-PANI and PA-BTA-PANI are more pronounced compared to HCl-PANI and PA-PANI. This enhancement is attributed to the N=C=N stretching vibration of quinone diimide and the joint stretching vibration of C=C and C=N. A possible explanation is that BTA changes the conjugation effect and the charge delocalization of PANI, leading to more chemical bonds with higher and wider characteristic peaks [43].
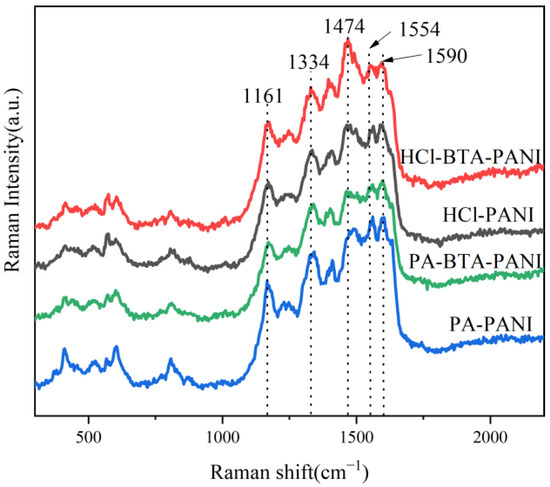
Figure 2.
Raman spectra of polyaniline materials and BTA.
3.1.3. Microscopic Morphology
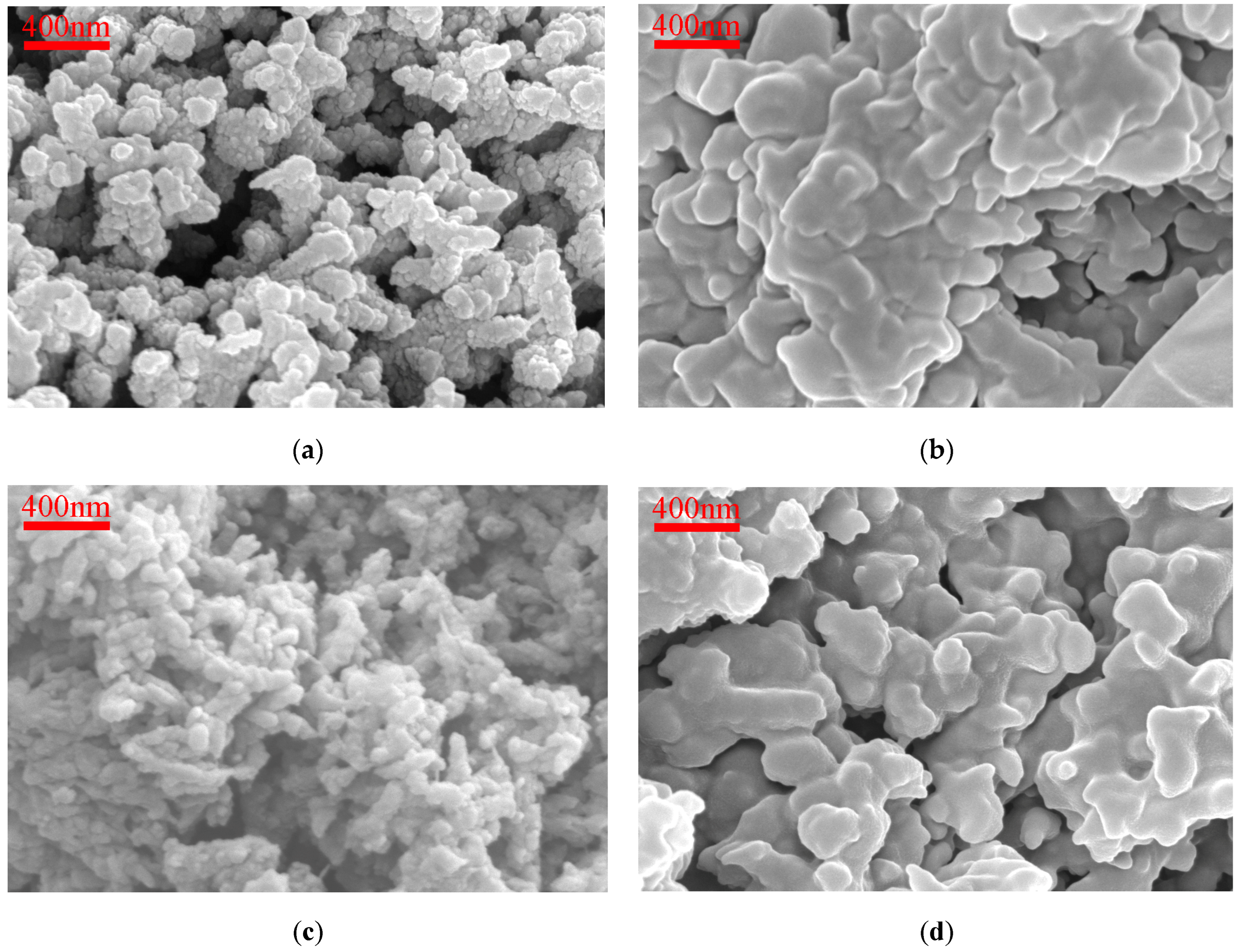
Different preparation methods and conditions can produce PANI with various morphologies. When PANI is of short oligomer, its morphology is mainly nanoplate [44], leaf-like structure [45], or nanosheet [46]. PANI appears in an amorphous phase when it is of higher oligomer, commonly observed under conditions involving strong oxidizing agents and high concentrations of aniline [47,48]. Figure 3 displays SEM images of HCl-PANI, PA-PANI, HCl-BTA-PANI, and PA-BTA-PANI magnified 40,000 times. In the images, PANI appears as an assembly of small, nonhomogeneous nanospheres stacked together to form short nanofibers [49], exhibiting an irregular recombination that results in a coral-shaped microstructure. The HCl-PANI nanofibers are less than 200 nm, presenting a short rod-like structure with a rough surface and a looser structure, which enhances the accommodation of BTA. PA-PANI fibers exhibit a tightly bonded coral-like structure with a smooth surface, providing a challenging environment for BTA adherence. As can be seen from the figure, the microscopic morphology of PANI shows minimal alteration before and after loading with BTA.
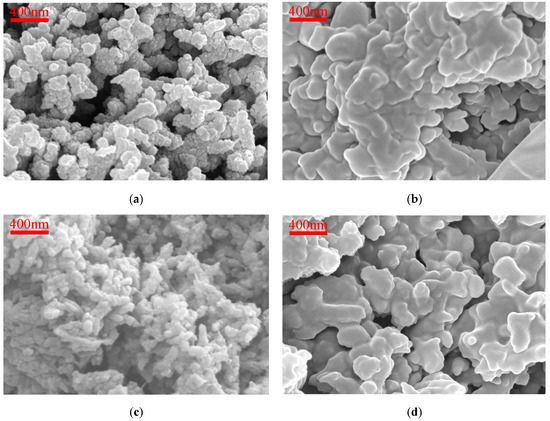
Figure 3.
SEM images (40,000×) for (a) HCl-PANI; (b) PA-PANI; (c) HCl-BTA-PANI; (d) PA-BTA-PANI.
3.1.4. Thermal Analysis
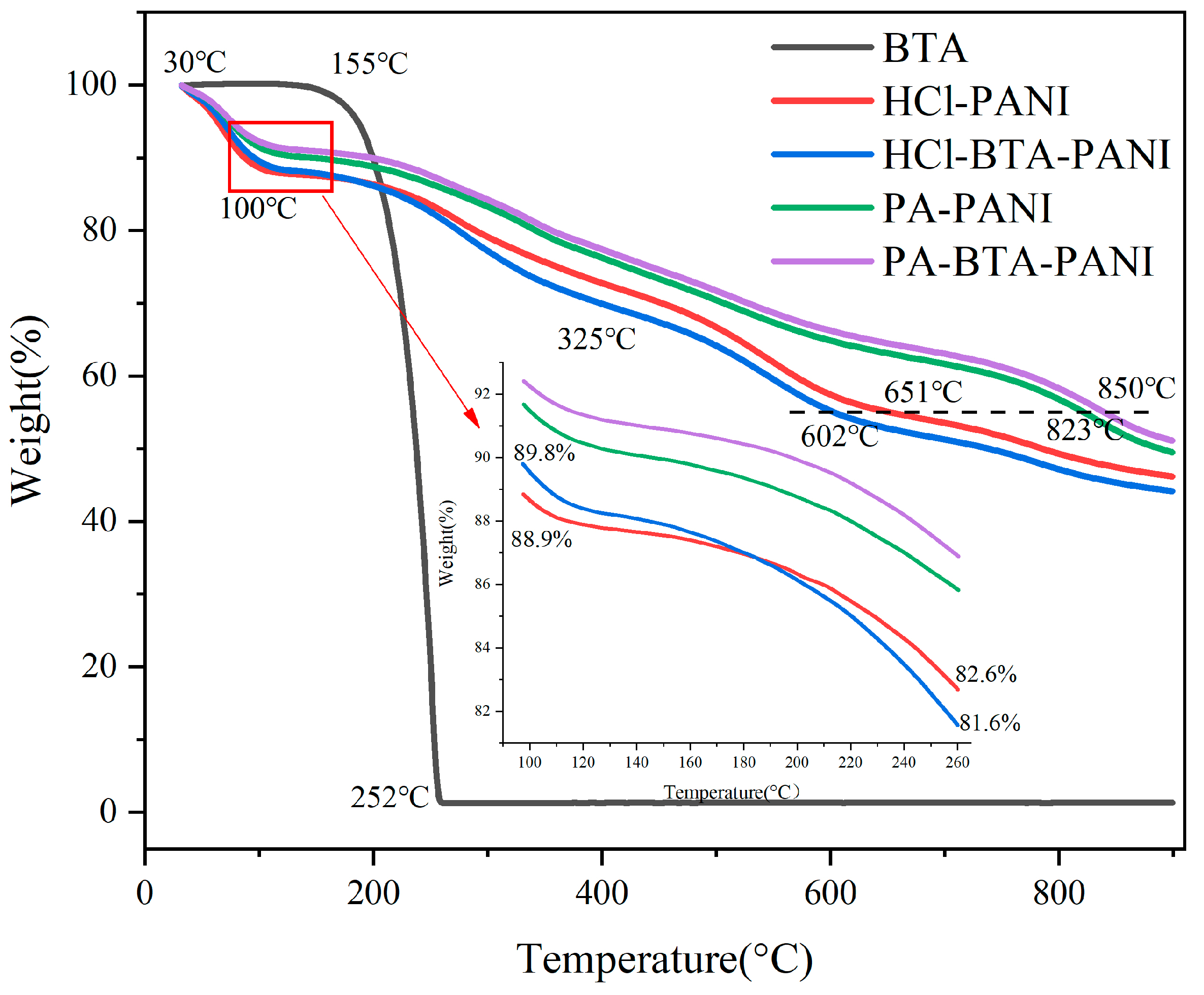
Figure 4 exhibits the thermogravimetric loss curve (TGA) of BTA, HCl-PANI, PA-PANI, HCl-BTA-PANI, and PA-BTA-PANI.
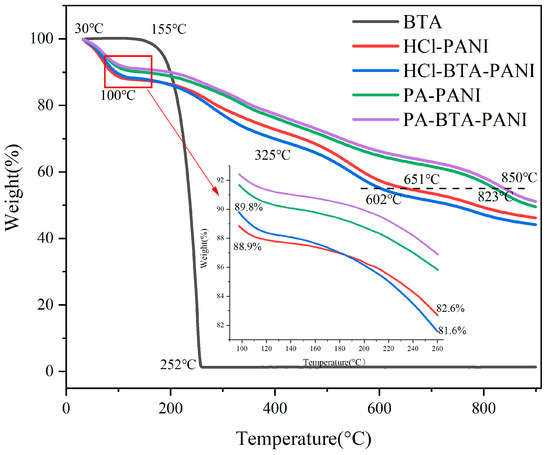
Figure 4.
Thermogravimetric profiles of PANI materials and BTA.
According to the TGA curve, all four kinds of PANI materials exhibit a significant weight reduction of approximately 8% to 12% within the temperature range of 30 °C to 100 °C. This decline is attributed to the volatilization of water in PANI powder [50]. Beyond 100 °C, each subsequent stage corresponds to the dedoping of acid from PANI and the decomposition of PANI [51]. For PA-PANI and PA-BTA-PANI, the mass loss observed between 160 °C and 350 °C can be attributed to the condensation dehydration of the P-OH groups in the phytic acid. Upon heating beyond 500 °C, the phytic acid molecules begin to decompose. By comparing the residual weights of four kinds of PANI materials at the same temperature, it is observed that at 899 °C, HCl-PANI, HCl-BTA-PANI, PA-PANI, and PA-BTA-PANI retain the weights of 46.18%, 44.15%, 49.51%, and 51.11%, respectively. This indicates that PA-PANI and PA-BTA-PANI exhibit higher residual weights. When the weight reduction is 10%, the temperatures of PA-BTA-PANI, HCl-PANI, HCl-BTA-PANI, and PA-PANI are 87 °C, 95 °C, 143 °C, and 197 °C, respectively. Further analysis of the decomposition temperatures of PANI materials with the same residual weight reveals that at a residual mass of 55%, HCl-PANI, HCl-BTA-PANI, PA-PANI, and PA-BTA-PANI have decomposition temperatures of 651 °C, 602 °C, 823 °C, and 850 °C, respectively. These comparisons reveal that PA-PANI and PA-BTA-PANI present greater resistance to decomposition compared to HCl-PANI and HCl-BTA-PANI. This suggests that macromolecular organic acid is more challenging to remove than inorganic acid, resulting in a superior thermal stability for the former PANI materials [40,52].
