Improving the Wear Resistance Properties of 7A04 Aluminum Alloy with Three Surface Modification Coatings
Abstract
1. Introduction
2. Test Materials and Methods
2.1. Test Material
2.2. Test Method
3. Results and Discussion
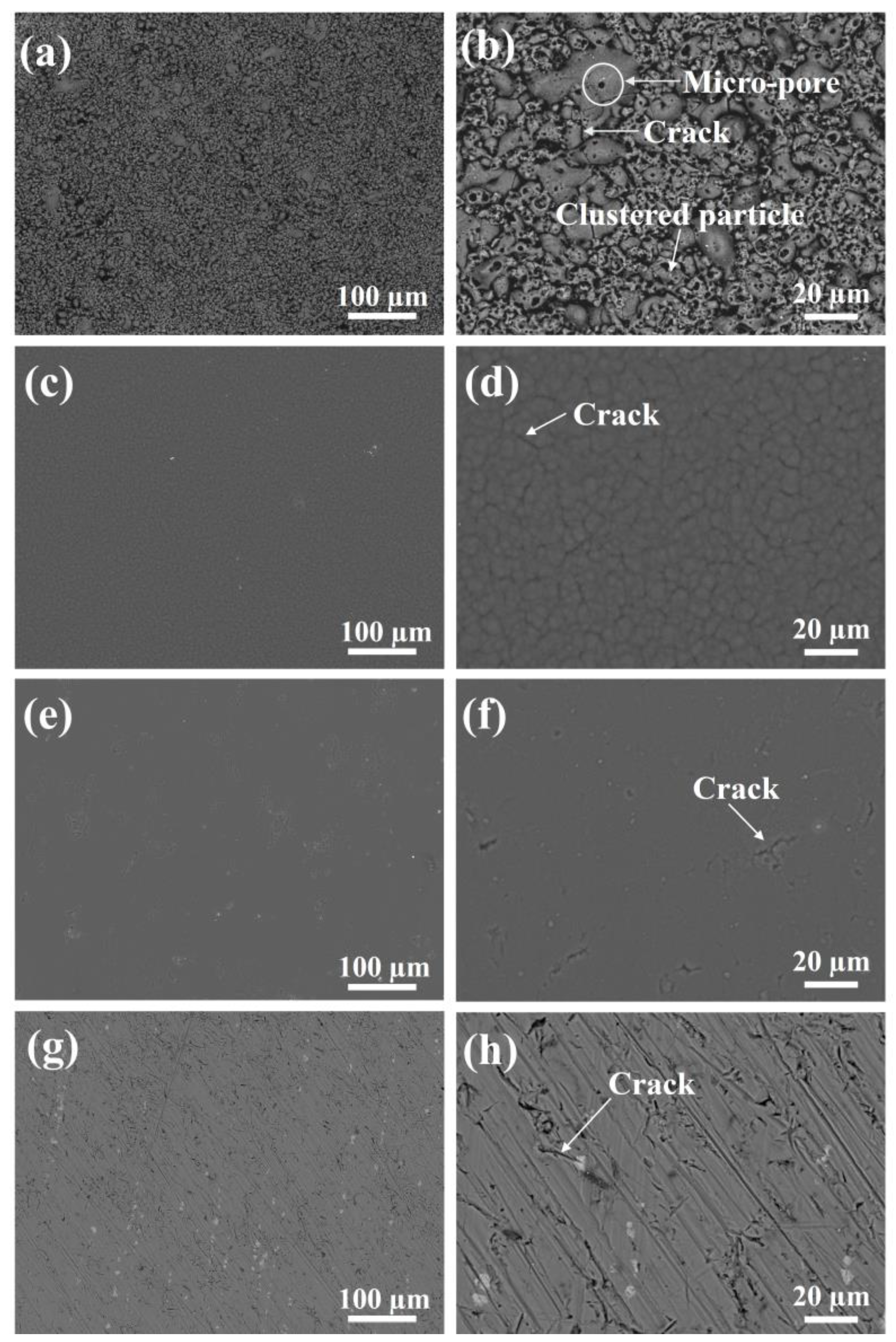
