Corrosion Behavior of Hybrid Zinc Coatings Based on Chitosan and Corrosion Inhibitor BTA: Effect of the Molecular Weight and ζ-Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation Procedure
2.2. Methods—Particle Synthesis
2.3. Characterization of the Electrokinetic Charge and Size of the Composite Liposomes
2.4. Determination of the Encapsulated Amount of Corrosion Inhibitor
2.5. Stability of the Uploaded and BTA-Loaded Particles at the Corrosion Conditions
2.6. Transmission Electron Microscopy (TEM)
2.7. Electrodeposition of Hybrid Coatings with Incorporated Chitosan Particles of Low- and High-Polymer Molecular Weight
2.8. Surface Morphology
2.9. Corrosion Characterization and CVA Studies
2.10. Atomic Force Microscopy (AFM) and Water Contact Angle Measurements
2.11. Test Medium and Reproducibility
3. Results and Discussion
3.1. Characterization of the Chitosan-TPP Particles
3.2. Estimation of the Loaded Amount from the Corrosion Inhibitor BTA
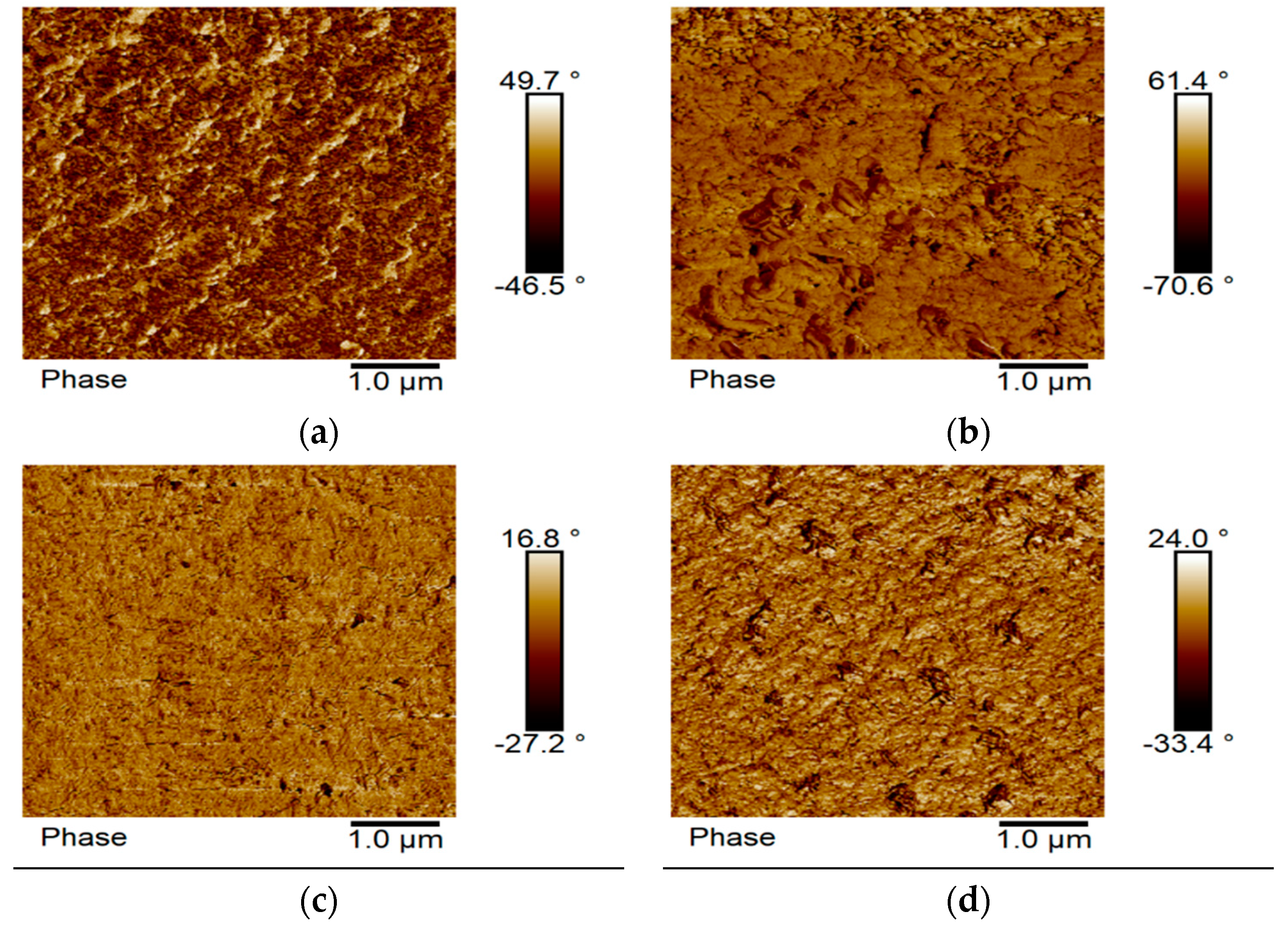