Due to the volatilization of water molecules, the weight of PANI powder decreases at temperatures between 30 and 100 °C. In contrast, BTA, being a hydrophobic material, experiences minimal weight loss before reaching 100 °C. BTA begins to decompose at 155 °C to 160 °C, and its weight is reduced by 10% at about 199 °C. The weight loss rate of BTA decreases sharply up to 0% at about 252 °C, indicating that BTA has decomposed completely. According to the TGA curve, the weight reduction in HCl-BTA-PANI is faster than that in HCl-PANI at 100 °C to 260 °C. In detail, the residual weight of HCl-PANI decreases from 88.9% to 82.6%, and that of HCl-BTA-PANI decreases from 89.8% to 81.6%. The weight of HCl-PANI and HCl-BTA-PANI decreases by 6.3% and 8.2%, respectively, which can be explained by the volatilization of BTA at about 190 °C. Before the decomposition of BTA, the mass decline rate of the two substances appears to be comparable. However, after the decomposition of BTA, the mass decline of HCL-BTA-PANI accelerates in comparison to HCl-PANI. Consequently, the two curves intersect at 180 °C, with the mass loss on the blue curve exceeding that on the red curve thereafter. The TGA curves of PA-PANI and PA-BTA-PANI present that the weight loss rates of these two powders exhibit no significant difference. This similarity arises from the limited quantity of BTA loaded onto PA-BTA-PANI, and the weight loss from the thin layer of BTA has a negligible impact on the overall weight fraction of PA-BTA-PANI.
3.1.5. Release of Inhibitor from PANI
The concentration of BTA has a high linear correlation coefficient with the UV-Vis absorbance, so the linear curve of BTA concentration–absorbance was established to calculate the BTA concentration released from PANI. The absorbance of each BTA solution with different concentration was tested using the UV-Vis spectrophotometer to monitor the peak at 258 nm, which is the characteristic absorbance of BTA [17]. Further details of UV-Vis spectra for BTA at different concentrations can be found in the Supplementary Materials Text S1.
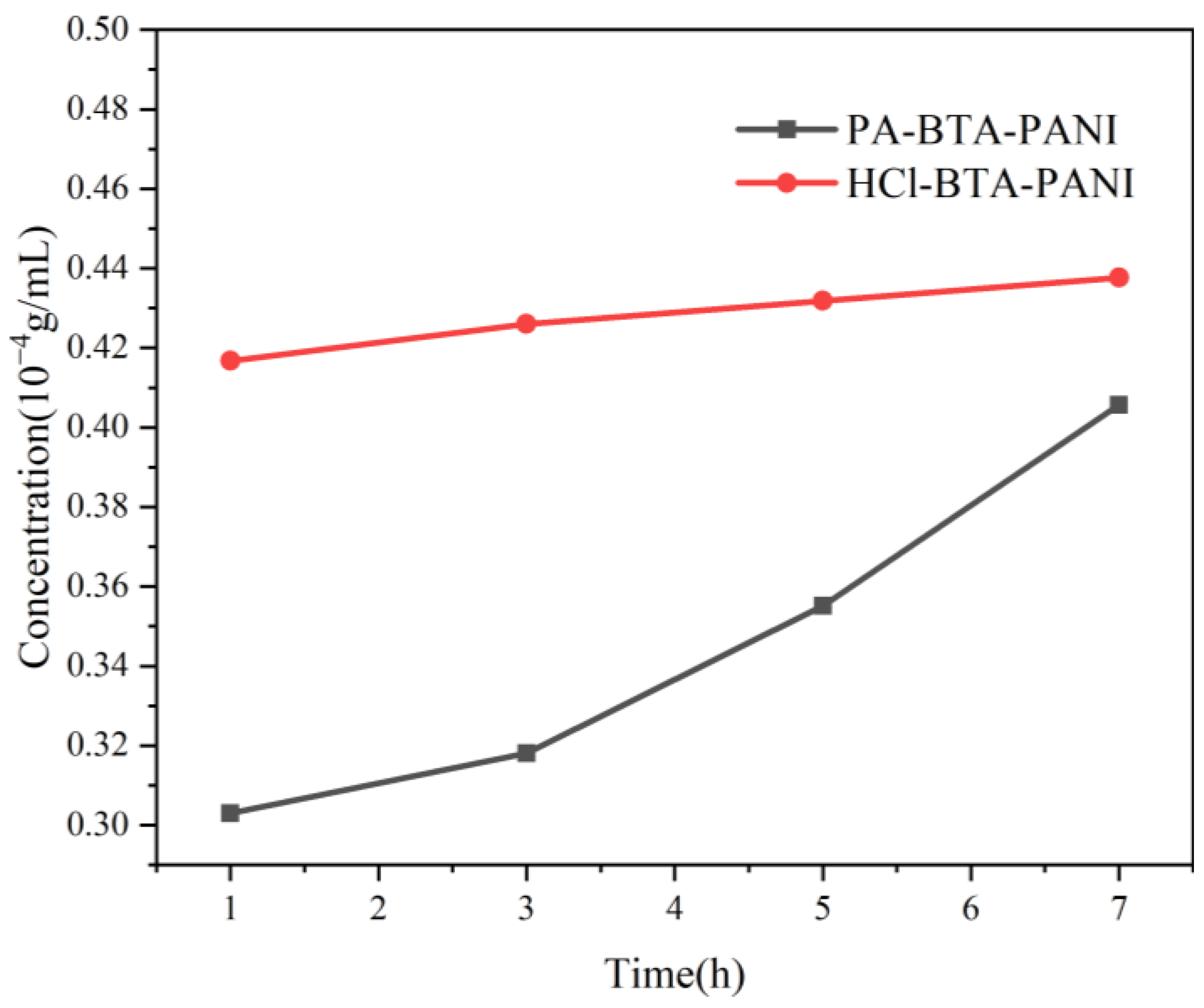
The time-dependent release of BTA from 0.015 g of HCl-BTA-PANI and 0.015 g of PA-BTA-PANI is presented in Figure 5. As shown in Figure 5, after stirring for 1 h, the BTA concentration released from HCl-BTA-PANI is 0.417 10−4 g/mL, and the concentration changes slightly with the increase in stirring time. The concentration of BTA released from PA-BTA-PANI is about 0.303 10−4 g/mL, which is lower than that of HCl-BTA-PANI, but it increases rapidly after stirring for 3 h and the overall release rate is faster. After stirring for 7 h, the BTA release rates of HCl-BTA-PANI and PA-BTA-PANI are 0.438 10−4 g/mL and 0.406 10−4 g/mL, respectively.
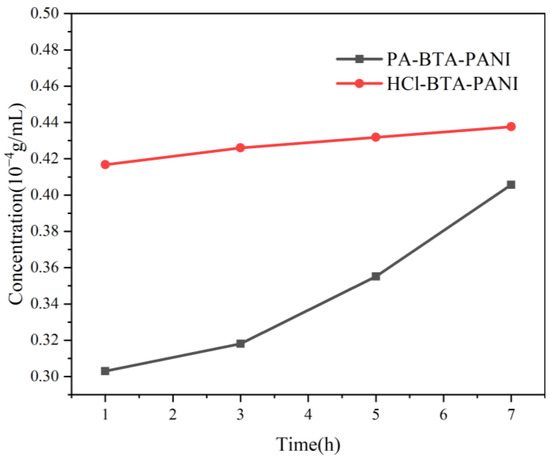
Figure 5.
Time dependency of BTA released from HCl-BTA-PANI and PA-BTA-PANI in 3.5 wt% NaCl solution.
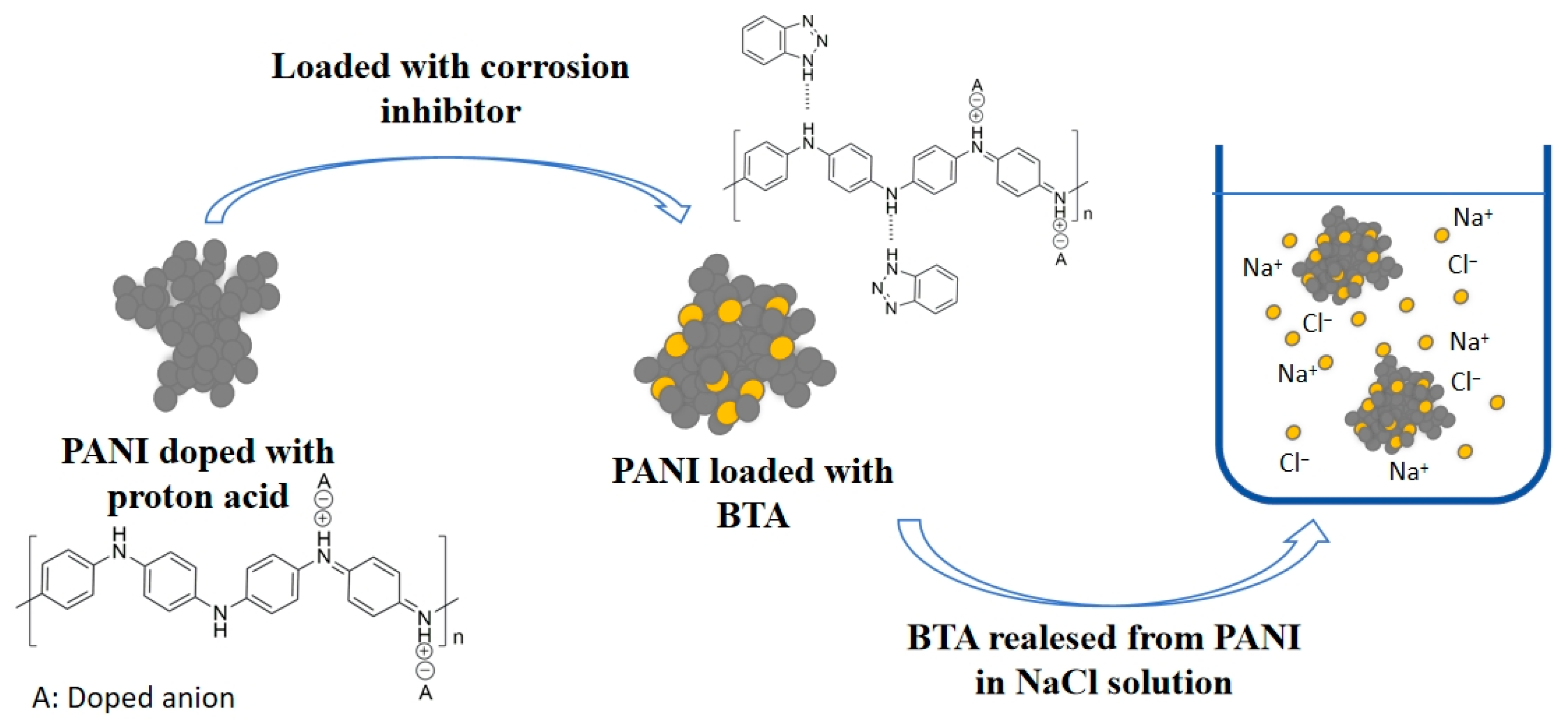
According to the reference [53], the N atom of BTA adsorbs onto the main chain of PANI via hydrogen bonding. NaCl enhances the dynamic movement of PANI, promoting the breaking of hydrogen bonds and thereby releasing BTA. The diagram of BTA release from PANI in NaCl solution is shown in Figure 6.
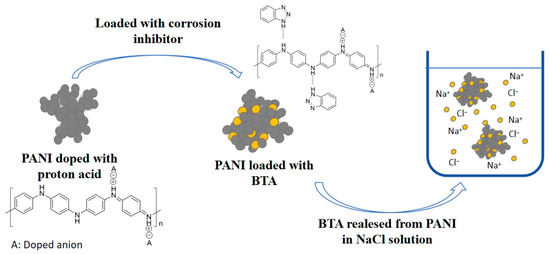
Figure 6.
Diagram of BTA release from PANI in NaCl solution.
After stirring for one hour, BTA is rapidly released from HCl-BTA-PANI, and then stabilizes in the subsequent hours. In contrast, for PA-BTA-PANI, due to initially low BTA release concentrations, it is subsequently released at a faster rate. The difference in BTA concentration in the solution contributes to the disparate release rates between the two.