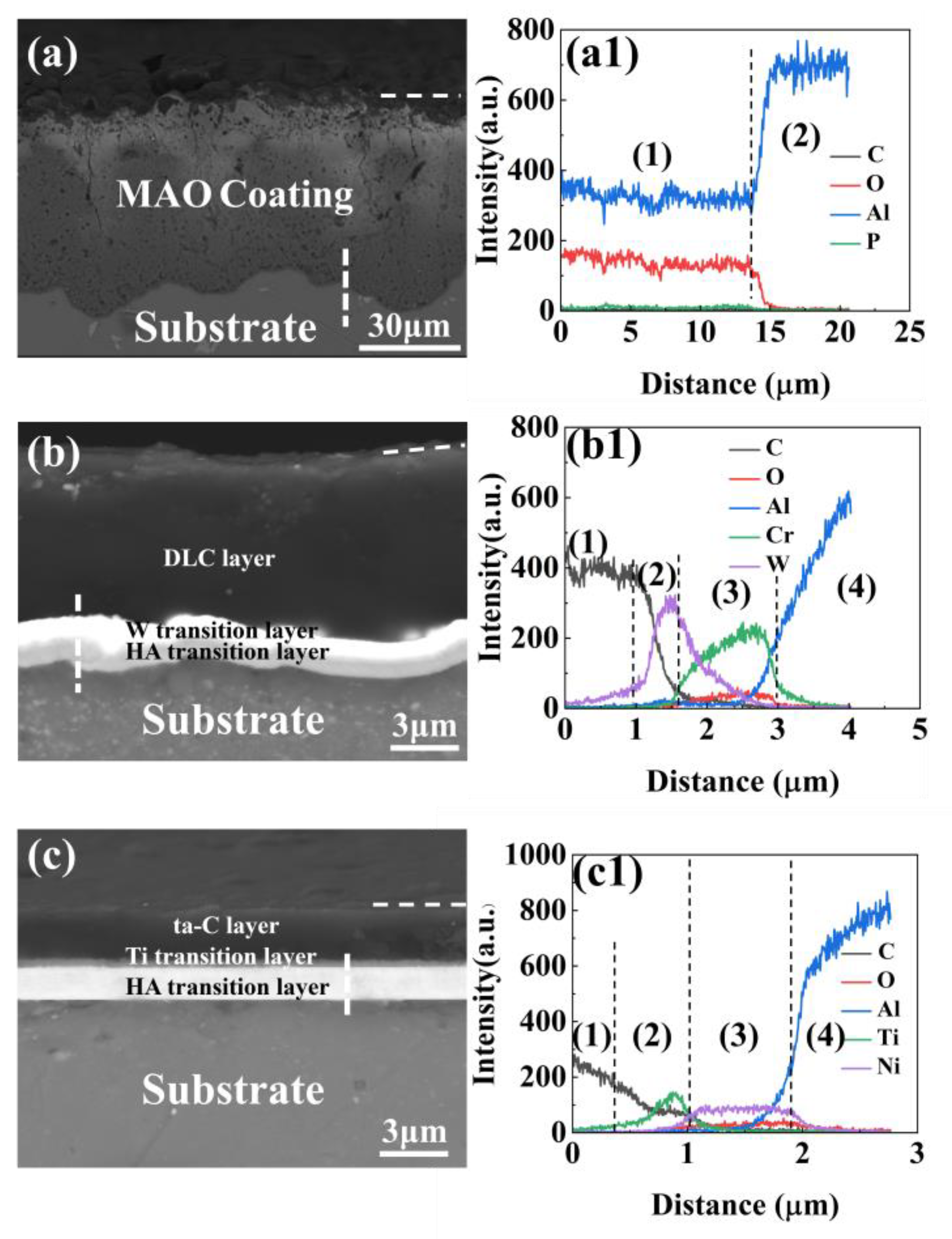
3.1. Pre-Wear Microscopic Morphology Analysis
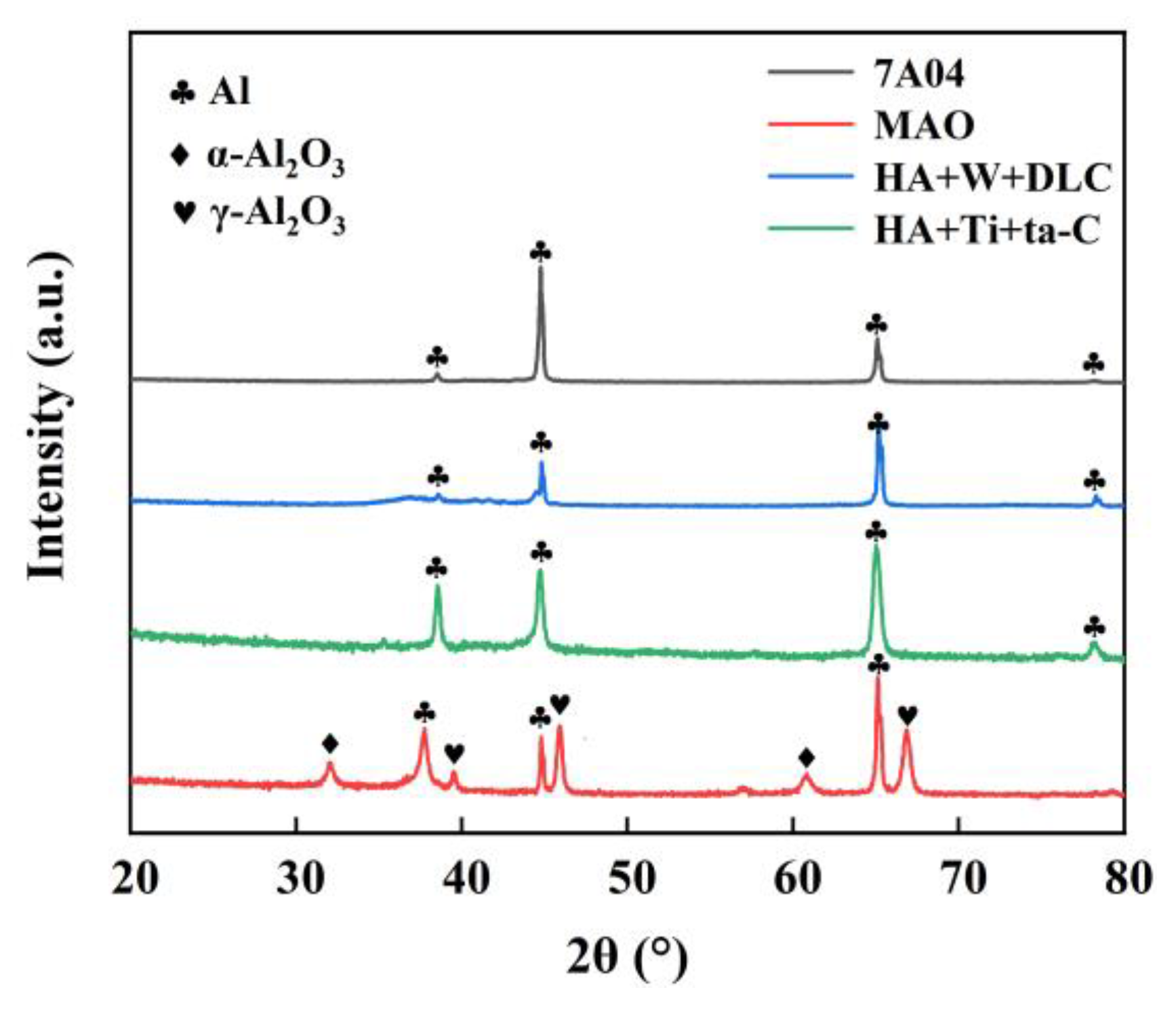
3.2. XRD Patterns
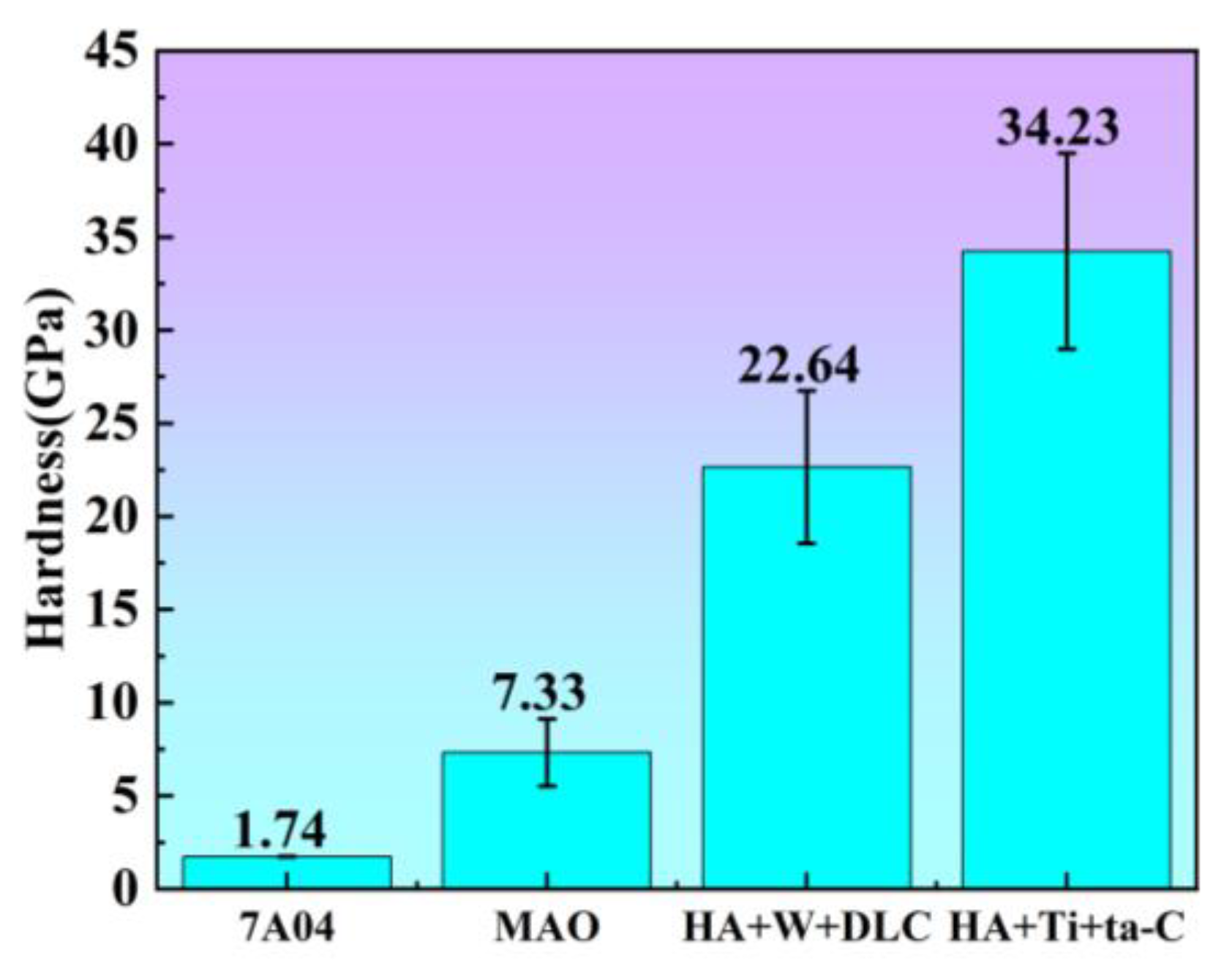
3.3. Hardness Analysis
3.4. Wear Test
3.4.1. Friction Coefficient and Wear Rate