3.3. Surface Morphology
3.4. Behavior of the Polymer Particles at the Corrosion Conditions
3.5. Cyclic Voltammetry (CVA) Studies
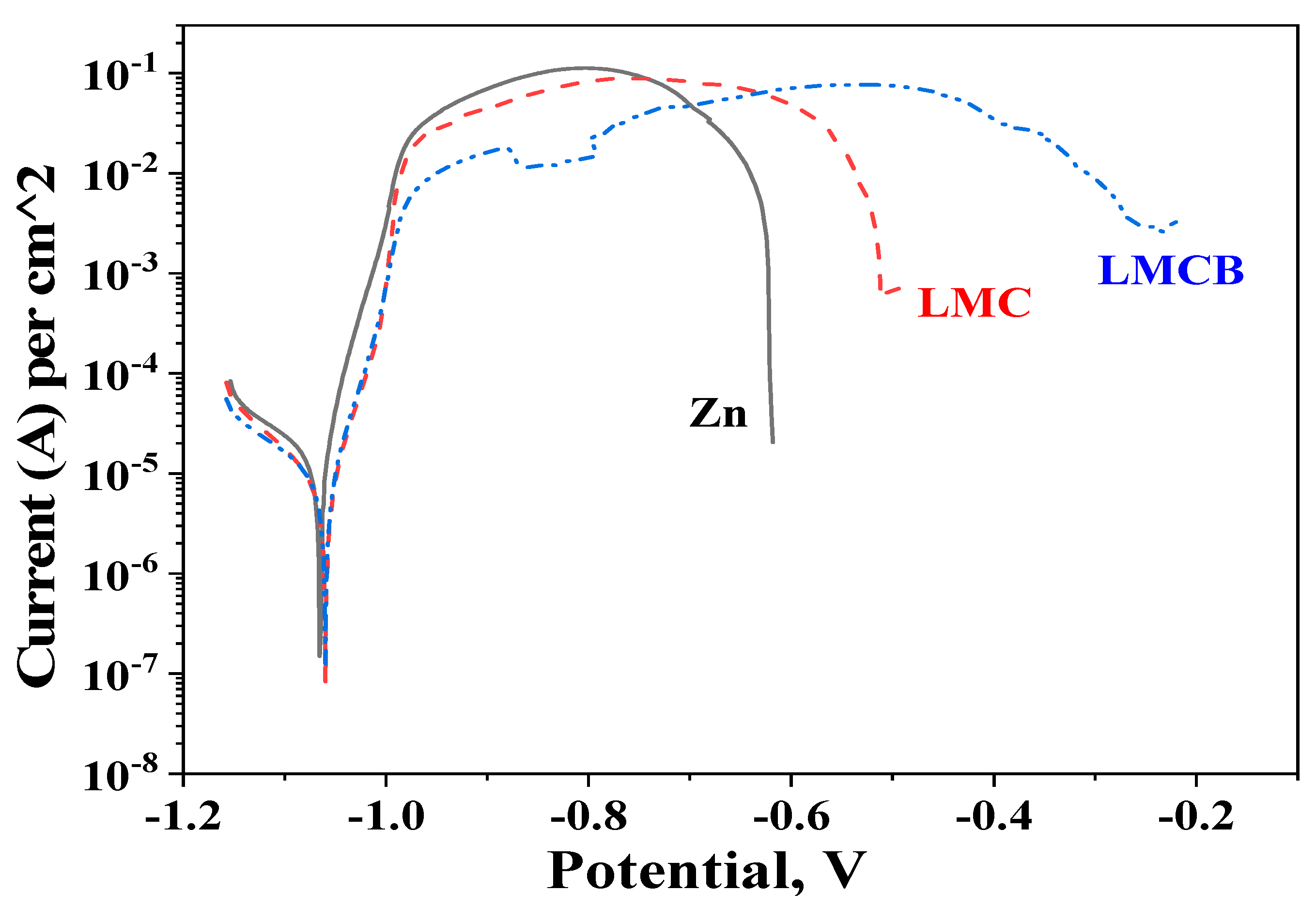
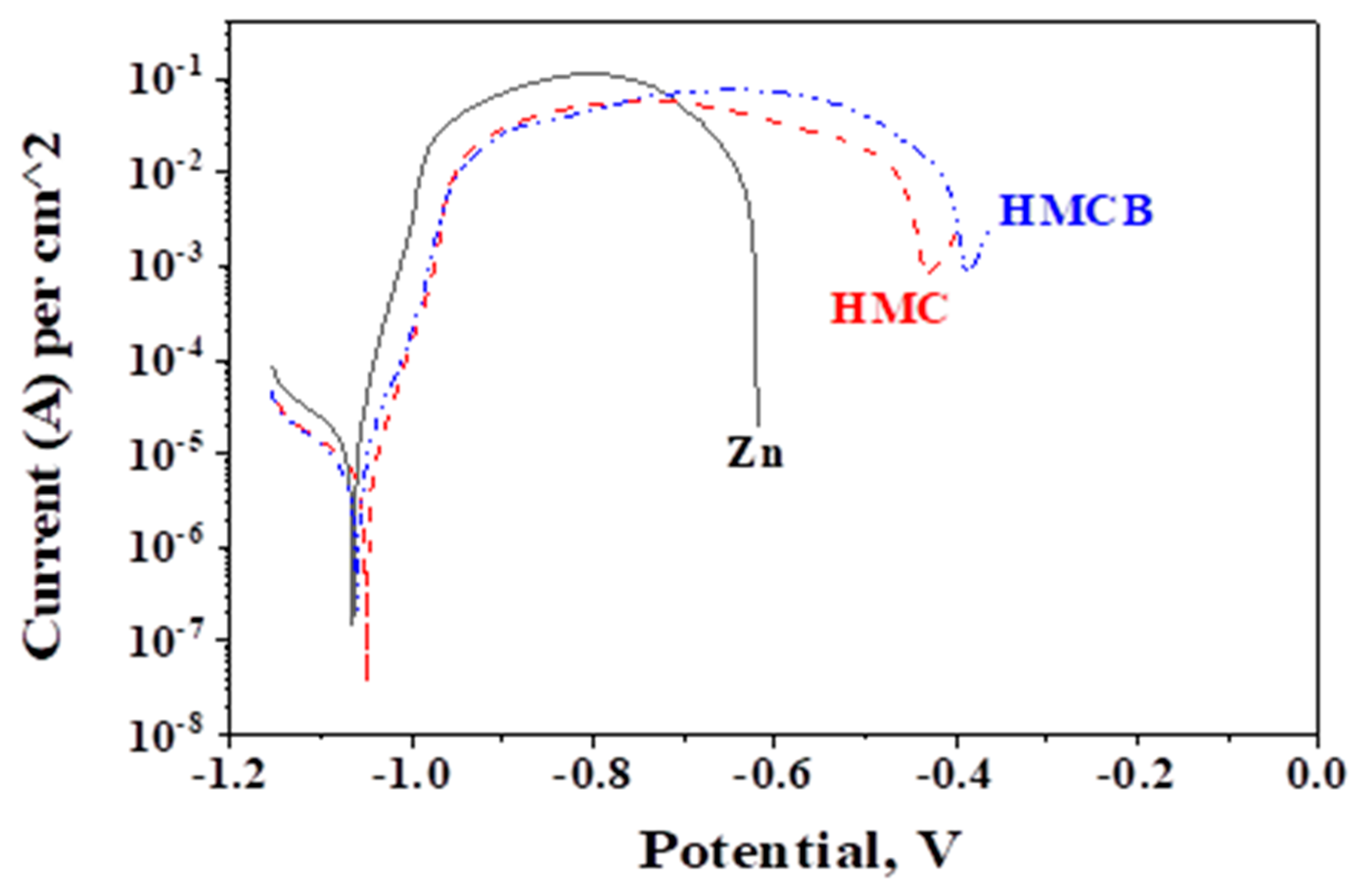
3.6. Potentiodynamic Polarization (PDP) Curves
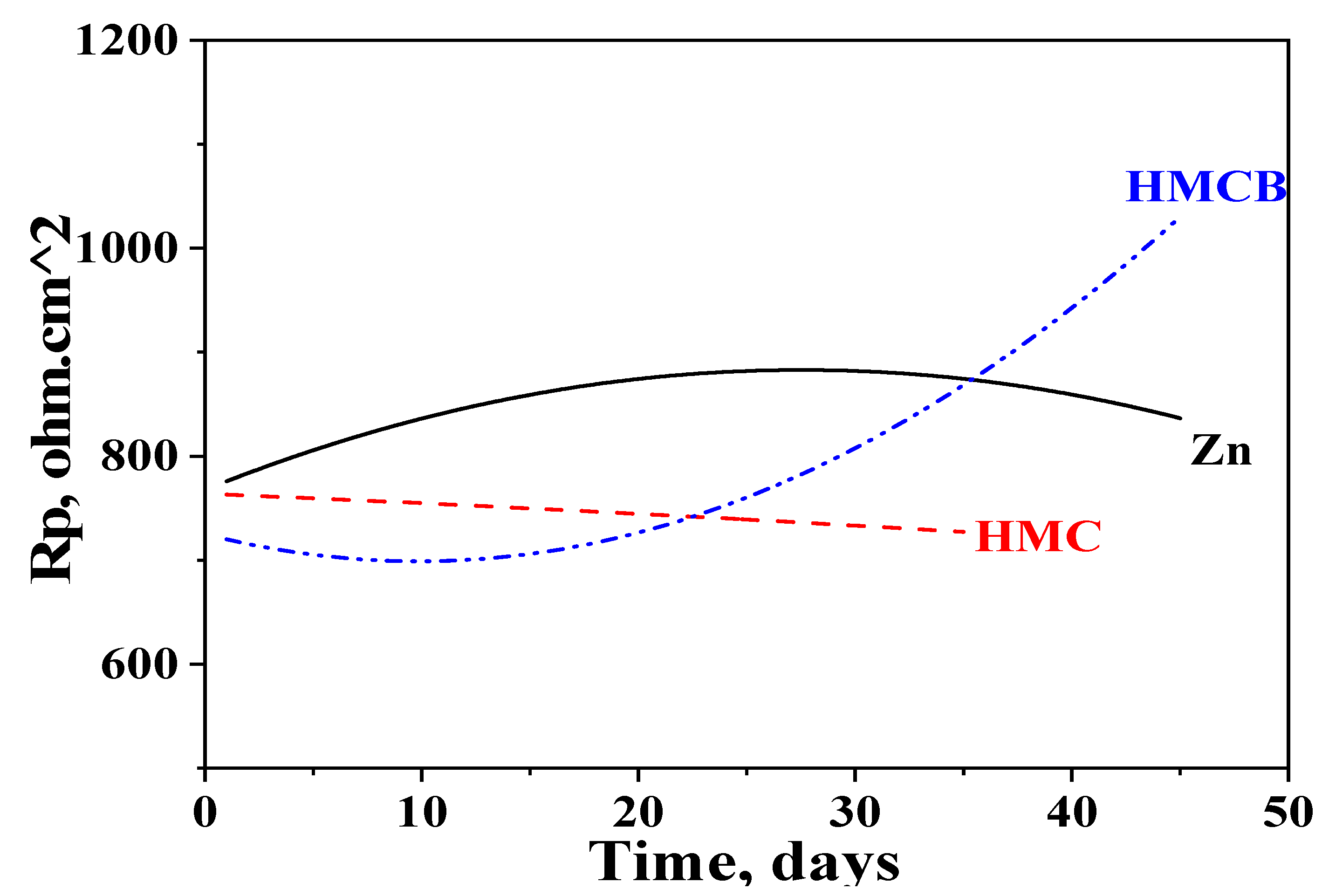
3.7. Polarization Resistance (Rp) Measurements
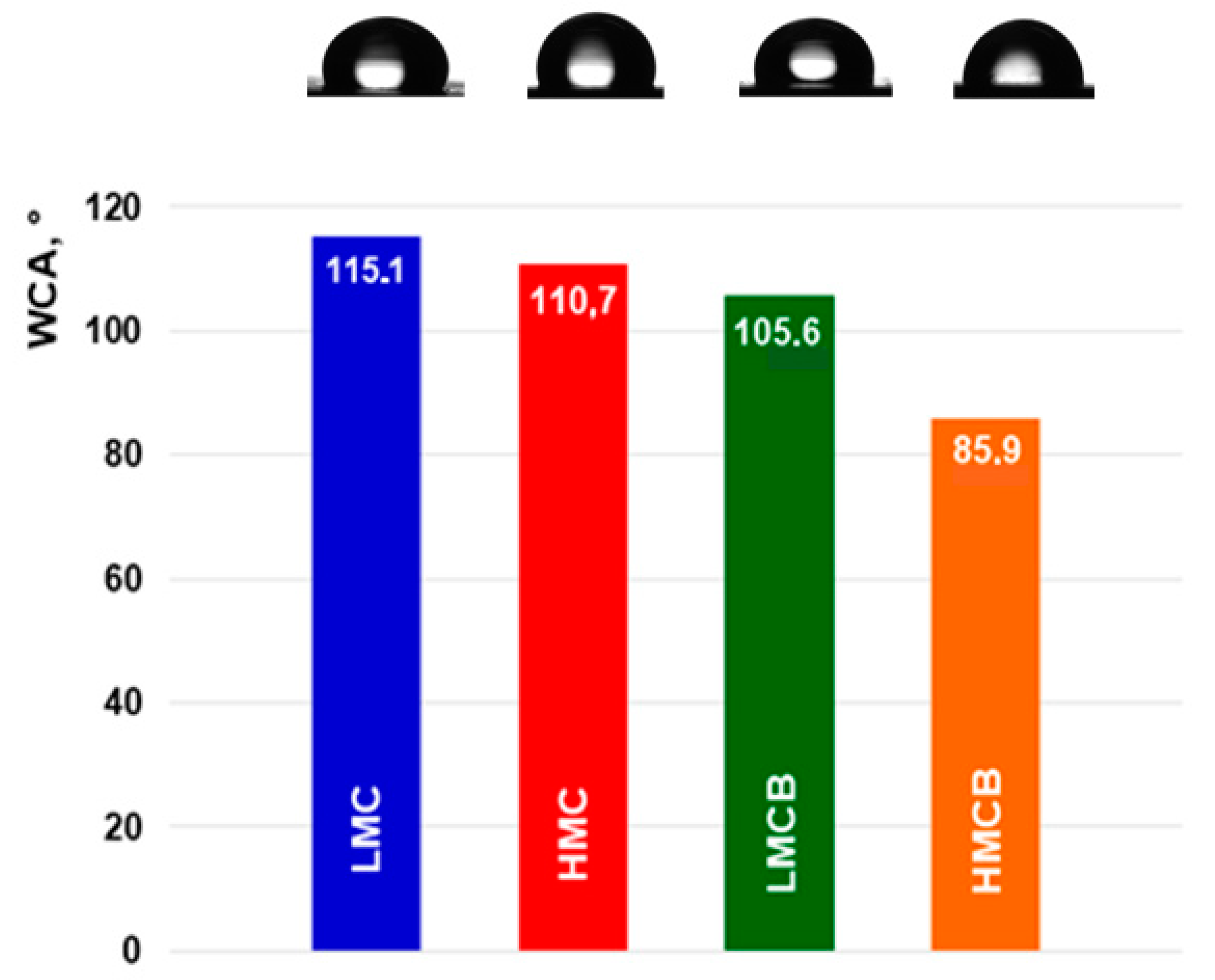
3.8. Surface Topography and Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzzarelli, R.A.A. Natural Chelating Polymers: Alginic Acid, Chitin and Chitosan; Pergamon Press Oxford: New York, NY, USA, 1973; p. 254. [Google Scholar]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Roman, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466–467, 1047–1059. [Google Scholar]
- Rabae, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhaoa, Q.; Guoa, J.; Cuia, G.; Hana, T.; Wu, Y. Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf. B Biointerfaces 2020, 194, 111150. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Feng, H.; Chen, N.; Lu, Y.; Li, S.; Wang, P. Polyaniline/chitosan as a corrosion inhibitor for mild steel in acidic medium. RSC Adv. 2019, 9, 9211–9217. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Wen, F.; Wei, Y.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar]
- Mazancová, P.; Némethová, V.; Treľová, D.; Kleščíková, L.; Lacík, I.; Rázga, F. Dissociation of chitosan/tripolyphosphate complexes into separate components upon pH elevation. Carbohydr. Polym. 2018, 192, 104–110. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.G.; Magalhães, T.C.; Teixeira, N.M.; Gondim, B.L.C.; Carlo, H.L.; Dos Santos, R.L.; De Oliveira, A.R.; Denadai, Â.M.L. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C 2019, 104, 109885. [Google Scholar]
- Huang, Y.; Lapitsky, Y. Monovalent salt enhances colloidal stability during the formation of chitosan/tripolyphosphate microgels. Langmuir 2011, 27, 10392–10399. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef] [PubMed]
- Popoola, P.A.I.; Malatji, N.; Fayomi, O.S. Fabrication and properties of zinc composite coatings for mitigation of corrosion in coastal and marine zone. In Applied Studies of Coastal and Marine Environments; InTech: London, UK, 2016; Volume 32, pp. 137–144. [Google Scholar]
- De La Fuente, D.; Castano, J.G.; Morcillo, M. Long-term atmospheric corrosion of zinc. Corros. Sci. 2007, 9, 1420–1436. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Corrosion performance of electrodeposited zinc and zinc-alloy coatings in marine environment. Corros. Mater. Degrad. 2021, 2, 163–189. [Google Scholar] [CrossRef]
- Koch, G.; Brongers, M.; Thomson, N.; Virmani, Y.; Payer, J. Corrosion Cost and Preventive Strategies in the United States; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Plenum Press: New York, NY, USA, 1996; ISBN 978-1-4757-9877-7. [Google Scholar]
- Sorensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Banfield, J.F. Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876–14884. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.K.; Ji, N.G.; Prakash, R. Chitosan based new nanocomposites for corrosion protection of mild steel in aggressive chloride media. Int. J. Biol. Macromol. 2019, 140, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Boshkova, N.; Kamburova, K.; Radeva, T.; Simeonova, S.; Grozev, N.; Shipochka, M.; Boshkov, N. Comparative corrosion characterization of hybrid zinc coatings in Cl--contaning medium and artificial sea water. Coatings 2022, 12, 1798. [Google Scholar] [CrossRef]
- Varoqui, R.; Dejardin, P. Hydrodynamic thickness of adsorbed polymers. J. Chem. Phys. 1977, 66, 4395–4399. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Vitkova, S.; Nemska, S.; Raichevski, G. Composition of the corrosion products of galvanic coatings Zn-Co and their influence on the protective ability. Surf. Coat. Technol. 2002, 157, 171–178. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Kovacheva, D.; Vitkova, S.; Nemska, S. Influence of the alloying component on the protective ability of some zinc galvanic coatings. Electrochim. Acta 2005, 51, 77–84. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Hybrid zinc coating with CuO nanocontainers containing corrosion Inhibitor for combined protection of mild Steel from corrosion and biofouling. Coatings 2022, 12, 1254. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Atanassova, G.; Radeva, T. Hybrid zinc coatings for corrosion protection of steel using polyelectrolyte nanocontainers loaded with benzotriazole. Colloids Surf. A 2018, 559, 243–250. [Google Scholar] [CrossRef]
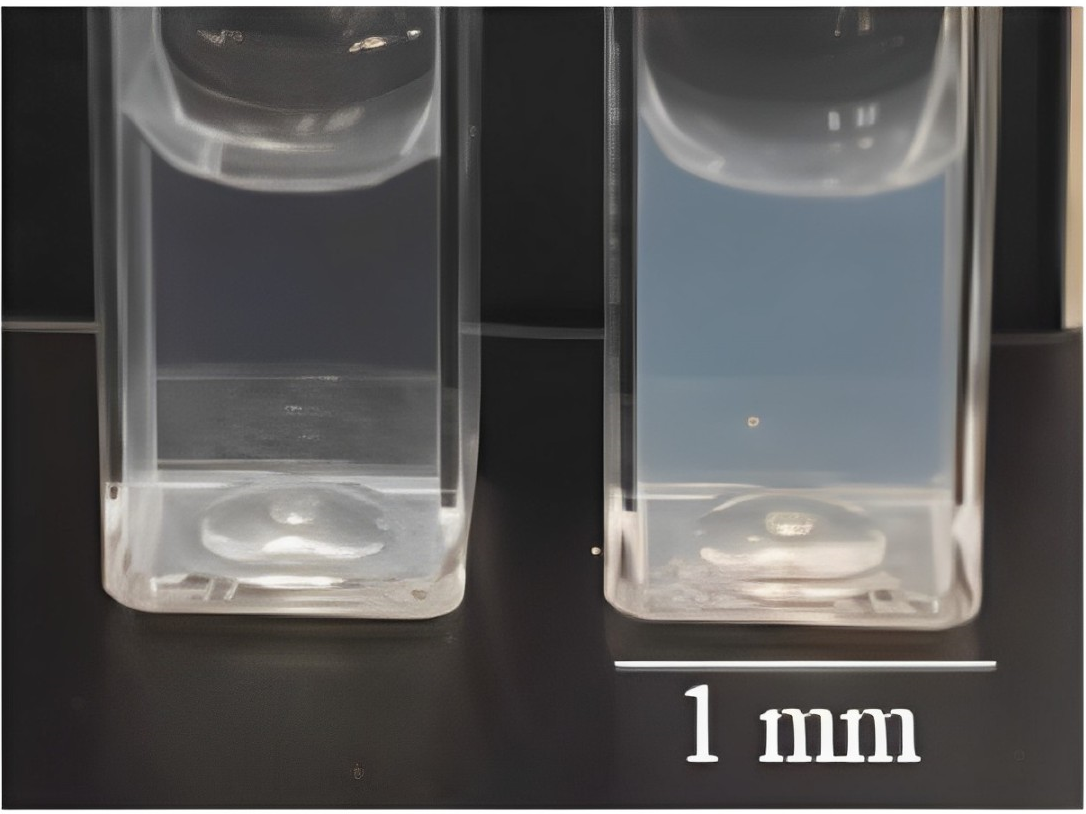
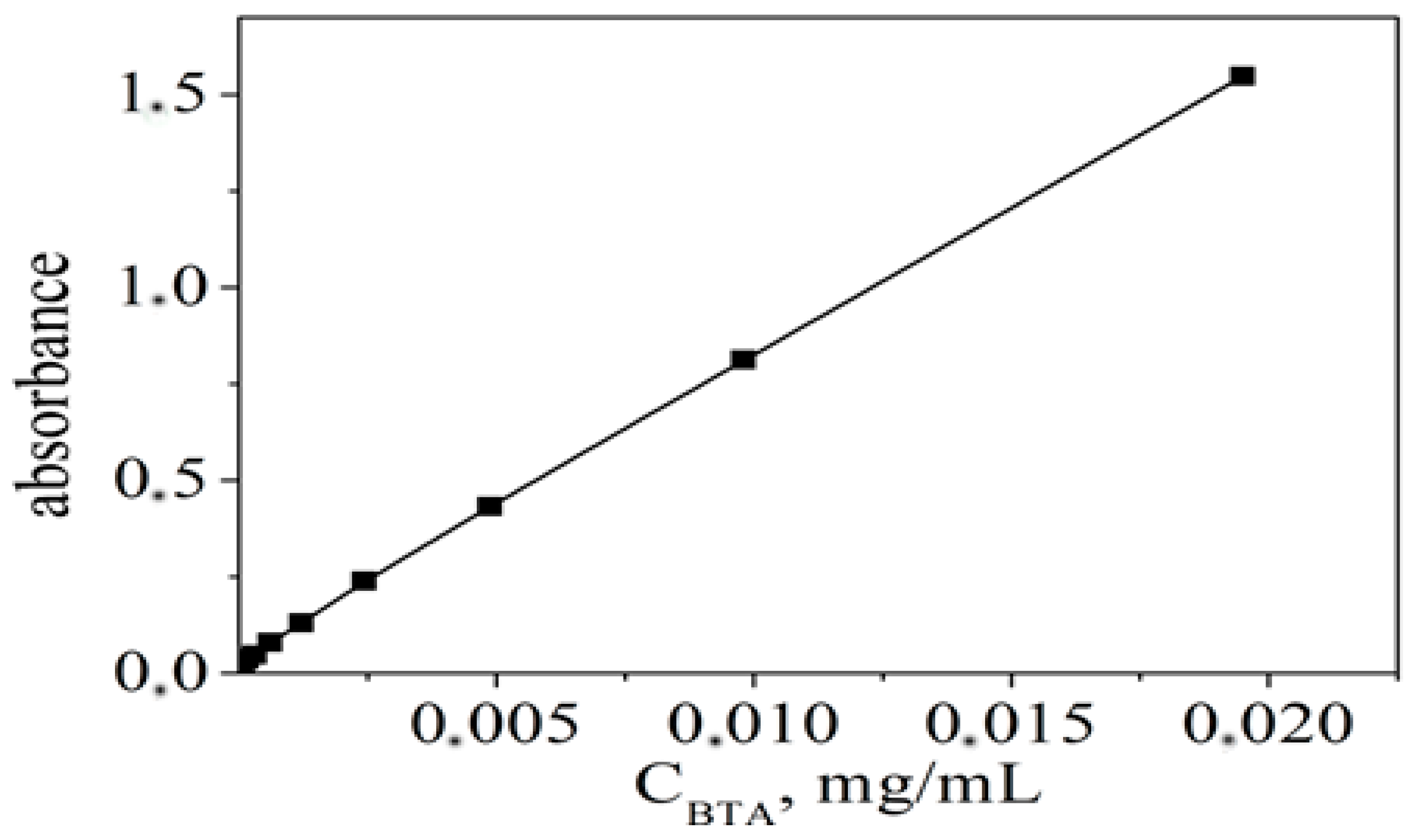
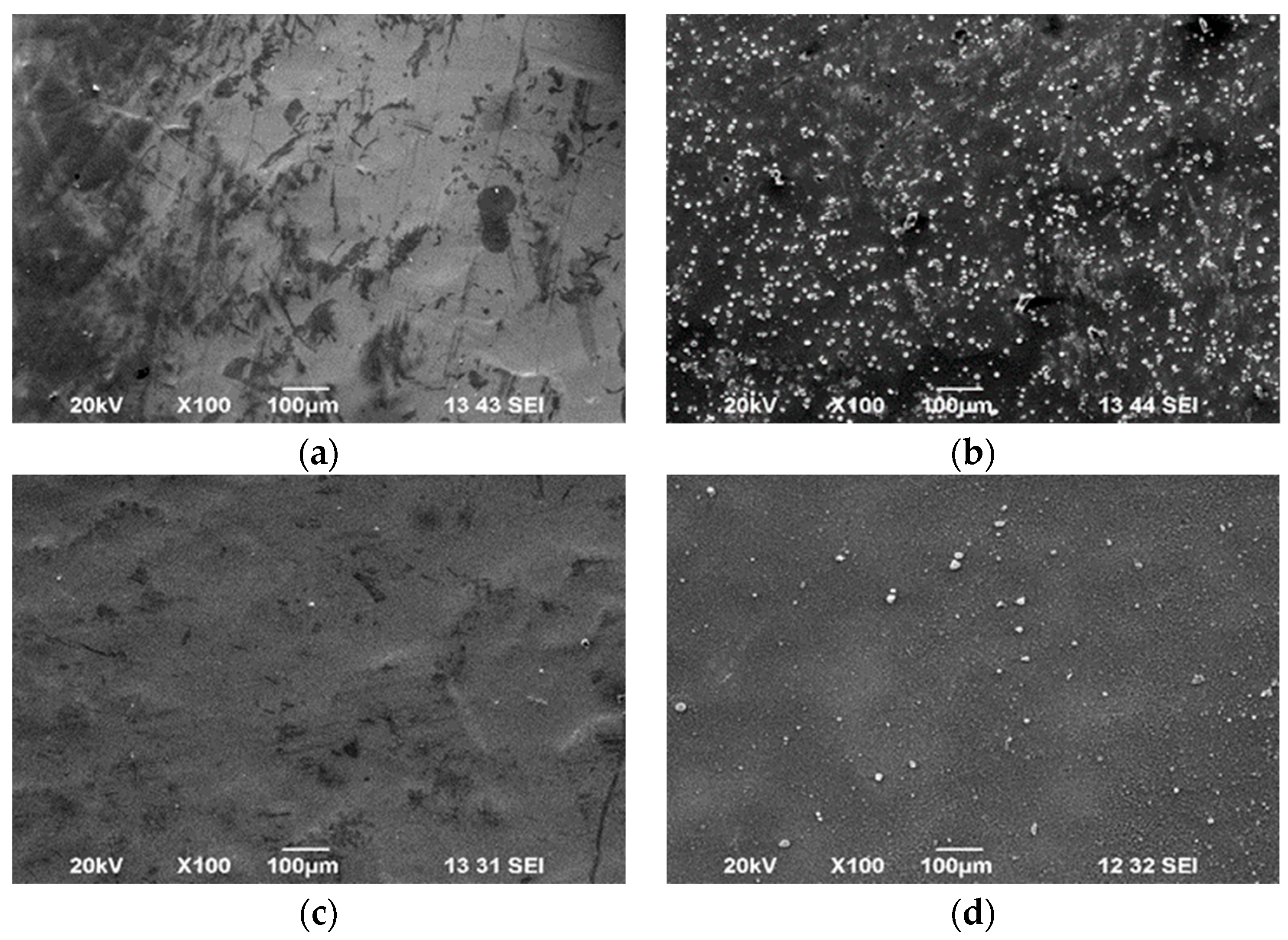
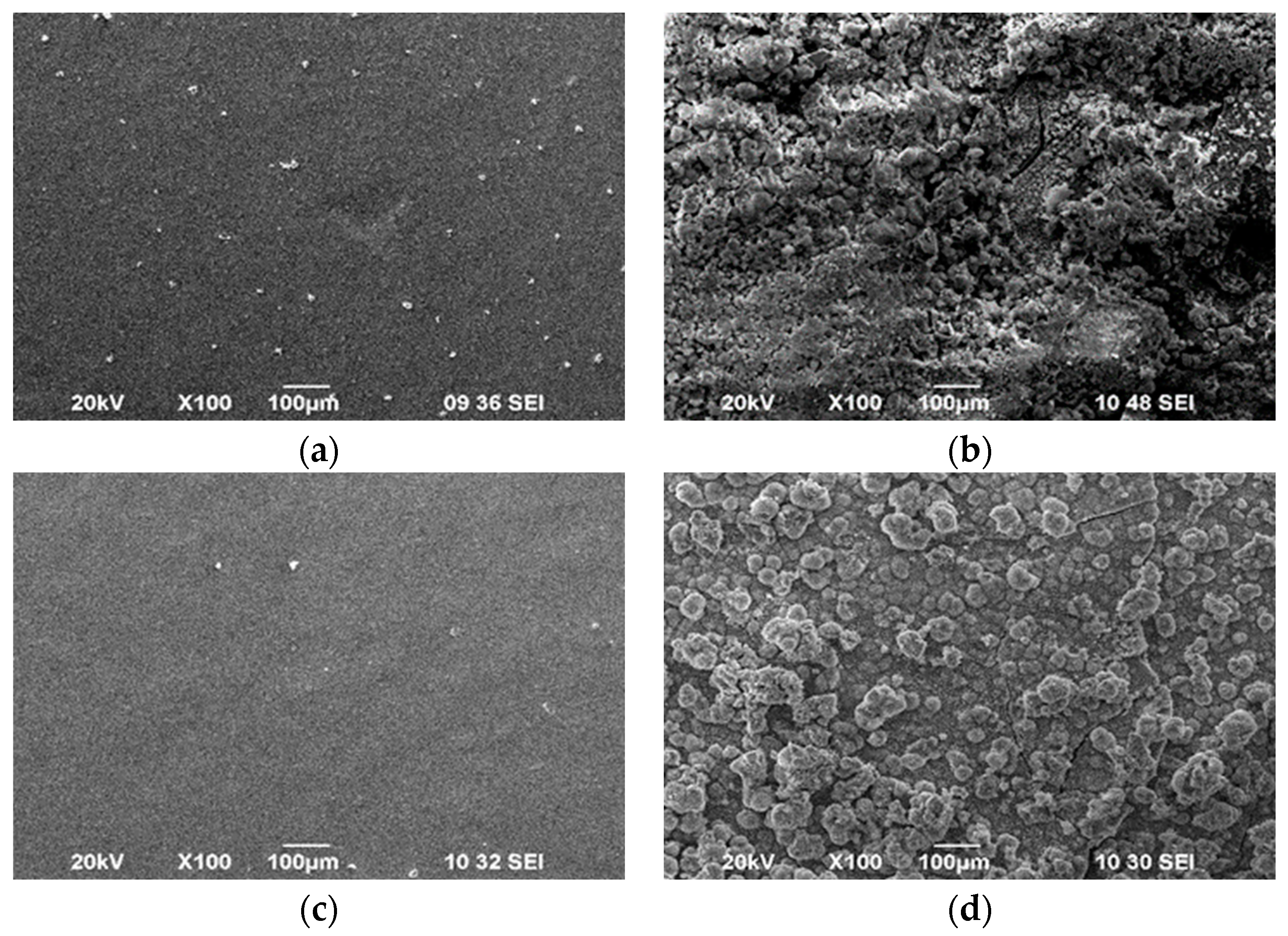
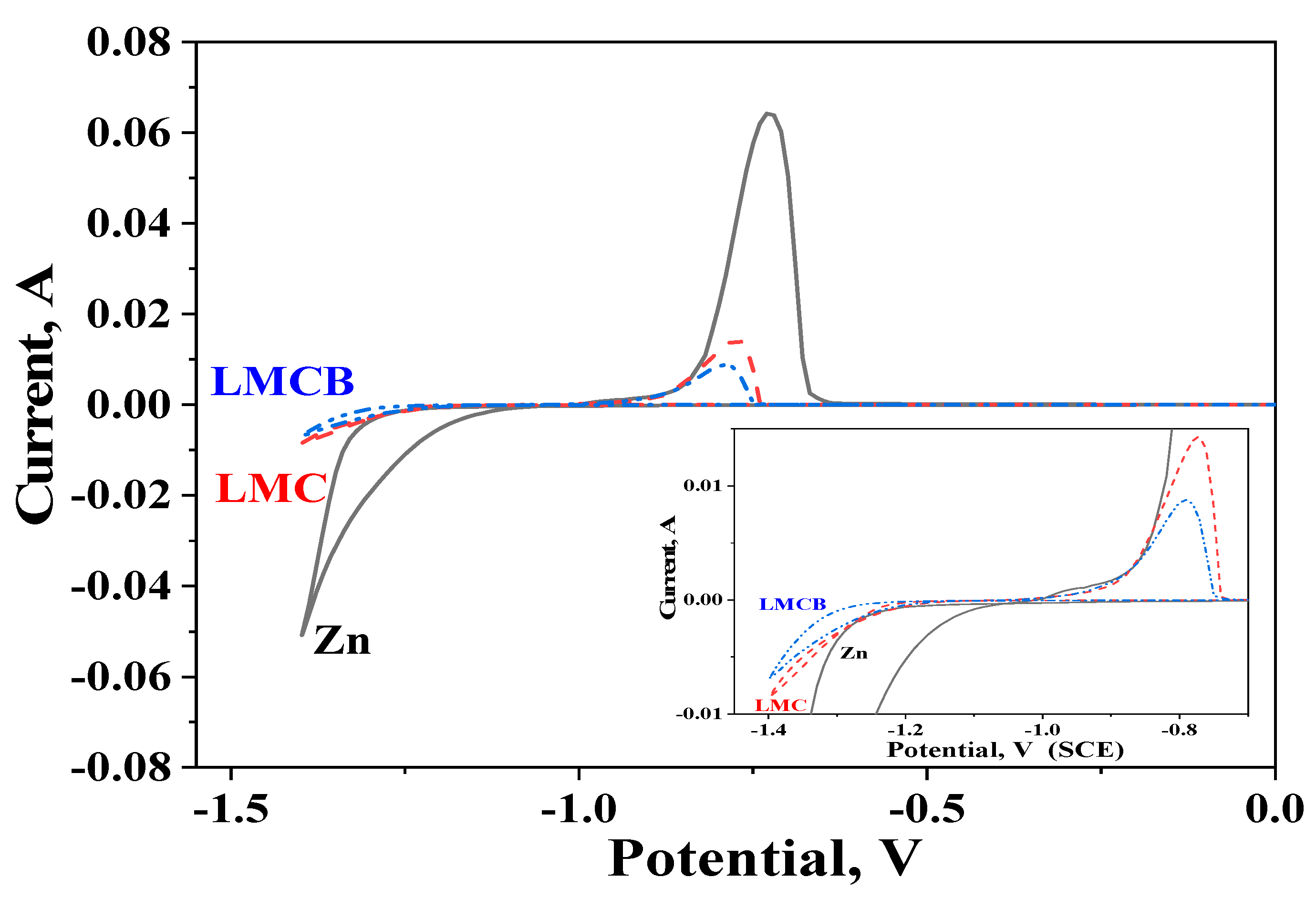
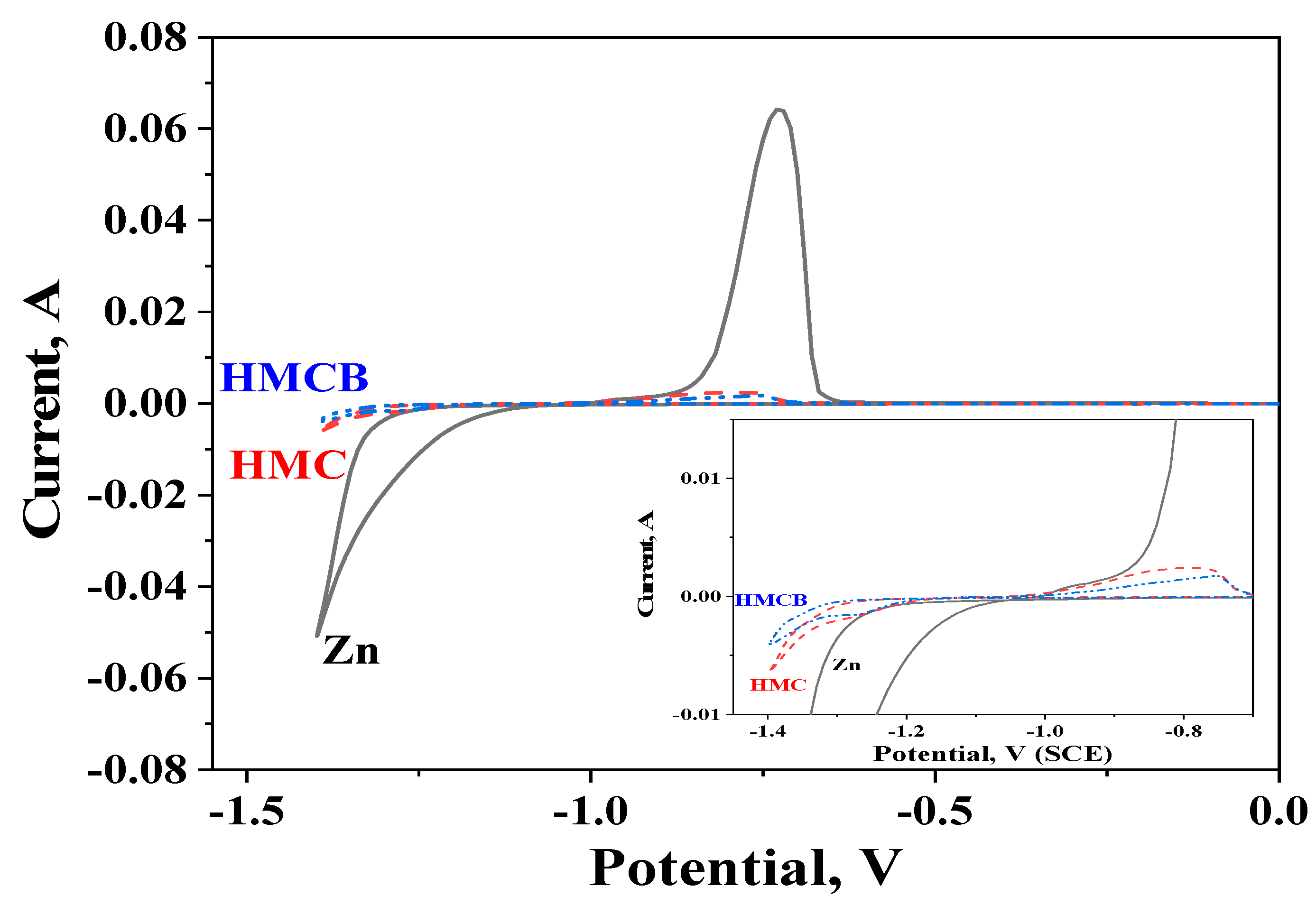


| Sample | D *, nm | ζ-Potential, mV |
|---|---|---|
| CS50-TPP (LMC) | 223.4 ± 8.20 | 46.8 ± 1.05 |
| CS50-TPP-BTA (LMCB) | 130.8 ± 1.85 | 45.9 ± 0.46 |