3.2. Physical Performance of Coatings
3.2.1. The Wettability of the Coatings
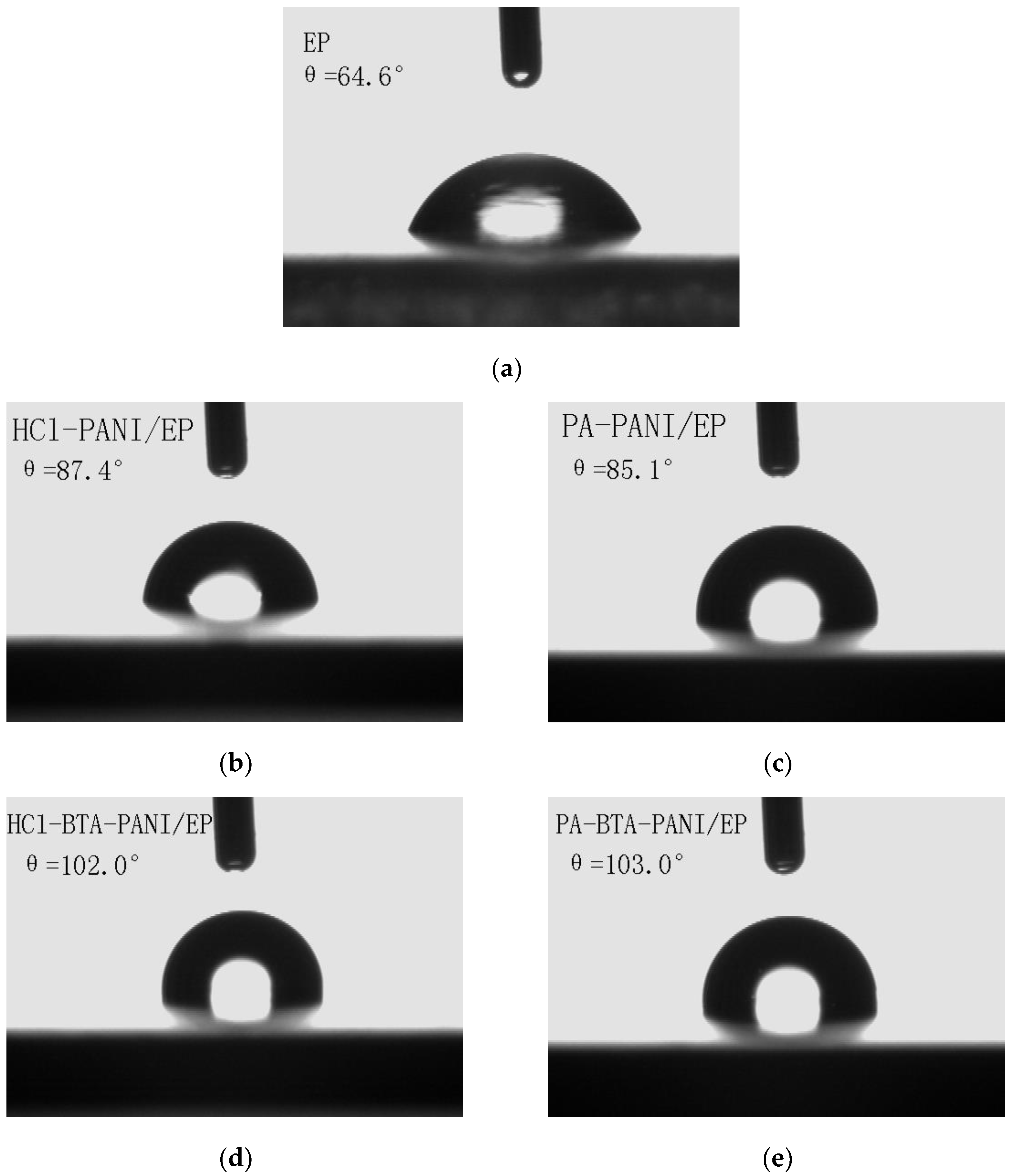
According to Figure 7, the contact angles of water droplets on the surfaces of HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP, and PA-BTA-PANI/EP are 87.4°, 85.1°, 102.0°, and 103.0°, respectively.
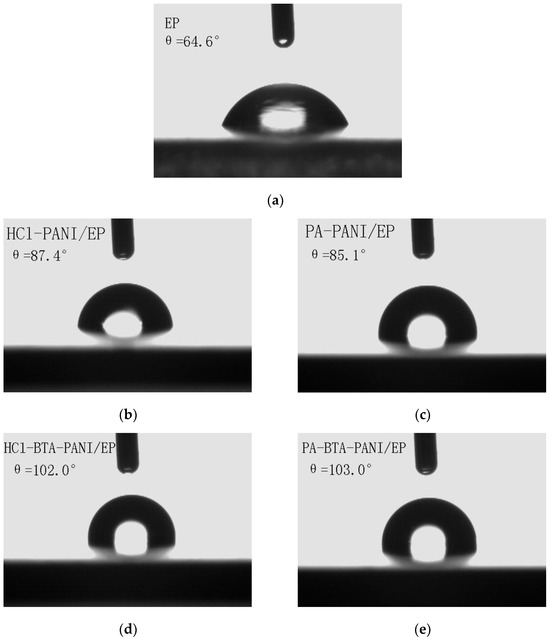
Figure 7.
Contact angle images for (a) EP; (b) HCl-PANI/EP; (c) PA-PANI/EP; (d) HCl-BTA-PANI/EP; and (e) PA-BTA-PANI/EP.
The improved hydrophobicity of coatings containing BTA may be attributed to BTA adsorbing via hydrogen bonding around the PANI conjugated chains, with its hydrophobic groups facing outward from the main chain. Simultaneously, interchain interactions affect the structure of PANI, thereby enhancing the material’s hydrophobicity [54].
Therefore, the hydrophobic coating can avoid the surface being wet by liquid and reduce the possibility of the metal matrix being corroded by corrosive media.
3.2.2. The Water Absorption of the Coatings
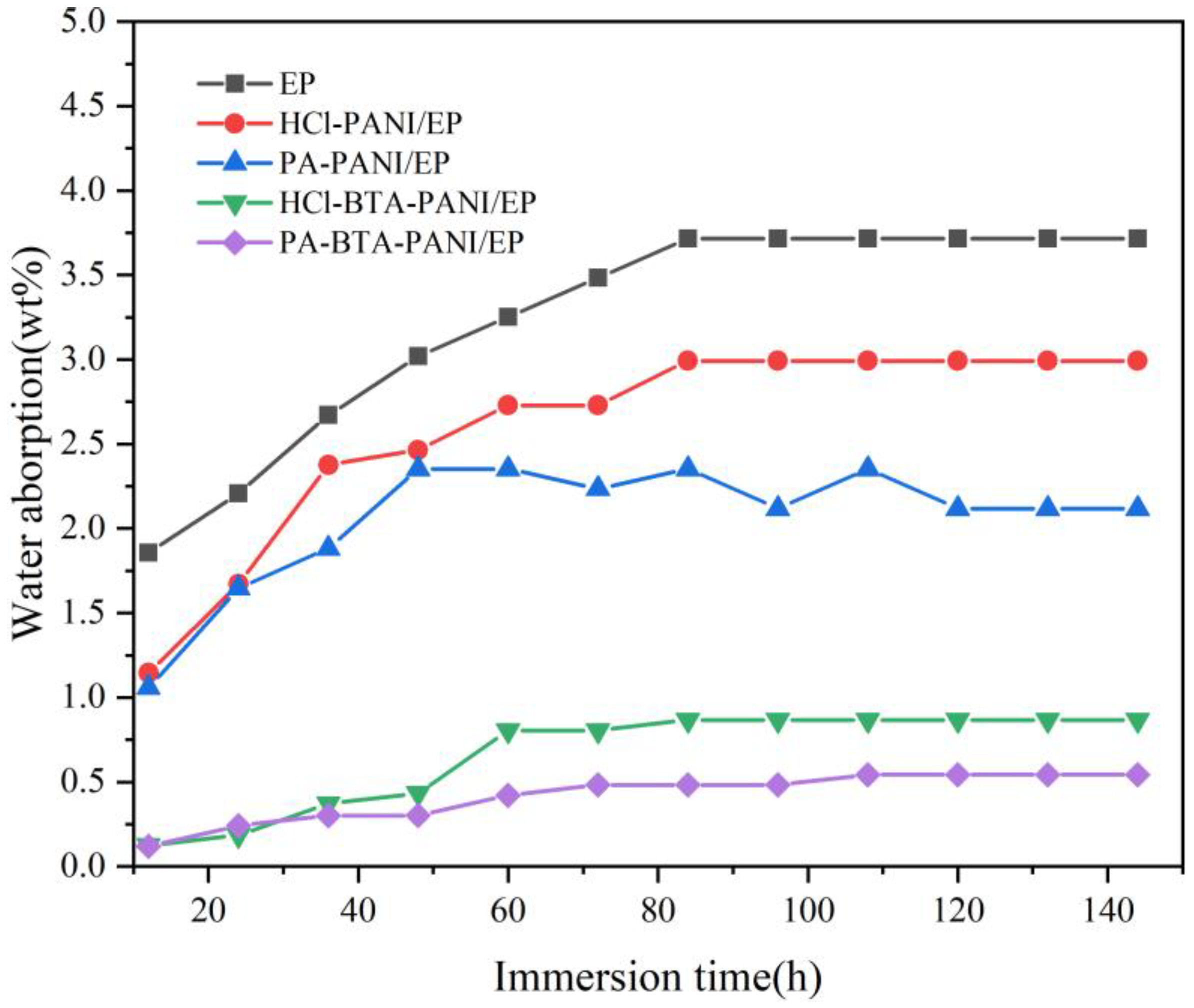
The penetration of water and oxygen in the coating is faster than that of other corrosive media such as chloride ions. When water and oxygen penetrate the coating on the surface of the metal substrate, chemical reaction occurs, and the diffusion of corrosion product becomes a decisive factor affecting the corrosion rate of the metal substrate [55]. Therefore, the saturated water absorption of the coating reflects its ability to absorb water and its resistance to corrosive media.
The water absorptions of HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP, and PA-BTA-PANI/EP are displayed in Figure 8. This allows the investigation of the permeation of water and the anticorrosive performance. The absorption and diffusion of water molecules in the epoxy coating are related to the composition and structure of the epoxy coating. The free volume and polar groups are decisive factors [56]. The water absorption rate of the coatings in the early stage of immersion was relatively high, which was due to the high concentration of polar groups from the doped polyaniline. After soaking in deionized water for about 110 h, the weight of the coatings remained stable indicating that the water absorption of the coating had reached saturation. The saturated water absorption rates of HCl-BTA-PANI/EP and PA-BTA-PANI/EP were 0.86% and 0.54%, respectively, which were lower than those of HCl-PANI/EP (2.9%) and PA-PANI/EP (2.1%). This could be explained by BTA improving the hydrophobicity of the coatings and blocking water molecules from entering the coatings, which was the main reason for the low saturation water absorption of HCl-BTA-PANI/EP and PA-BTA-PANI/EP.
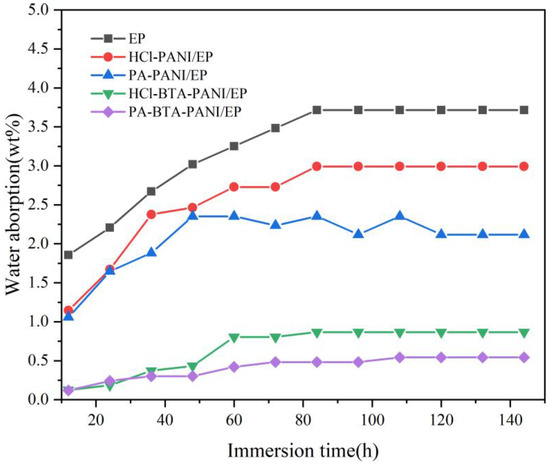
Figure 8.
Water absorption of different coatings over immersion time.
3.3. Corrosion Resistance of the Coatings
3.3.1. Immersion Test of Coatings
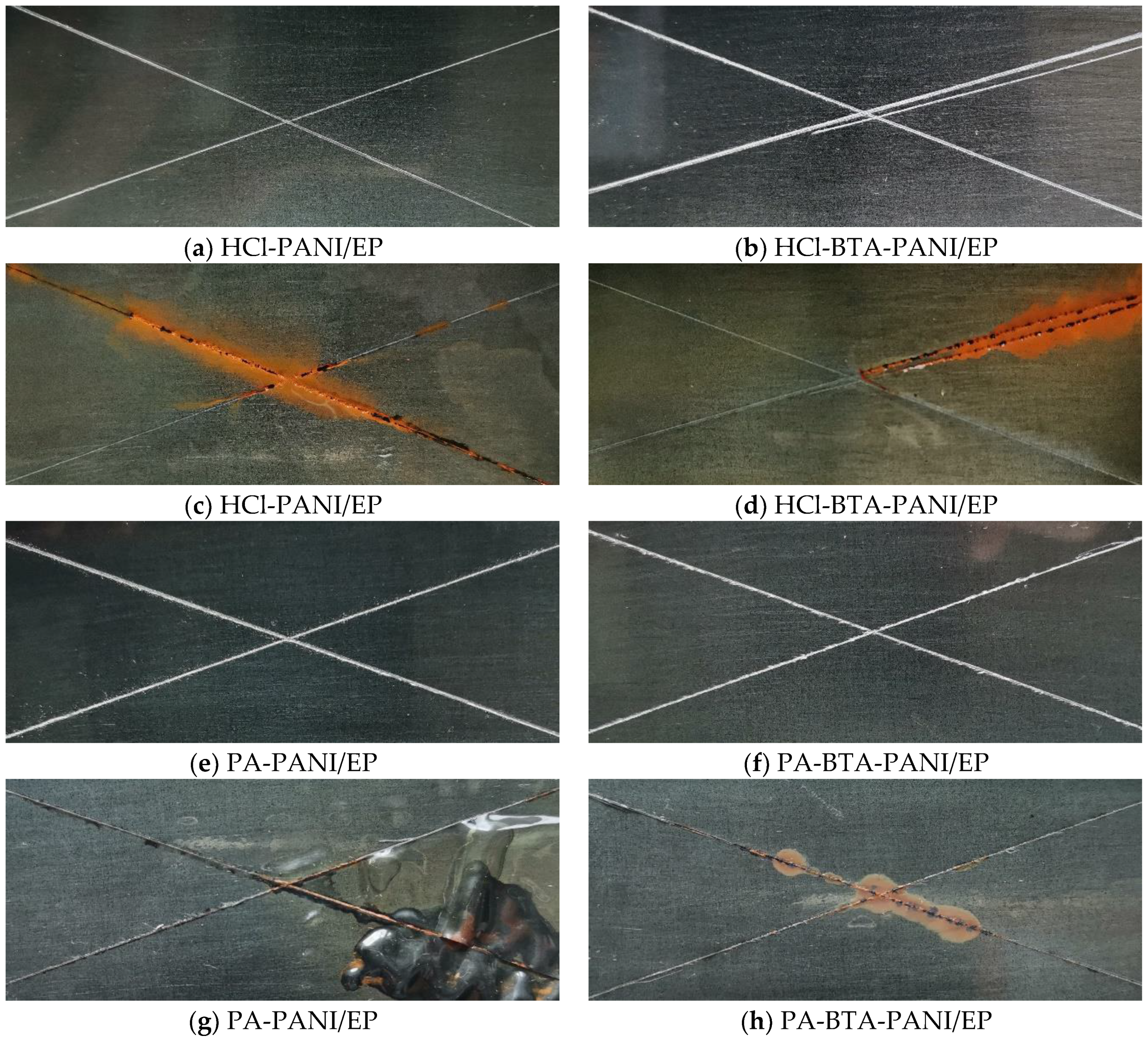
Q235 mild steel with a size of 120 mm × 50 mm × 1.5 mm was selected as the substrate in immersion test of coatings. As can be seen from Figure 9, the surfaces of the coatings before immersion were smooth and flat. After crossing the surfaces of the coatings under the same force, they remained firmly adhered to the metal substrate without detachment. Following an 18-day immersion in a 3.5 wt% NaCl solution, discernible changes occurred on the scratched areas of the four coatings, which manifested as yellow rust and black corrosion products. The HCl-PANI/EP coating displayed a substantial area of rust on its scratches, accompanied by an uneven surface and slight bubbling. In contrast, the HCl-BTA-PANI/EP coating exhibited minimal bubbling, presenting a flatter overall surface compared to the former. The PA-PANI/EP coating experienced a pronounced bulging away from the metal substrate, featuring extensive bubbling after immersion. The metal substrate beneath this coating displayed signs of rust and exhibited blackening. However, the PA-BTA-PANI/EP coating showed only slight rust at the intersections of scratches, maintaining a relatively smooth and flat surface without significant bubbling or detachment from the metal substrate. After the immersion tests of the four coatings, it is evident that the corrosive medium causes less damage to the coating with BTA. This suggests that BTA imparts resistance to the erosive effects of the corrosive medium to the coatings.