3.4.2. Three-Dimensional Morphology
3.4.3. Wear Surface Morphology Analysis
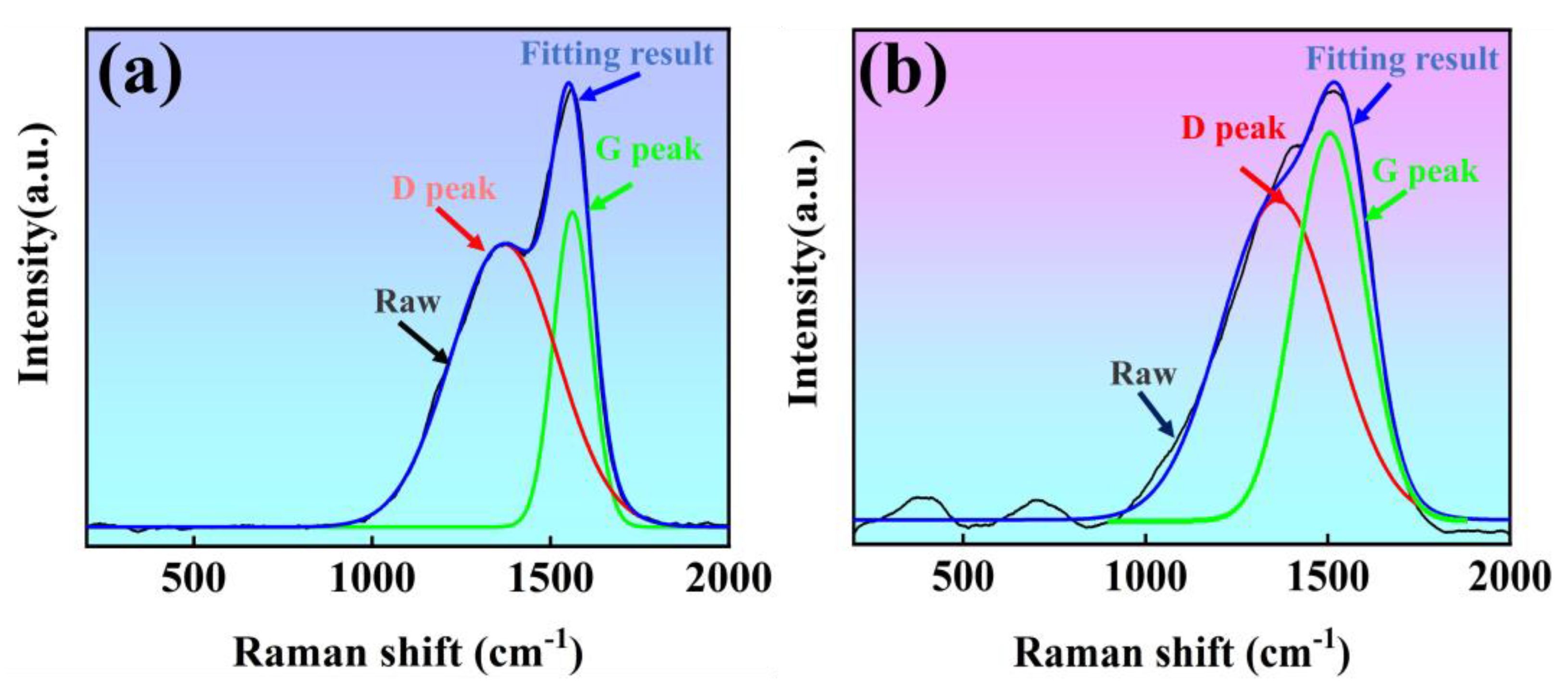
3.4.4. Raman Spectroscopy
4. Conclusions
- (1)
- In this work, MAO, HA+W+DLC, and HA+Ti+ta-C coatings were successfully prepared on a 7A04 aluminum alloy substrate. The surface hardness was significantly improved after coating. The hardnesses of three coated samples were 7.33 GPa, 22.64 GPa, and 34.23 GPa, respectively. The highest hardness of the HA+Ti+ta-C coatings resulted from the high sp3 C-C bond content.