| CS190-TPP (HMC) | 218.7 ± 8.65 | 39.4 ± 0.57 |
| CS190-TPP-BTA (HMCB) | 139.8 ± 2.43 | 39.6 ± 0.32 |
| CS50-TPP (LMC) | 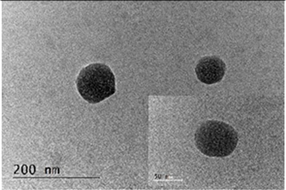 |
| CS50-TPP-BTA (LMCB) | 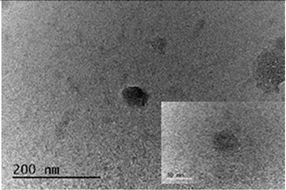 |
| CS190-TPP (HMC) |  |
| CS190-TPP-BTA (HMCB) | 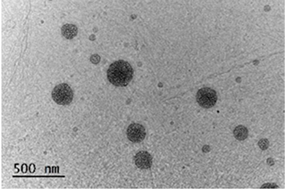 |
| Sample | Icorr, A·cm−2 | Ecorr, V |
|---|---|---|
| Zn | 1.7 × 10−5 | –1.07 |
| LMC | 7.9 × 10−6 | –1.06 |
| LMCB | 8.1 × 10−6 | –1.06 |
| Sample | Icorr, A·cm−2 | Ecorr, V |
|---|---|---|
| Zn | 1.7 × 10−5 | –1.07 |
| HMC | 6.9 × 10−6 | –1.07 |
| HMCB | 6.1 × 10−6 | –1.05 |
| Sample | LMC | LMCB | HMC | HMCB |
|---|---|---|---|---|
| Image (µm × µm) | 5 × 5 | 5 × 5 | 5 × 5 | 5 × 5 |
| Rq (nm) | 26.1 | 44.5 | 11.7 | 18.1 |
| Ra (nm) | 20.7 | 34.2 | 8.9 | 13.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milkova, V.; Boshkova, N.; Grancharov, G.; Stoilova, O.; Boshkov, N. Corrosion Behavior of Hybrid Zinc Coatings Based on Chitosan and Corrosion Inhibitor BTA: Effect of the Molecular Weight and ζ-Potential. Coatings 2024, 14, 495. https://doi.org/10.3390/coatings14040495
Milkova V, Boshkova N, Grancharov G, Stoilova O, Boshkov N. Corrosion Behavior of Hybrid Zinc Coatings Based on Chitosan and Corrosion Inhibitor BTA: Effect of the Molecular Weight and ζ-Potential. Coatings. 2024; 14(4):495. https://doi.org/10.3390/coatings14040495
Chicago/Turabian StyleMilkova, Viktoria, Nelly Boshkova, Georgy Grancharov, Olya Stoilova, and Nikolai Boshkov. 2024. "Corrosion Behavior of Hybrid Zinc Coatings Based on Chitosan and Corrosion Inhibitor BTA: Effect of the Molecular Weight and ζ-Potential" Coatings 14, no. 4: 495. https://doi.org/10.3390/coatings14040495
APA StyleMilkova, V., Boshkova, N., Grancharov, G., Stoilova, O., & Boshkov, N. (2024). Corrosion Behavior of Hybrid Zinc Coatings Based on Chitosan and Corrosion Inhibitor BTA: Effect of the Molecular Weight and ζ-Potential. Coatings, 14(4), 495. https://doi.org/10.3390/coatings14040495