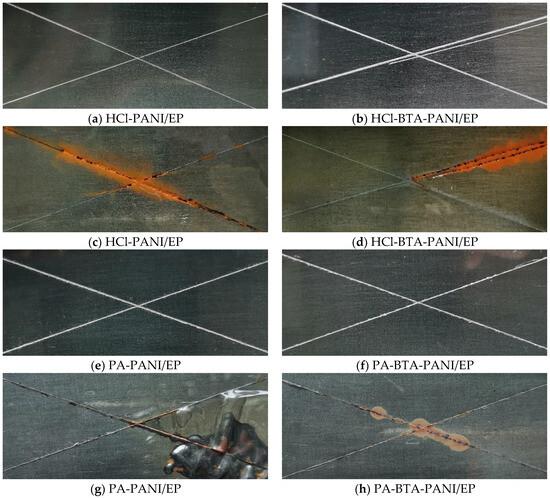
Figure 9.
Images showing HCl-PANI/EP, HCl-BTA-PANI/EP, PA-PANI/EP, and PA-BTA-PANI/EP before immersion (a,b,e,f) and after immersion for 18 days (c,d,g,h) in 3.5 wt% NaCl solution.
3.3.2. Corrosion Morphology of the Substrate under the Coatings
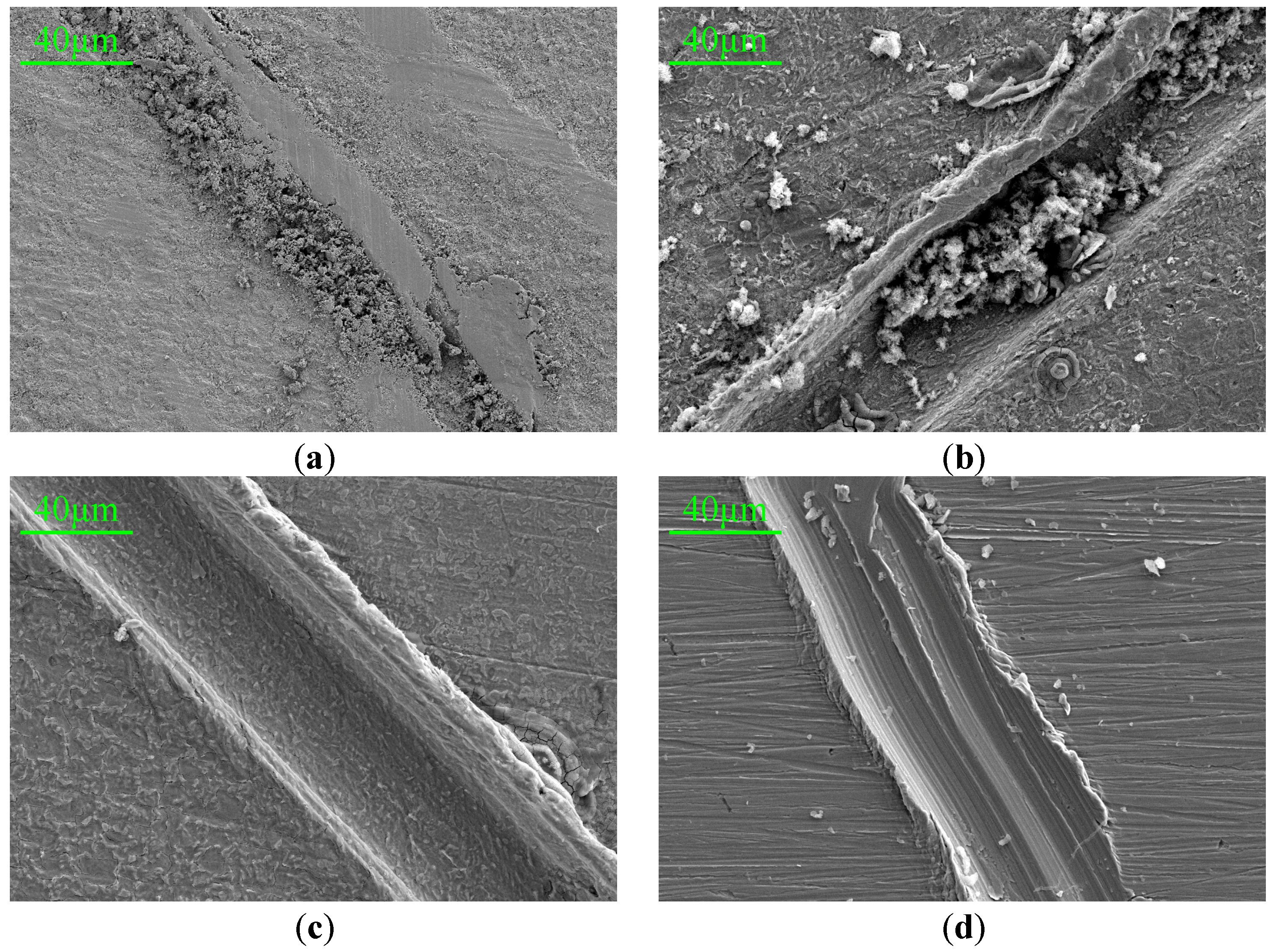
Q235 mild steel with a size of 10 mm × 10 mm × 1.5 mm was selected as the substrate for observing the corrosion morphology. After the substrate surface was polished and cleaned, various PANI paints were uniformly applied to the metal substrate. When the coatings were completely dried, the surface of the coating was scratched under the same force, and then the coating samples were immersed in 3.5 wt% NaCl solution for 7 days. After that, the coatings were removed and the metal substrates were observed by a scanning electron microscope. Figure 10 shows the SEM images of HCl-PANI/EP, HCl-BTA-PANI/EP, PA-PANI/EP, and PA-BTA-PANI/EP after being immersed in 3.5 wt% NaCl solution for 7 days, with a magnification of 500 times.
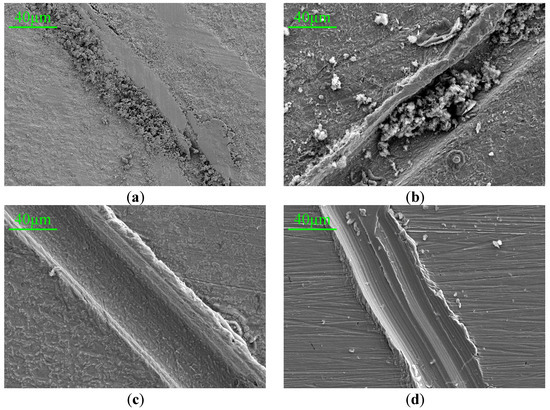
Figure 10.
SEM images (500×) for scratches of (a) HCl-HCl-PANI/EP, (b) PA-PANI/EP, (c) HCl-BTA-PANI/EP and (d) PA-BTA-PANI/EP immersed in 3.5 wt% NaCl solution for 7 days.
From the figure, it can be clearly seen that the metal substrate under the HCl-PANI/EP coating exhibited a rough surface with a layer of rust, and the scratch was filled with corrosion products displaying a dense and amorphous structure. The surface of the metal substrate under PA-PANI/EP appeared rough with large particles distributed on it. The corrosion product was scattered and easy to separate from the substrate, which was different from the former case.
The surface of the metal substrate beneath the HCl-BTA-PANI/EP coating showed slight corrosion, with a few corrosion products appearing in the scratch. Overall, the corrosion level was lower than the HCl-PANI/EP coating.
There were a limited number of large corrosion product particles on the metal substrate under the PA-BTA-PANI/EP coating, and fewer corrosion products were observed in the scratch compared with the PA-PANI/EP coating. Phytic acid may add complex polyvalent ions in solution and may thus “encourage” Fe3+/Fe2+ ions out of the metal. According to relevant references [57,58,59], phytic acid is capable of chelating with metal ions to generate stable complexes and can further form dense chemical conversion films on metal surfaces through chelation with surface metal atoms. Consequently, by attenuating the diffusion rate of corrosive agents towards the metal substrate surface, it effectively functions to inhibit metal corrosion.