- (2)
- During the ring-block wear tests under oil lubrication, both multilayer coatings exhibited excellent wear resistance. The average coefficient of friction and wear rate of HA+W+DLC and HA+Ti+ta-C were, respectively, 0.028 and 1.51 × 10−7 mm3/N·m, and 0.025 and 1.36 × 10−7 mm3/N·m. The higher surface hardness of the HA+Ti+ta-C coating led to better wear resistance, which suggests that the coating can be applied in the surface protection of aluminum alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Tisza, M.; Czinege, I. Comparative study of the application of steels and aluminium in lightweight production of automotive parts. Int. J. Lightweight Mater. Manuf. 2018, 1, 229–238. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Hui, L.; Zhou, S. Fatigue life prediction of aviation aluminium alloy based on quantitative pre-corrosion damage analysis. Trans. Nonferrous Met. Soc. China 2017, 27, 1353–1362. [Google Scholar] [CrossRef]
- Vigneshwar, M.; Selvamani, S.T.; Hariprasath, P.; Palanikumar, K. Analysis of mechanical, metallurgical and fatigue behavior of friction welded AA6061-AA2024 dissimilar aluminum alloys in optimized condition. Mater. Today Proc. 2018, 5, 7853–7863. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, H.; Miao, J.; Du, J. Effect of oxidation time on the microstructure and properties of ceramic coatings prepared by microarc oxidation on 7A04 superhard aluminum alloy. Int. J. Mod. Phys. B 2017, 31, 1744026. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, B.; Yao, W.; Hong, X.; Song, S. Atmospheric Corrosion of Anodized Pure Al 1060, Al-alloys 2A12 and 7A04 Exposed to Polluted Atmospheric Environment at Jiangjin Region. J. Chin. Soc. Corros. Prot. 2017, 37, 273–278. [Google Scholar]
- Su, Y.; Gui, X.; Xie, D.; Li, S.Y.; Sun, H.; Leng, Y.; Huang, N. The effect of a TiN interlayer on the tribological properties of diamond-like carbon films deposited on 7A04 aluminum alloy. IEEE Trans. Plasma Sci. 2011, 39, 3144–3148. [Google Scholar] [CrossRef]
- Chen, X.W.; Song, H.; Pu, H.; Zheng, R.; Zhang, M.; Zhang, D.F. Study of wear and corrosion resistance of ATO nanoparticle–doped micro-arc oxide film layers. Int. J. Appl. Ceram. Technol. 2024, 21, 1078–1093. [Google Scholar] [CrossRef]
- Cheng, C.; Ngan, A.H.W. Chemo-mechanical softening during in situ nanoindentation of anodic porous alumina with anodization processing. J. Appl. Phys. 2013, 113, 184903. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. IMF 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Shchedrina, I.; Rakoch, A.G.; Henrion, G.; Martin, J. Non-destructive methods to control the properties of MAO coatings on the surface of 2024 aluminium alloy. Surf. Coat. Technol. 2014, 238, 27–44. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Pukha, V.E.; Maleyev, M.V.; Kim, D.E. Ultra-thin nano-patterned wear-protective diamond-like carbon coatings deposited on glass using a C60 ion beam. Carbon 2014, 80, 534–543. [Google Scholar] [CrossRef]
- Yokota, T.; Sawa, T.; Yokouchi, M.; Tozawa, K.; Anzai, M.; Aizawa, T. Frictional properties of diamond-like carbon coated tool in dry intermittent machining of aluminum alloy 5052. Precis. Eng. 2014, 38, 365–370. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180, 76–84. [Google Scholar] [CrossRef]
- Volosova, M.; Grigoriev, S.; Metel, A.; Shein, A. The role of thin-coating vacuum-plasma coatings and their influence on the efficiency of ceramic cutting inserts. Coatings 2018, 8, 287. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Volosova, M.A.; Fedorov, S.V.; Mosyanov, M. Influence of DLC coatings deposited by PECVD technology on the wear resistance of carbide end mills and surface roughness of AlCuMg2 and 41Cr4 workpieces. Coatings 2020, 10, 1038. [Google Scholar] [CrossRef]
- Hilbert, J.; Mangolini, F.; McClimon, J.B.; Lukes, J.R.; Carpick, R.W. Si doping enhances the thermal stability of diamond-like carbon through reductions in carbon-carbon bond length disorder. Carbon 2018, 131, 72–78. [Google Scholar] [CrossRef]
- Soffritti, C.; Fortini, A.; Nastruzzi, A.; Sola, R.; Merlin, M.; Garagnani, G.L. Dry sliding behavior of an aluminum alloy after innovative hard anodizing treatments. Materials 2021, 14, 3281. [Google Scholar] [CrossRef]
- Kwolek, P.; Obłój, A.; Kościelniak, B.; Buszta, R.; Tokarski, T.; Krupa, K.; Gradzik, A.; Nowak, W.J.; Wojnicki, M.; Motyka, M. Wear resistance of hard anodic coatings fabricated on 5005 and 6061 aluminum alloys. Arch. Civ. Mech. Eng. 2024, 24, 51. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Guo, Y.Q.; Xiang, N.; Lu, X.Y.; Hu, Q.; Song, R.G. Sliding wear behaviour and microstructure of PEO coatings formed on aluminium alloy. Mater. Sci. Technol. 2016, 32, 1559–1566. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, J.; Cui, S.; Chen, P.; Wu, Z.; Ma, Z.; Fu, R.K.Y.; Tian, X.; Chu, P.K.; Wu, Z. Wear and corrosion resistant coatings prepared on LY12 aluminum alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2021, 409, 126885. [Google Scholar] [CrossRef]
- Zou, Y.S.; Zhou, K.; Wu, Y.F.; Yang, H.; Cang, K.; Song, G.H. Structure, mechanical and tribological properties of diamond-like carbon films on aluminum alloy by arc ion plating. Vacuum 2012, 86, 1141–1146. [Google Scholar] [CrossRef]