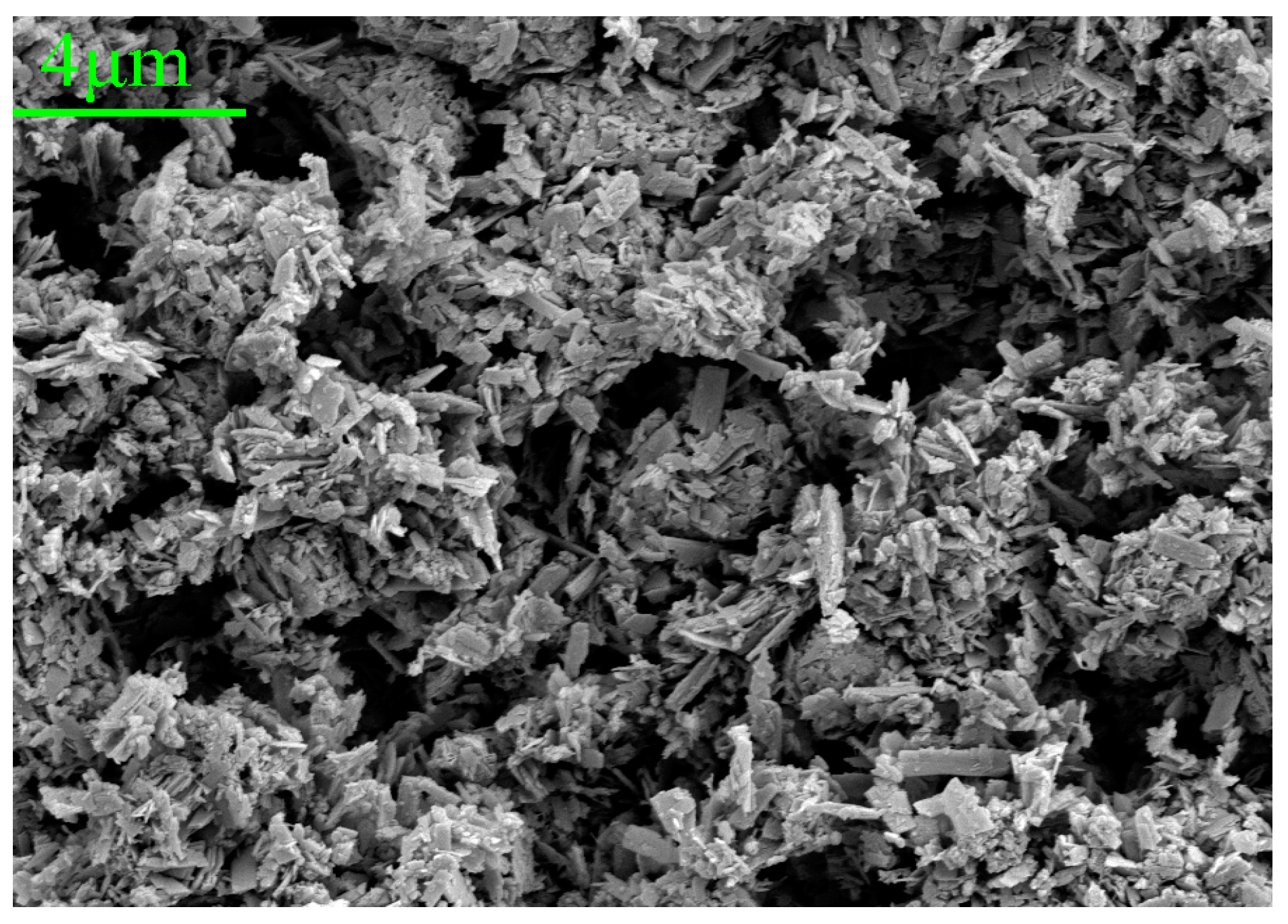
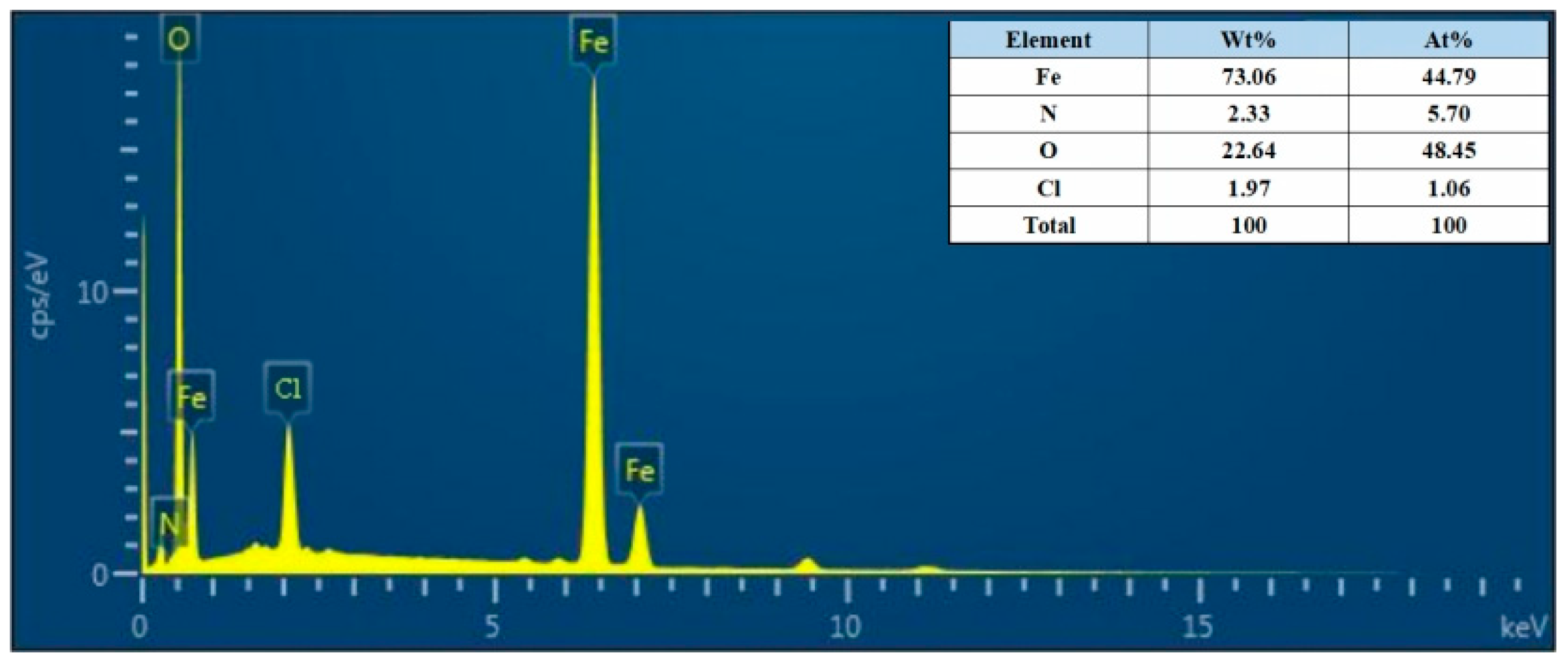
According to the research on the anti-corrosion mechanism of PANI, PANI can form Fe3O4/Fe2O3 oxide layer on the surface of mild steel. This occurs as the quinone ring in PANI attracts electrons and the metal charge diffuses to the substrate surface, resulting in the formation of a positively charged surface area between the metal and PANI. Simultaneously, Fe3O4 in this region tends to stabilize due to the loss of electrons, making it more difficult to further oxidize iron ions. As a result, the low carbon steel substrate exhibits increased resistance to corrosion [60]. To further investigate the products within the scratches, the corrosion products of the HCl-PANI/EP coating were selected for analysis. Figure 11 displays the SEM images of the corrosion products at 5000 times magnification, and Figure 12 is the energy dispersive X-ray spectroscopy (EDX) results. Figure 11 indicates that the corrosion products possess a closely arranged spinel structure, while the crystal structure of Fe3O4 and Fe2O3 is spinel [61]. Combined with EDX diagram and the proportion of Fe and O atoms in the corrosion products, it was speculated that the corrosion product at the substrate scratch of HCl-PANI/EP may be a mixture of Fe3O4 and Fe2O3. This indicates that the existence of PANI promoted the formation of stable Fe3O4/Fe2O3 oxide layer on the substrate surface. This layer impedes the diffusion of Fe2+ through the metal substrate to the interface and the diffusion of O2− from the surface down to the interface, thereby reducing the corrosion rate of the substrate. PANI enhances the enduring shielding and corrosion resistance of the coating, providing protection to the substrate even in the event of coating damage [62].
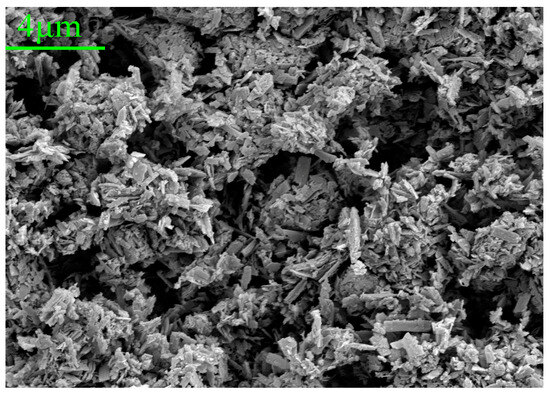
Figure 11.
SEM images (5000×) for the corrosion products of the HCl-PANI/EP coating.
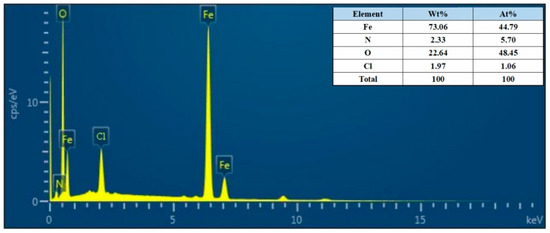
Figure 12.
EDX of the corrosion products of the HCl-PANI/EP coating.
3.3.3. Electrochemical Impedance Spectroscopy for the Coatings
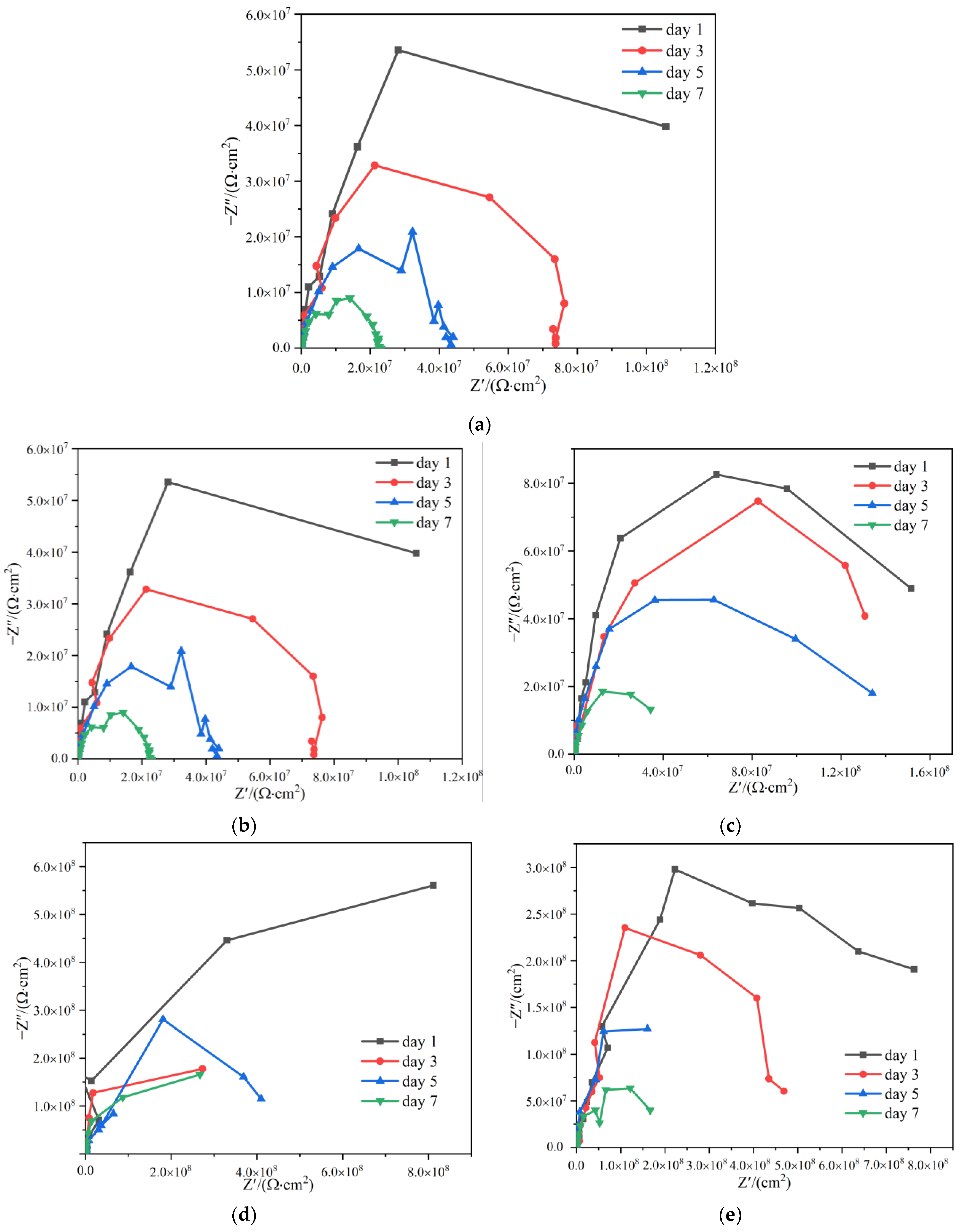
The working electrode of the electrochemical test was a Q235 carbon steel sample; one side of the samples was coated with paints with a thickness of (120 20) m, and the surrounding and the back sides were sealed with waterproof tape. Figure 13 showed the Nyquist plots of HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP, and PA-BTA-PANI/EP samples immersed in 3.5 wt% NaCl solution for 1 day, 3 days, 5 days, and 7 days.
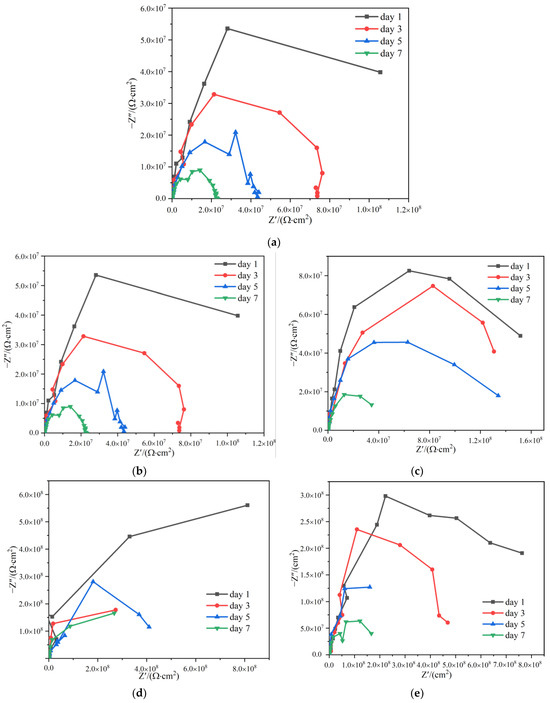
Figure 13.
Nyquist plots for Q235 steel covered by (a) EP; (b) HCl-PANI/EP; (c) PA-PANI/EP; (d) HCl-BTA-PANI/EP; (e) PA-BTA-PANI/EP immersed in 3.5 wt% NaCl solution for different durations.
According to previous studies, PANI and BTA promotes the formation of a passivation film in the coating, and the capacitive arc of the Nyquist curve in the high-frequency region can reflect the performance of the passive film; the larger the capacitive arc radius, the larger the impedance modulus of the passivation film, indicating that the charge transfer is more difficult and the test sample has a stronger barrier ability to corrosive substances [63]. In the Nyquist plots of the four coatings, only one capacitive arc appeared, and the shapes of them were not complete semicircles, which may be caused by the dispersion effect of the coatings [64,65].