- Srinivasan, N.; Bhaskar, L.K.; Kumar, R.; Baragetti, S. Residual stress gradient and relaxation upon fatigue deformation of diamond-like carbon coated aluminum alloy in air and methanol environments. Mater. Des. 2018, 160, 303–312. [Google Scholar] [CrossRef]
- Sui, X.; Liu, J.; Zhang, S.; Yang, J.; Hao, J. Microstructure, mechanical and tribological characterization of CrN/DLC/Cr-DLC multilayer coating with improved adhesive wear resistance. Appl. Surf. Sci. 2018, 439, 24–32. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, J.; Liao, B.; Zhang, X.; Zhao, Y.; Zeng, X.; Chen, L.; Pang, P.; Bao, F. Tribocorrosion and tribological behavior of Ti-DLC coatings deposited by filtered cathodic vacuum arc. Diam. Relat. Mater. 2022, 125, 108985. [Google Scholar] [CrossRef]
- Yi, M.; Wang, T.; Liu, Z.; Lei, J.; Qiu, J.; Xu, W. Tribological Performance of Steel/W-DLC and W-DLC/W-DLC in a Solid–Liquid Lubrication System Additivated with Ultrathin MoS2 Nanosheets. Lubricants 2023, 11, 433. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kalscheuer, C.; Thiex, M. Formation of tribochemical reaction layers on a metal modified amorphous carbon coating a-C: H: Zr (ZrCg). Tribol. Int. 2019, 135, 152–160. [Google Scholar] [CrossRef]
- Mohammadinia, E.; Elahi, S.M.; Shahidi, S. Structural and optical properties of Ni-embedded hydrogenated diamond-like carbon (Ni-DLC) prepared by co-deposition of RF-Sputtering and RF-PECVD method. Mater. Sci. Semicond. Process. 2018, 7, 7–12. [Google Scholar] [CrossRef]
- Sun, L.; Guo, P.; Ke, P.; Li, X.; Wang, A. Synergistic effect of Cu/Cr co-doping on the wettability and mechanical properties of diamond-like carbon films. Diam. Relat. Mater. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Wang, K.; Koo, B.-H.; Lee, C.-G.; Kim, Y.-J.; Lee, S.-H.; Byon, E. Effects of electrolytes variation on formation of oxide layers of 6061 Al alloys by plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2009, 19, 866–870. [Google Scholar] [CrossRef]
- Wen, S.; Huang, P. Principles of Tribology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Li, X.J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.G.; Guo, Y.Y.; Wang, Y.X. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Xie, H.; Cheng, Y.; Li, S.; Cao, J.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]
- Martini, C.; Ceschini, L.; Tarterini, F.; Paillard, J.M.; Curran, J.A. PEO layers obtained from mixed aluminate–phosphate baths on Ti–6Al–4V: Dry sliding behaviour and influence of a PTFE topcoat. Wear 2010, 269, 747–756. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhao, P.H.; Zhao, J.M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, W.; Hou, H.; Jin, Y.; Zeng, Z.; Wu, X.; Xue, Q. Achieving excellent anti-corrosion and tribological performance by tailoring the surface morphology and chemical composition of aluminum alloys. RSC Adv. 2014, 4, 60307–60315. [Google Scholar] [CrossRef]