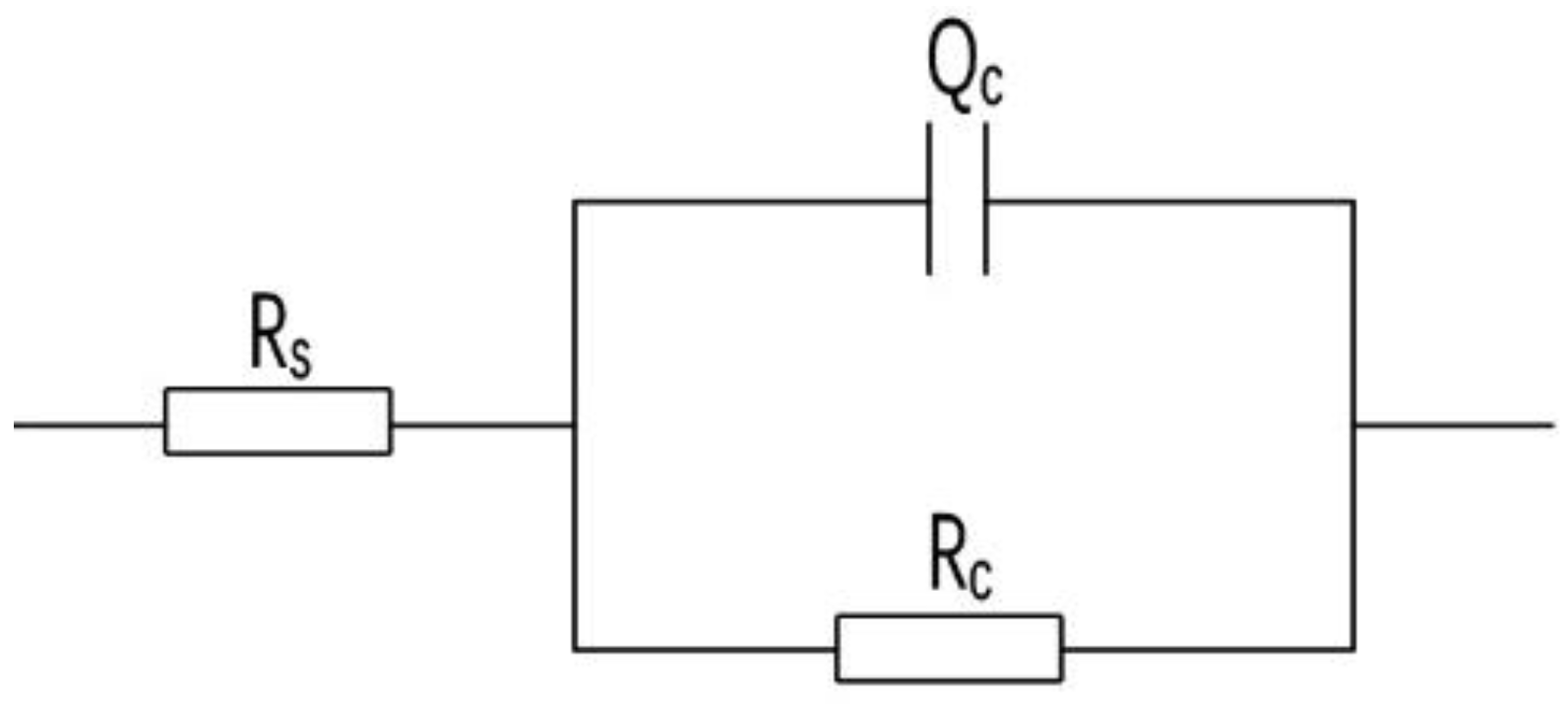
The Nyquist plots of the four coatings showed a single capacitive arc with one time constant on impedance plane. Figure 14 was chosen as their fitted equivalent electrical circuit, in which the symbol Rs represents the solution resistance and the symbol Rc represents the coating resistance. Since the dispersion effect on the electrode surface will cause the coating capacitance to deviate from the ideal capacitance, a constant phase angle element Qc is introduced to characterize the dispersion effect, and n is the dispersion index of the dispersion effect; the closer n is to 0, the closer the element is to pure resistance, and the closer n is to 1, the closer the element is to pure capacitance. The coating resistance Rc reflects the resistance of charge transfer between the electrolyte and the metal interface. If the Rc value is large, it indicates that the electrolyte is not easy to penetrate into the coating and the metal substrate, and the coating has good corrosion resistance. After the electrochemical impedance spectra curves of each coating are fitted by ZsimpWin software (Version 3.30), the fitting parameters of each component of the coating can be obtained, and the data are shown in Table 1.
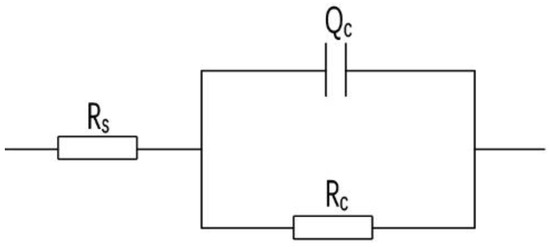
Figure 14.
Equivalent electrical circuits for the coatings.

Table 1.
Fitting electrochemical parameters of the applied coatings in 3.5 wt% NaCl solution.
With the increase in soaking time, the arc radius of several coatings decreased. The Nyquist diagrams of the four coatings all showed one time constant, which indicated that the corrosive medium in the coating had not reaching the interface between the coating and the metal substrate at this time, and the coatings played a role in preventing the contact between water and the metal substrate [66,67]. After being immersed for 1 day, the coating resistance Rc of EP, HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP, and PA-BTA-PANI/EP were 2.893 × 106 Ω·cm2, 1.270 × 108 Ω·cm2, 1.904 × 108 Ω·cm2, 1.125 × 109 Ω·cm2, and 8.095 × 108 Ω·cm2, respectively. When the immersing time was 1 day, the coating resistances of HCl-PANI/EP and PA-PANI/EP were higher than that of EP, and the coating impedance was greatly improved after BTA was introduced into the two coatings. After 3 to 7 days of immersion, all coating resistances decreased slightly, the coating impedance values of HCl-PANI/EP and PA-PANI/EP were reduced to the seventh power of ten, but the coating impedance values of HCl-BTA-PANI/EP and PA-BTA-PANI/EP were still maintained at the eighth power of ten. When the sample was immersed for 7 days, the coating resistance Rc of HCl-BTA-PANI/EP and PA-BTA-PANI/EP were 3.42 × 108 Ω·cm2 and 1.423 × 108 Ω·cm2, respectively. The corrosion resistance of HCl-BTA-PANI/EP and PA-BTA-PANI/EP was better than that of coatings without BTA, whether it was the resistance value of coating after soaking or the decline rate of resistance of coating after soaking 7 days.
At the initial stage of immersion, the organic coating acted as a barrier between water and the metal substrate, and the coating could be regarded as an insulating layer with a small capacitance but a large resistance value. Due to the swelling of the coating, the agglomeration of PANI powder, the volatilization of the thinner, and other reasons, the channel for electrolyte solution to penetrate into the coating was formed. With the increase in immersing time, the penetration of the solution became stronger, which led to the coating capacitance becoming larger and the coating resistance becoming smaller. At the middle stage of immersion, the dispersion index n in the constant phase angle element Qc of HCl-BTA-PANI/EP and PA-BTA-PANI/EP reached 1, and the element could be regarded as a pure capacitor at this time. At this stage, NaCl solution destroyed the adhesion of the epoxy coating and reached the metal surface, resulting in corrosion reaction at the interface between the coating and metal substrate, the protective performance of the coating declined. However, no small holes and bubbling on the surface of the coating were observed on a macroscopic level.
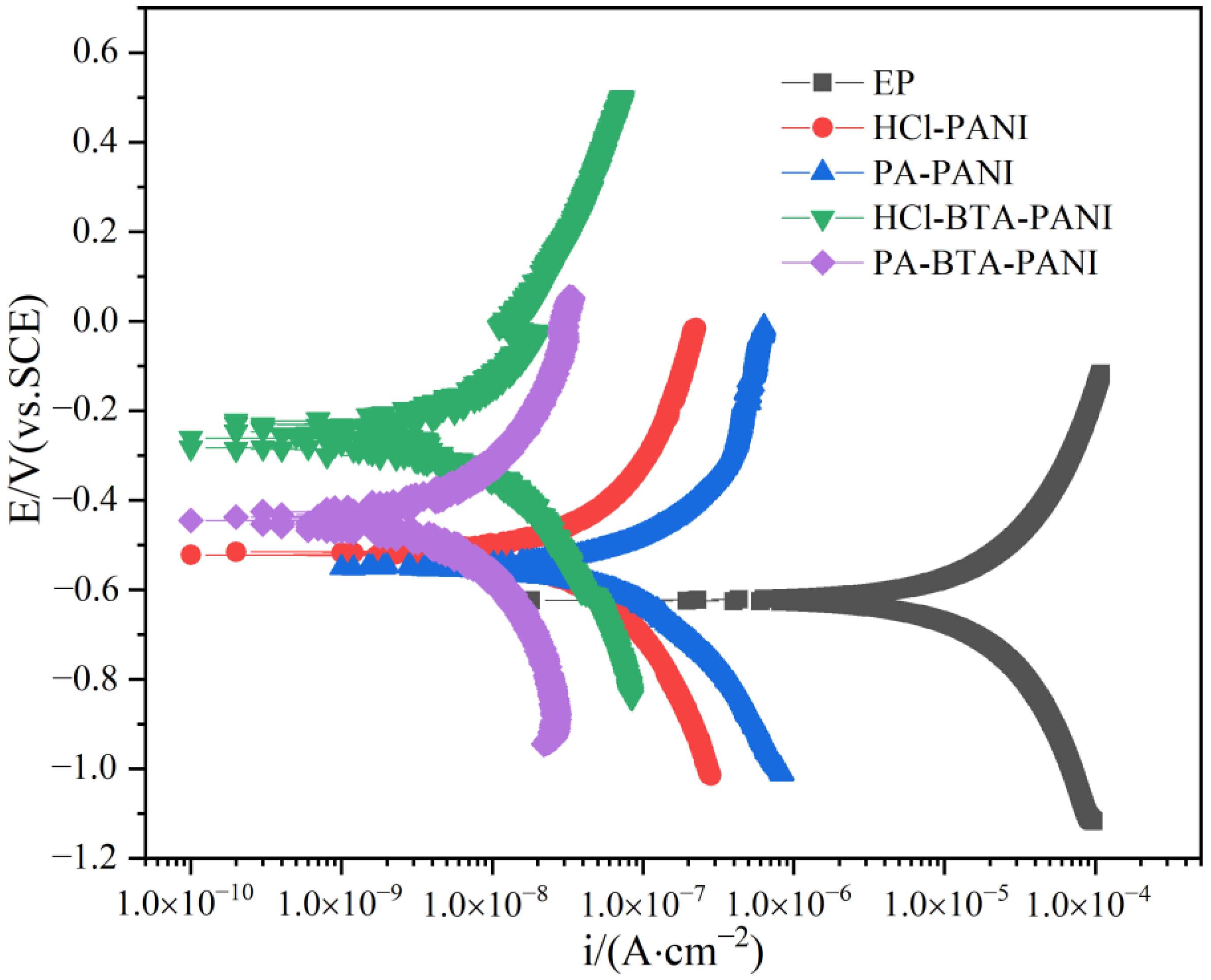
3.3.4. Potentiodynamic Polarization Measurements for the Coatings
Before potentiodynamic polarization measurements, one side of the samples was coated with paints with a thickness of (120 20) m, and the surrounding and the back sides were sealed with waterproof tape, and the samples were soaked in 3.5 wt% NaCl solution for 14 days. Figure 15 shows the polarization curves of EP, HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP and PA-BTA-PANI/EP, and Table 2 shows the fitting results of the polarization curves of the coatings. As shown in the figure, the polarization curves of HCl-PANI/EP and PA-PANI/EP are lower to the right than the other polarization curves, which corresponded to more negative corrosion potential (Ecorr) and higher corrosion current (Icorr). After calculation, the Ecorr values of EP, HCl-PANI/EP, PA-PANI/EP, HCl-BTA-PANI/EP, and PA-BTA-PANI/EP were −0.622 V, −0.517 V, −0.527 V, −0.262 V, and −0.430 V, respectively, and the Icorr of them were 2.704 10−5 Acm−2, 7.208 10−8 Acm−2, 1.301 10−8 Acm−2, 9.795 10−8 Acm−2, and 8.379 10−8 Acm−2, respectively. Compared with HCl-PANI/EP and PA-PANI/EP, the cathodic polarization curves of HCl-BTA-PANI/EP and PA-BTA-PANI/EP moved towards the position with higher corrosion potential and lower corrosion current density, which demonstrated that the effect of BTA on corrosion inhibition in metal dissolution reaction at anode and hydrogen evolution reaction at cathode. In the Tafel polarization curves, the Ecorr of HCl-BTA-PANI is higher than that of PA-BTA-PANI, but the Icorr of PA-BTA-PANI is lower than that of HCl-BTA-PANI. According to the reference literature, Ecorr is mainly used to describe the thermodynamic corrosion tendency of coatings and therefore cannot be used to evaluate the anticorrosive ability of coatings [68,69], while lower Icorr indicates a lower corrosion rate, meaning better anticorrosive performance [70]. Therefore, the anticorrosive ability of PA-BTA-PANI is stronger. PANI enhances the anticorrosion performance of pure epoxy coating, and the BTA further elevates the anticorrosion performance of PANI in the coating, which can illustrate the synergistic effect of PANI and BTA.
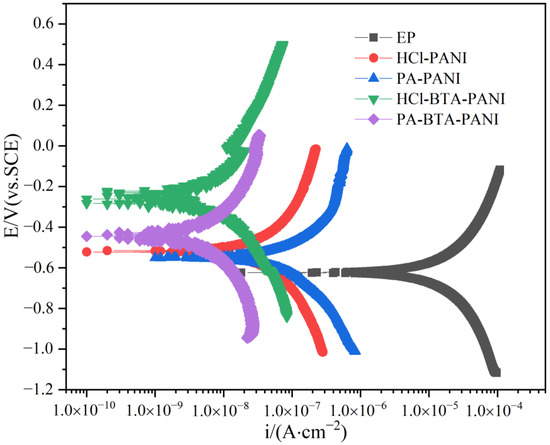
Figure 15.
Potentiodynamic polarization curves measured for the coatings after immersion in 3.5 wt% NaCl solution for 14 days.

Table 2.
Polarization parameters of the applied coatings in 3.5 wt% NaCl solution.
When calculating the corrosion efficiency of the inhibitor, it is necessary to know the corrosion rate of the metal in the corrosion medium with and without the inhibitor. In electrochemistry, the data measured by the potentiodynamic polarization curve can be used to express the corrosion inhibition efficiency of the coating on the metal [71,72], and the calculation formula is shown in Equation (2) as follows:
where is the corrosion inhibition efficiency, Icorr is the corrosion current density tested before adding the inhibitor, and I′corr is the corrosion current density tested after adding the inhibitor. After calculation, the inhibition efficiency of BTA in HCl-BTA-PANI/EP is 86.4%, and that in PA-BTA-PANI/EP is 93.6%.
3.4. Inhibition Mechanism
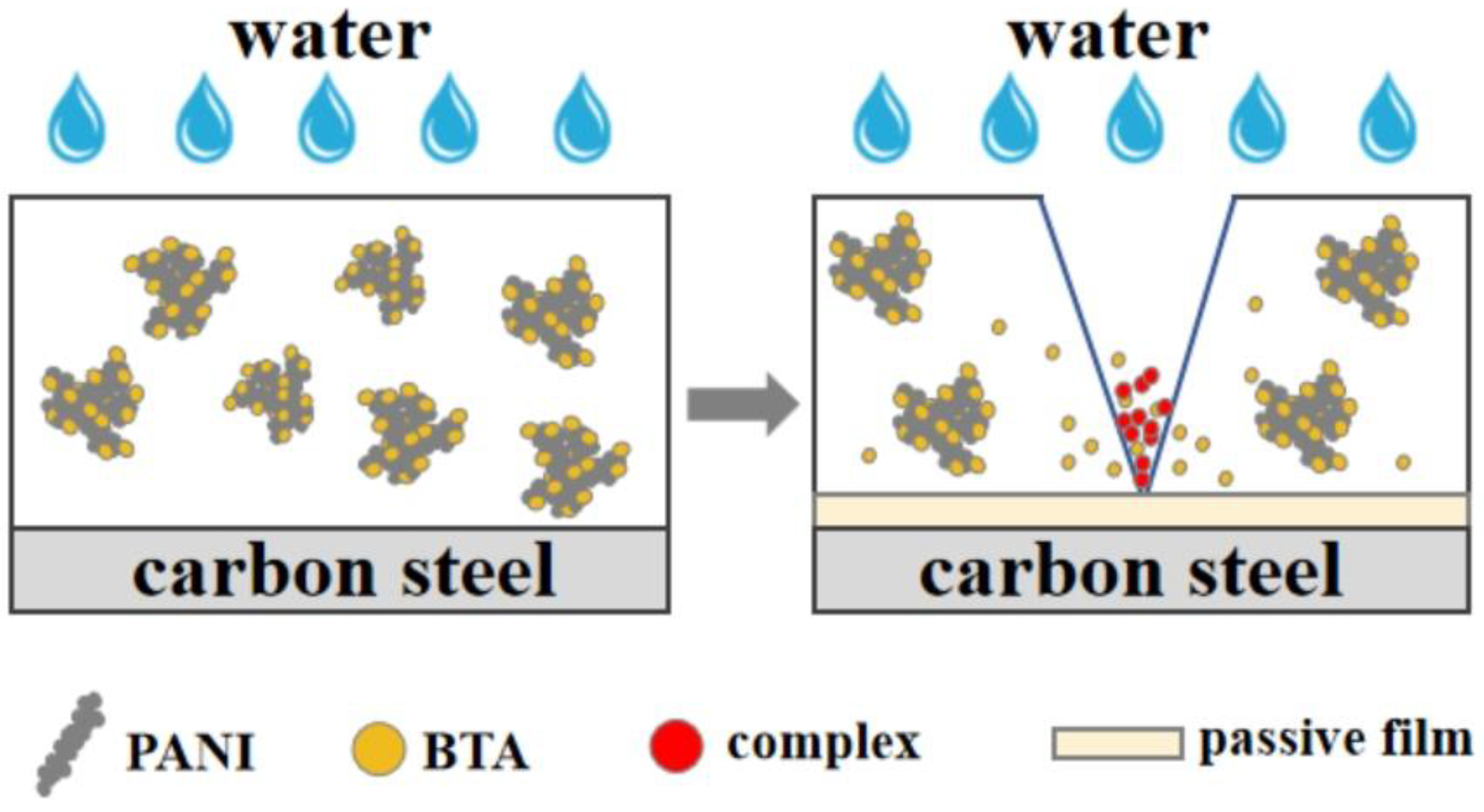
After the immersion test, electrochemical test, and observation of substrate corrosion, it is found that the coatings containing BTA exhibit superior corrosion resistance compared to the coatings without BTA. The inhibition mechanism of PANI has been described in the previous section; based on the inhibition effect of PANI, BTA demonstrates a synergistic role. In the event of coating damage, BTA adsorbed on the surface of PANI dissolves in water and permeates through the gap channel to reach the metal substrate surface. It reacts with Fe2+ to form a complex [BTA-Fe-BTA]n, which deposits in the damaged coating region, preventing further diffusion of the corrosive medium. BTA forms a thin protective film on the substrate beneath the coating, preventing the undamaged area from being damaged [73]. The formation mechanism of BTA forming complex is shown in Equations (3) and (4) [29].
According to Equation (3), hydrogen ions are generated during the formation of the complex [BTA-Fe-BTA]n. These hydrogen ions are then adsorbed on the surface of the complex or combined to form meteorological hydrogen molecules [74]. In addition to the formation of complex [BTA-Fe-BTA]n, another protective mechanism of BTA is that BTA migrates to the substrate surface and reacts with Fe to form a protective layer, blocking the channel of water diffusion [29].
The experimental results show that PANI, serving as the carrier loaded with BTA, exhibits excellent anticorrosion performance whether the coating is intact or damaged. Other inhibitor carriers such as SiO2 microspheres can be used as the container to load BTA and improve the hydrophobicity of the coating. However, SiO2 microspheres are easy to agglomerate together and reduce the density of the coating. Moreover, SiO2 microspheres only function as the container for loading BTA, offering limited protection against corrosive media when the coating is compromised [75,76]. Meroxysilalite has anticorrosion effects and can improve the protective effect of the coating when combined with BTA. However, despite its lamellar structure having good compatibility with epoxy resin, it is not conducive to improving the loading of benzotriazole [77]. SEM analysis reveals that the coral-like structure of HCl-PANI and PA-PANI, with its rough surface, enhances the adsorption capacity of corrosion inhibitors, making it an ideal carrier for BTA. Release rate tests demonstrate that BTA can be gradually released from PANI, with the released concentration surpassing that of other corrosion inhibitors [19]. Electrochemical tests show that the combination of BTA and PANI achieves a high corrosion inhibition efficiency, indicating that PANI is effective as a carrier of inhibitors. The epoxy coating, benefiting from the unique corrosion inhibition mechanism of PANI and BTA, effectively shields the metal. The inhibition mechanism of the epoxy coating containing PANI loaded with BTA is illustrated in Figure 16.
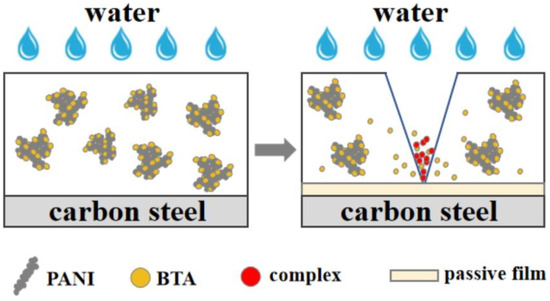
Figure 16.
Inhibition mechanism of epoxy coating containing PANI loaded with BTA.
4. Conclusions
In this study, PANI doped with HCl and PA were synthesized by chemical oxidation method and used as a carrier for BTA loading, which were confirmed by FTIR, Raman, SEM and TGA. The corrosion inhibition effect of 0.6 wt% of HCl-BTA-PANI and PA-BTA-PANI in epoxy coating was investigated by electrochemical measurements and a series of physical properties tests. The following observations were made:
- FTIR and Raman results showed that the PANI doped by HCL and PA have the characteristic structure of PANI, and the SEM images of PANI showed that it is a coralliform structure with a rough surface. BTA does not change the morphology and structure of PANI, but its presence enhances the characteristic peaks associated with the same chemical bonds in PANI.
- HCl-PANI can load more BTA than PA-PANI. After stirring for 7 h, the BTA release rates of HCl-BTA-PANI and PA-BTA-PANI are 0.438 × 10−4 g/mL and 0.406 × 10−4 g/mL, respectively.
- HCl-PANI and PA-PANI with and without BTA have good compatibility with epoxy resin, and BTA can improve the hydrophobicity and reduce the water absorption rate of the coating.
- After immersion in 3.5 wt% NaCl solution, PANI loaded with BTA showed a good anticorrosion effect in the epoxy coating because of their synergistic inhibition effect. Particularly, PA-BTA-PANI has better inhibition efficiency. The calculated inhibition efficiency of HCl-BTA-PANI was 86.4%, and that of PA-BTA-PANI was 93.6%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings14040456/s1.
Author Contributions
J.B.: Data curation, formal analysis, writing—original draft preparation, and writing—review and editing. J.L.: Conceptualization, investigation, and methodology. H.W.: Project administration and resources. J.W.: Funding acquisition G.L.: Supervision, validation, and writing—original draft preparation (supporting). J.H.: Supervision, validation, and writing—original draft preparation (supporting). C.Z.: Supervision, validation, and writing—original draft preparation (supporting). S.L.: Supervision, validation, and writing—original draft preparation (supporting). All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of the Funding of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW-2022-08-08) is acknowledged. This is a key project of Guangdong Province for promoting high-quality economic development (Marine Economic Development) in 2022: Research and development of key technology and equipment for Marine vibroseis system (GDNRC[2022]29).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All results of this study are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Han, E.H.; Chen, J.M.; Su, Y.J.; Liu, M. Corrosion Protection Techniques of Marine Engineering Structure and Ship Equipment—Current Status and Future Trend. Mater. China 2014, 33, 6576. [Google Scholar]
- Ma, C.L.; Ma, R.P.; Bai, Y.H. Characteristics of Atmospheric Environment in China’s Coastal Areas and Atmospheric Corrosion in Typical Coastal Regions. Equip. Environ. Eng. 2017, 14, 65–69. [Google Scholar]
- Morcillo, M.; Diaz, I.; Cano, H.; Chico, B.; De la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Al Ashhab, A.; Sweity, A.; Bayramoglu, B.; Herzberg, M.; Gillor, O. Biofouling of reverse osmosis membranes: Effects of cleaning on biofilm microbial communities, membrane performance, and adherence of extracellular polymeric substances. Biofouling 2017, 33, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Liu, W.L.; Xie, W.C. Influence of Sodium Lignosulfonate on the Corrosion-Inhibition Behavior of Q235 Steel in Simulated Concrete Pore Solutions. Int. J. Electrochem. Sci. 2020, 15, 7136–7151. [Google Scholar] [CrossRef]
- Fazayel, A.; Khorasani, M.; Sarabi, A. The effect of functionalized polycarboxylate structures as corrosion inhibitors in a simulated concrete pore solution. Appl. Surf. Sci. 2018, 441, 895–913. [Google Scholar] [CrossRef]
- Wu, M.; Ma, H.F.; Shi, J.J. Enhanced corrosion resistance of reinforcing steels in simulated concrete pore solution with low molybdate to chloride ratios. Cem. Concr. Compos. 2020, 110, 103589. [Google Scholar] [CrossRef]
- Asipita, S.A.; Ismail, M.; Majid, M.Z.A.; Majid, Z.A.; Abdullah, C.; Mirza, J. Green Bambusa Arundinacea leaves extract as a sustainable corrosion inhibitor in steel reinforced concrete. J. Clean. Prod. 2014, 67, 139–146. [Google Scholar] [CrossRef]
- Montemor, M. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Weishaar, A.; Carpenter, M.; Loucks, R.; Sakulich, A.; Peterson, A.M. Evaluation of self-healing epoxy coatings for steel einforcement. Constr. Build. Mater. 2018, 191, 125–135. [Google Scholar] [CrossRef]
- Heidarshenas, B.; Nasehi, M.; Hammoud, A.; Yuan, Y.J. Fabrication and characterization of incorporated nickel coatings with different loadings of tungsten carbide microparticles: Investigation of mechanical and chemical properties. Mater. Chem. Phys. 2023, 310, 128465. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Athanasopoulou, E.; Snihirova, D.; Martins, B.; Koklioti, M.A.; Montem, M.F.; Kordas, G.; Charitidis, C.A. Multifunctional epoxy coatings combining a mixture of traps and inhibitor loaded nanocontainers for corrosion protection of AA2024-T3. Corros. Sci. 2014, 85, 147–159. [Google Scholar] [CrossRef]
- Liu, H.W.; Xu, D.K.; Yang, K.; Liu, H.F.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Siva, T.; Sathiyanarayanan, S. Self-healing coatings containing dual active agent loaded urea formaldehyde (UF) microcapsules. Prog. Org. Coat. 2015, 82, 57–67. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Surface-Engineered Nanocontainers for Entrapment of Corrosion Inhibitors. Adv. Funct. Mater. 2007, 17, 1451–1458. [Google Scholar] [CrossRef]
- Liu, W.L.; Li, J.S.; Huang, X.Q.; Bi, J.Y. Corrosion Protection of Q235 Steel Using Epoxy Coatings Loaded with Calcium Carbonate Microparticles Modified by Sodium Lignosulfonate in Simulated Concrete Pore Solutions. Materials 2021, 14, 1982. [Google Scholar] [CrossRef] [PubMed]
- Cotting, F.; Aoki, I.V. Smart protection provided by epoxy clear coating doped with polystyrene microcapsules containing silanol and Ce (III) ions as corrosion inhibitors. Surf. Coat. Technol. 2016, 303 Pt B, 310–318. [Google Scholar] [CrossRef]
- Fang, J.J.; Xu, K.; Zhu, L.H.; Zhou, Z.X.; Tang, H.Q. A study on mechanism of corrosion protection of polyaniline coating and its failure. Corros. Sci. 2007, 49, 4232–4242. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wei, X.X.; Zhang, B. Oxide film formed on Al alloy beneath sulfosalicylic acid doped polyaniline incorporated into epoxy organic coating. Appl. Surf. Sci. 2020, 512, 144840. [Google Scholar] [CrossRef]
- Deberry, D.W. Modification of the Electrochemical and Corrosion Behavior of Stainless Steels with an Electroactive Coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Corrosion protection of steel by polyaniline blended coating. Electrochim. Acta 2006, 51, 6313–6319. [Google Scholar] [CrossRef]
- Kinlen, P.J.; Ding, Y.; Silverman, D.C. Corrosion Protection of Mild Steel Using Sulfonic and Phosphonic Acid-Doped Polyanilines. Corrosion 2016, 58, 490–497. [Google Scholar] [CrossRef]
- Bairi, V.G.; Warford, B.A.; Bourdo, S.E.; Biris, A.S.; Viswanathan, T. Synthesis and characterization of tanninsulfonic acid doped polyaniline–metal oxide nanocomposites. J. Appl. Polym. Sci. 2012, 124, 3320–3328. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Xie, X.J.; He, J.; Lv, K.; Pan, L. Study on the synergistic inhibitive effect of an imidazole derivative and BTA on copper in deionised water, Anti-Corros. Methods Mater. 2009, 56, 271–274. [Google Scholar]