- Cao, H.; Ye, X.; Li, H.; Qi, F.; Wang, Q.; Ouyang, X.; Zhao, N.; Liao, B. Microstructure, mechanical and tribological properties of multilayer Ti-DLC thick films on Al alloys by filtered cathodic vacuum arc technology. Mater. Des. 2021, 198, 109320. [Google Scholar] [CrossRef]
- Krishna, L.R.; Gupta, P.; Sundararajan, G. The influence of phase gradient within the micro arc oxidation (MAO) coatings on mechanical and tribological behaviors. Surf. Coat. Technol. 2015, 269, 54–63. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered amorphous and diamond like carbon. Phys. Rev. B Condens. Matter 2001, 64, 5414–5426. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Katagiri, G.; Ishida, H.; Ishitani, A.; Akamatsu, T. Raman spectra of diamondlike amorphous carbon films. Solid State Commun. 1988, 66, 1177–1180. [Google Scholar] [CrossRef]


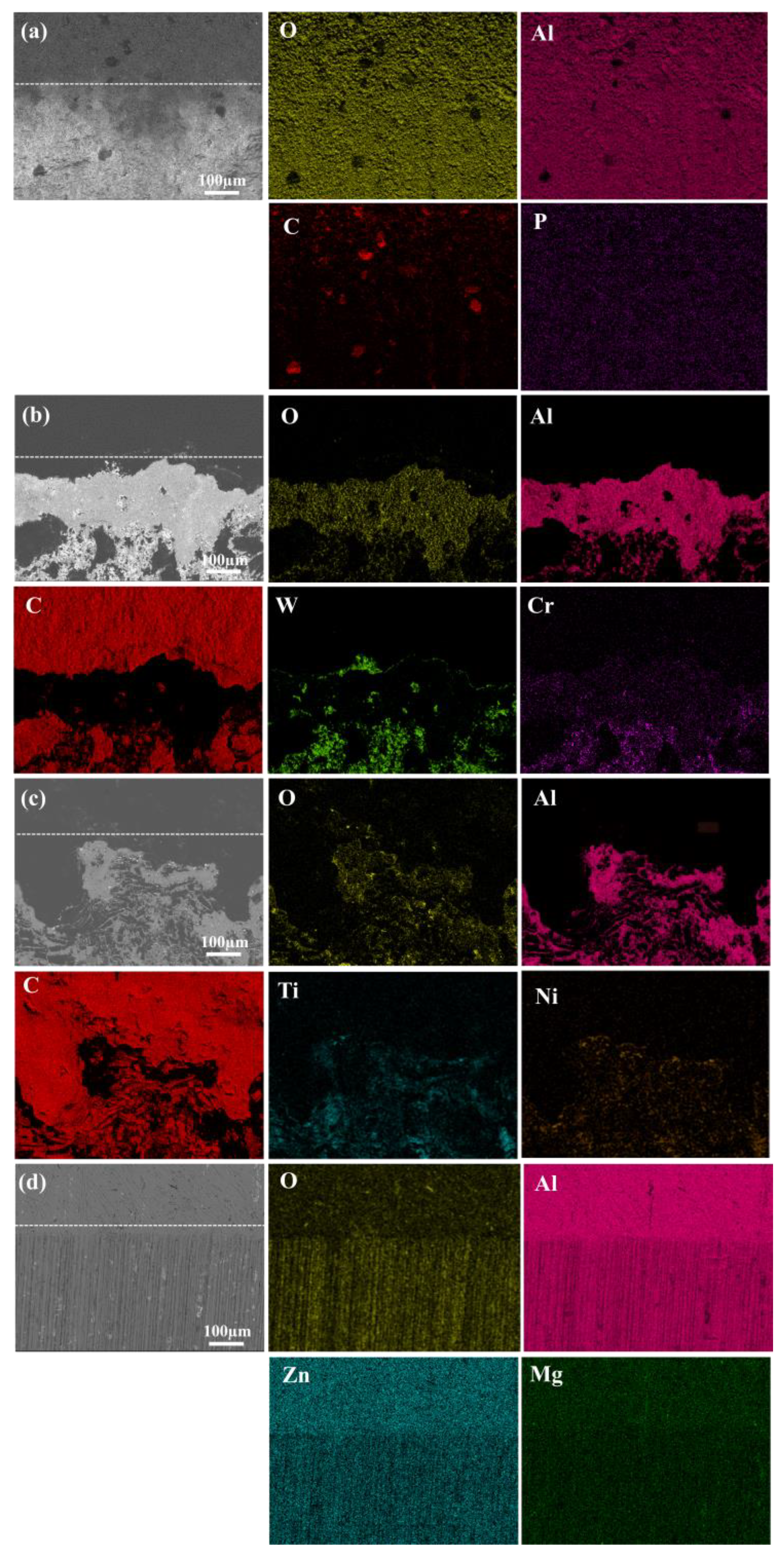
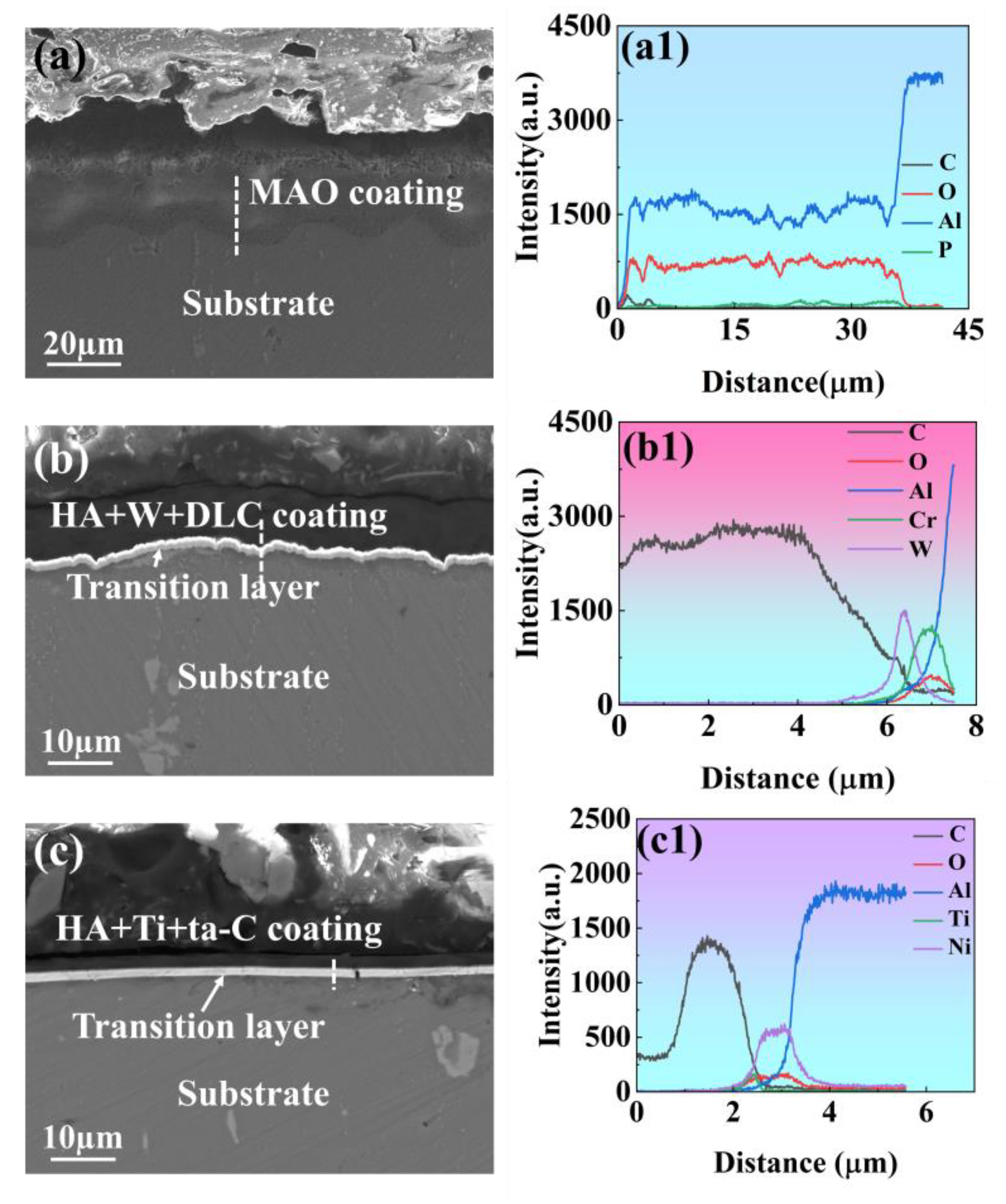
| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| content | 0.5 | 0.5 | 1.4–2.0 | 0.2–0.6 | 1.8–2.8 | 0.1–0.25 | 5.0–7.0 | 0.1 | Balance |
| Element | C | Si | Mn | Cr | Mo | Ni | Nb | Zr | V |
|---|---|---|---|---|---|---|---|---|---|
| content | 0.28 | <0.1 | 0.18 | 3.03 | 2.94 | 0.55 | 0.14 | 0.0012 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhang, C.; Wang, X.; Meng, X.; Dou, C.; Yu, H.; Wang, C.; Xue, J.; Qiao, Z.; Jiang, T. Improving the Wear Resistance Properties of 7A04 Aluminum Alloy with Three Surface Modification Coatings. Coatings 2024, 14, 476. https://doi.org/10.3390/coatings14040476
Hu J, Zhang C, Wang X, Meng X, Dou C, Yu H, Wang C, Xue J, Qiao Z, Jiang T. Improving the Wear Resistance Properties of 7A04 Aluminum Alloy with Three Surface Modification Coatings. Coatings. 2024; 14(4):476. https://doi.org/10.3390/coatings14040476
Chicago/Turabian StyleHu, Jinmeng, Cheng Zhang, Xiaodong Wang, Xiaobo Meng, Caihong Dou, Hua Yu, Changji Wang, Jun Xue, Ziping Qiao, and Tao Jiang. 2024. "Improving the Wear Resistance Properties of 7A04 Aluminum Alloy with Three Surface Modification Coatings" Coatings 14, no. 4: 476. https://doi.org/10.3390/coatings14040476
APA StyleHu, J., Zhang, C., Wang, X., Meng, X., Dou, C., Yu, H., Wang, C., Xue, J., Qiao, Z., & Jiang, T. (2024). Improving the Wear Resistance Properties of 7A04 Aluminum Alloy with Three Surface Modification Coatings. Coatings, 14(4), 476. https://doi.org/10.3390/coatings14040476