- Matheswaran, P.; Ramasamy, A.K. Influence of Benzotriazole on Corrosion Inhibition of Mild Steel in Citric Acid Medium. J. Chem. 2010, 7, 1090–1094. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Ma, L.W.; Wang, J.K.; Zhang, D.W.; Li, X.G. Saline-responsive triple-action self-healing coating for intelligent corrosion control. Mater. Des. 2022, 214, 110381. [Google Scholar] [CrossRef]
- Vimalanandan, A.; Lv, L.P.; Tran, T.H.; Landfester, D.; Crespy, D.; Rohwerder, M. Redox-Responsive Self-Healing for Corrosion Protection. Adv. Mater. 2013, 48, 6980–6984. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Z.Y.; Wang, D.; Yu, F.J.; Du, B.S.; Gitsov, I. Improving the Protection Performance of Waterborne Coatings with a Corrosion Inhibitor Encapsulated in Polyaniline-Modified Halloysite Nanotubes. Coatings 2023, 13, 1677. [Google Scholar] [CrossRef]
- Hao, Y.S.; Sun, W.; Jiang, L.L.; Cui, J.T.; Zhang, Y.Y.; Song, L.X.; Zhang, Y.L. Self-healing effect of epoxy coating containing mesoporous polyaniline hollow spheres loaded with benzotriazole. Prog. Org. Coat. 2021, 159, 106445. [Google Scholar] [CrossRef]
- Hao, Y.S.; Zhao, Y.F.; Yang, X.X.; Hu, B.; Ye, S.W.; Song, L.X.; Li, R.G. Self-healing epoxy coating loaded with phytic acid doped polyaniline nanofibers impregnated with benzotriazole for Q235 carbon steel. Corros. Sci. 2019, 151, 175–189. [Google Scholar] [CrossRef]
- Bi, J.Y.; Li, J.S.; Wang, H.J.; Li, Y.M.; Lu, G.X. Study on corrosion resistance of epoxy coatings containing polyaniline doped with different acids. Electroplat. Finish. 2022, 41, 1435–1443. [Google Scholar]
- Hao, Y.S.; Zhao, Y.F.; Li, B.; Song, L.X.; Guo, Z. Self-healing effect of graphene@PANI loaded with benzotriazole for carbon steel. Corros. Sci. 2020, 163, 108246. [Google Scholar] [CrossRef]
- Dominis, A.J.; Spinks, G.M.; Kane-Maguire, L.A.P.; Wallance, G.G. A de-doping/re-doping study of organic soluble polyaniline. Synth. Met. 2002, 129, 165–172. [Google Scholar] [CrossRef]
- Titelman, G.I.; Siegmann, A.; Narkis, M.; Wei, Y. Morphology of polyaniline redoped by kneading with dodecylbenzene sulfonic acid. J. Appl. Polym. Sci. 1998, 69, 2205–8995. [Google Scholar] [CrossRef]
- Takashi, M.; Yukitoshi, T.; Azusa, I.; Takashi, S. Simulation of water absorption and desorption behavior for anti-corrosion coatings in existing and new accelerated corrosion tests. Prog. Org. Coat. 2018, 120, 71–78. [Google Scholar]
- Cruz-Silva, R.; Romero-García, J.; Angulo-Sánchez, J.L.; Flores-Loyola, E.; Farías, M.H.; Castillón, F.F.; Díaz, J.A. Comparative study of polyaniline cast films prepared from enzymatically and chemically synthesized polyaniline. Polymer 2004, 45, 4711–4717. [Google Scholar] [CrossRef]
- Zhou, S.P.; Zhang, H.M.; Zhao, Q.; Wang, X.H.; Li, J.; Wang, F.S. Graphene-wrapped polyaniline nanofibers as electrode materials for organic supercapacitors. Carbon 2013, 52, 440–450. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, H.J.; Li, J.S.; Li, G.Q.; Huang, X.Q.; Bi, J.Y. Preparation of polyanilines doped with different acids and their application as antifoulant in antifouling paints. Electroplat. Finish. 2020, 39, 323–328. [Google Scholar]
- Gao, X. Preparation and Properties of Silane Compositeanticorrosion Coatings on Mg-Li Alloy; Harbin Engineering University: Harbin, China, 2017. [Google Scholar]
- Ramezanzadeh, B.; Ghasemi, E.; Askari, F. Synthesis and characterization of a new generation of inhibitive pigment based on zinc acetate/benzotriazole: Solution phase and coating phase studies. Dyes Pigm. 2015, 122, 331–345. [Google Scholar] [CrossRef]
- Laska, J.; Girault, R.; Quillard, S.; Louarn, G.; Proń, A.; Lefrant, S. Raman spectroscopic studies of polyaniline protonation with bis(2-ethylhexyl) hydrogen phosphate. Synth. Met. 1995, 75, 69–74. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J. Organic/Inorganic Acids Synthesis and Properties of Conducting Polyaniline Doped with Compound. J. Chem. Enginecring Chin. Univ. 2009, 23, 984–989. [Google Scholar]
- Zhang, H.B.; Wang, J.X.; Wang, Z.; Wang, S.C. A Novel Strategy for the Synthesis of Sheet-Like Polyaniline. Macromol. Rapid Commun. 2009, 18, 1577–1582. [Google Scholar] [CrossRef]
- Han, J.; Song, G.; Guo, R. Nanostructure-Based Leaf-like Polyaniline in the Presence of an Amphiphilic Triblock Copolymer. Adv. Mater. 2007, 19, 2993–2999. [Google Scholar] [CrossRef]
- Shivani, K.; Rajeev, M. Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of Acid Green 25 dye from wastewater. Polym. Bull. 2021, 78, 2439–2463. [Google Scholar]
- Surwade, S.P.; Dua, V.; Manohar, N.; Manohar, S.K.; Manohar, E.; Ferraris, J.P. Oligoaniline intermediates in the aniline–peroxydisulfate system. Synth Met. 2009, 159, 445–455. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchova, M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog. Polym. Sci. 2010, 12, 1420–1481. [Google Scholar] [CrossRef]
- Sanches, E.A.; da Silva, J.M.; Ferreira, J.M.d.O.; Soares, J.C.; dos Santos, A.L.; Trovati, G.; Fernandes, E.G.; Mascarenhas, Y.P. Nanostructured Polyaniline Emeraldine-base form (EB-PANI): A structural investigation for different neutralization times. J. Mol. Struct. 2014, 1074, 732–737. [Google Scholar] [CrossRef]
- Wang, P.C.; Dan, Y.P.; Liu, L.H. Effect of thermal treatment on conductometric response of hydrogen gas sensors integrated with HCl-doped polyaniline nanofibers. Mater. Chem. Phys. 2014, 144, 155–161. [Google Scholar] [CrossRef]
- Han, M.G.; Cho, S.K.; Oh, S.G.; Im, S.S. Preparation and characterization of polyaniline nanoparticles synthesized from DBSA micellar solution. Synth. Met. 2002, 126, 53–60. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ng, S.C.; Ho, P.K.H. Polyanilines Doped with Phosphonic Acids: Their Preparation and Characterization. Macromolecules 1994, 8, 2159–2164. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Saline-Responsive and Hydrogen Bond Gating Effects in Self-Healing Polyaniline. ACS Appl. Polym. Mater. 2020, 2, 2311–2318. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, Z.; Guo, Y. Study on the Corrosion Resistance of Superhydrophobic Polyaniline Coating on Mercerized Wax. Paint. Coat. Ind. 2023, 53, 9–15. [Google Scholar]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Aronhime, M.T.; Peng, X.; Gillham, J.K.; Small, R.D. Effect of time-temperature path of cure on the water absorption of high Tg epoxy resins. J. Appl. Polym. Sci. 1986, 32, 3589–3626. [Google Scholar] [CrossRef]
- El-Sayed, A.R.; Harm, U.; Mangold, K.M.; Fuerbeth, W. Protection of galvanized steel from corrosion in NaCl solution by coverage with phytic acid SAM modified with some cations and thiols. Corros. Sci. 2012, 55, 339–350. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.H.; Yan, R.; Tian, H.W.; Ma, H.Y. Effect of zinc ion on the microstructure and electrochemical behavior of phytic acid based conversion coatings on Q235 steels. Surf. Coat. Technol. 2017, 325, 248–256. [Google Scholar] [CrossRef]
- Cui, X.F.; Li, Y.; Li, Q.F.; Wang, F.H. Influence of phytic acid concentration on performance of phytic acid conversion coatings on the AZ91D magnesium alloy. Mater. Chem. Phys. 2008, 111, 503–507. [Google Scholar] [CrossRef]
- Fahlman, M.; Jasty, S.; Epstein, A.J. Corrosion protection of iron/steel by emeraldine base polyaniline: An X-ray photoelectron spectroscopy study. Synth. Met. 1997, 85, 1323–1326. [Google Scholar] [CrossRef]
- Liu, Y.W. Influence of Seawater on the Carbon Steel Initial Corrosion Behavior. Int. J. Electrochem. Sci. 2019, 14, 1147–1162. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, Y.C.; Xu, H. Electrochemical Analyzing Methods for Protective Properties of Organic Coatings. Coat. Prot. 2023, 11, 40–47. [Google Scholar]
- Zuo, Y.; Pang, R.; Li, W.; Xiong, J.P.; Tang, Y.M. The evaluation of coating performance by the variations of phase angles in middle and high frequency domains of EIS. Corros. Sci. 2008, 50, 3322–3328. [Google Scholar] [CrossRef]
- Gupta, G.; Birbilis, N.; Cook, A.B.; Khanna, A.S. Polyaniline-lignosulfonate/epoxy coating for corrosion protection of AA2024-T3. Corros. Sci. 2013, 67, 256–267. [Google Scholar] [CrossRef]
- Tian, H.Y.; Sun, F.; Chu, F.H.; Wang, L.W.; Wang, X.; Cui, Z.Y. Passivation behavior and surface chemistry of 316 SS in the environment containing Cl− and NH4+. J. Electroanal. Chem. 2021, 886, 115138. [Google Scholar] [CrossRef]
- Gao, C. Investigation on Modification of Kraft Ligninand Its Corrosion Inhibition Performance; Qilu University of Technology: Jinan, China, 2020. [Google Scholar]
- Babic-Samardzija, K.; Lupu, C.; Hackerman, N.; Barron, A.R. Inhibitive properties, adsorption and surface study of butyn-1-ol and pentyn-1-olalcohols as corrosion inhibitors for iron in HCl. J. Electroanal. Chem. 2005, 15, 1908–1916. [Google Scholar]
- Wu, C.Q.; Liu, Q.; Chen, R.R.; Liu, J.Y.; Zhang, H.S.; Li, R.M.; Takahashi, K.; Liu, P.L.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef]
- Qiao, Z.; Lei, K.; Jiang, F.; Song, L.F. Durable superhydrophobic silica/epoxy resin coating for the enhanced corrosion protection of steel substrates in high salt and H2S environments. Colloids Surf. A 2022, 654, 130137. [Google Scholar]
- Vandana, A.M.; Abhijit, A.B.; Pravin, P.D. Graphene oxide-modified polyaniline pigment for epoxy based anti-corrosion coatings. Chem. Pap. 2017, 71, 1515–1528. [Google Scholar]
- Corrales Luna, M.; Le Manh, T.; Cabrera Sierra, R.; Medina Flores, J.V.; Lartundo Rojas, L.; Arce Estrada, E.M. Study of corrosion behavior of API 5L X52 steel in sulfuric acid in the presence of ionic liquid 1-ethyl 3-methylimidazolium thiocyanate as corrosion inhibitor. J. Mol. Liq. 2019, 289, 111106. [Google Scholar] [CrossRef]
- Chen, Y. Study on the Evaluation of Protective Coatings and the Inhibitor Technique; Zhejiang University: Hangzhou, China, 2013. [Google Scholar]
- Gattinoni, C.; Michaelides, A. Understanding corrosion inhibition with van der Waals DFT methods: The case of benzotriazole. Faraday Discuss. 2015, 180, 439–458. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Huang, L.; Zhang, G.A.; Qiu, Y.; Guo, X.P. Benzotriazole as a volatile corrosion inhibitor during the early stage of copper corrosion under adsorbed thin electrolyte layers. Corros. Sci. 2012, 65, 214–222. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, J. Preparation of waterborne intelligent anticorrosive coating materials with corrosion-inhibitor-loaded silicamicrocapsules and study on properties of the coatings obtained therefrom. Electroplat. Finish. 2018, 37, 231–235. [Google Scholar]
- Saremi, M.; Yeganeh, M. Application of mesoporous silica nanocontainers as smart host of corrosion inhibitor in polypyrrole coatings. Corros. Sci. 2014, 86, 159–170. [Google Scholar] [CrossRef]
- Wang, J.; Li, H. Study on Anticorrosion Properties of Magadiite in Coatings. Paint. Coat. Ind. 2021, 51, 18–23. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).