Preparation and Corrosion Resistance of Superhydrophobic Coatings on 7005 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Material and Sample Preparation
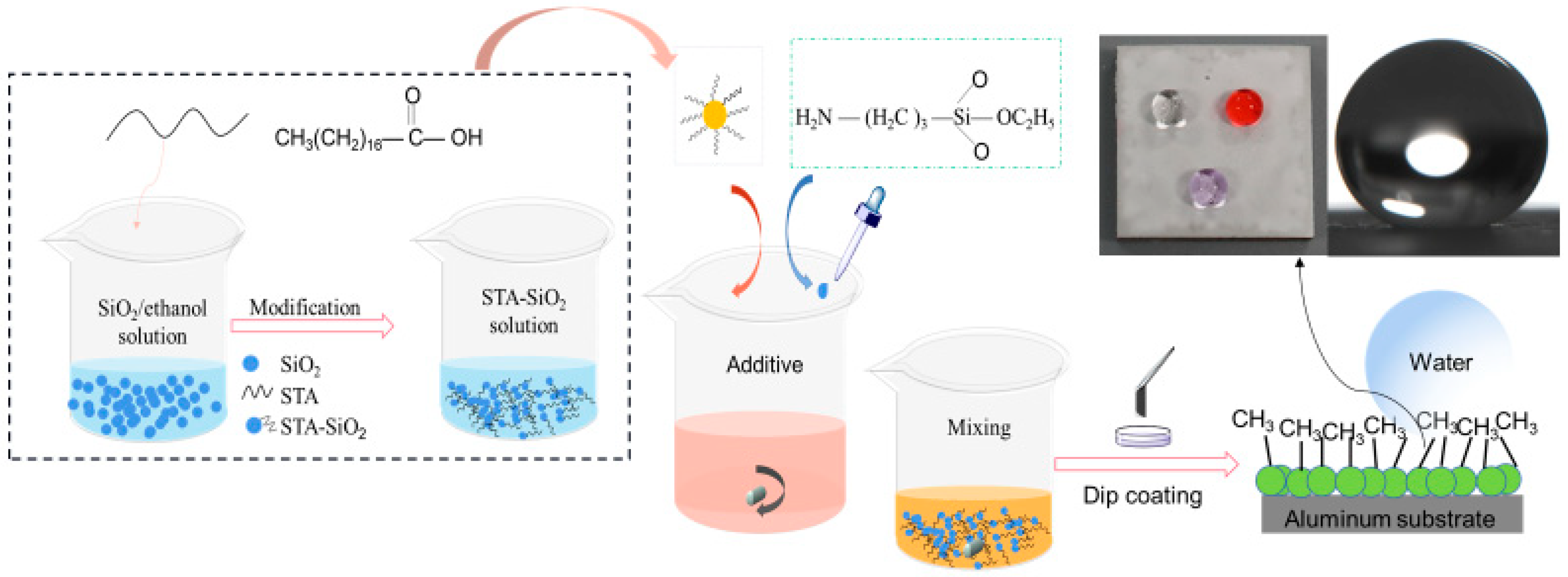
2.2. Superhydrophobic Coating Fabrication
2.3. Characterization
3. Results and Discussion
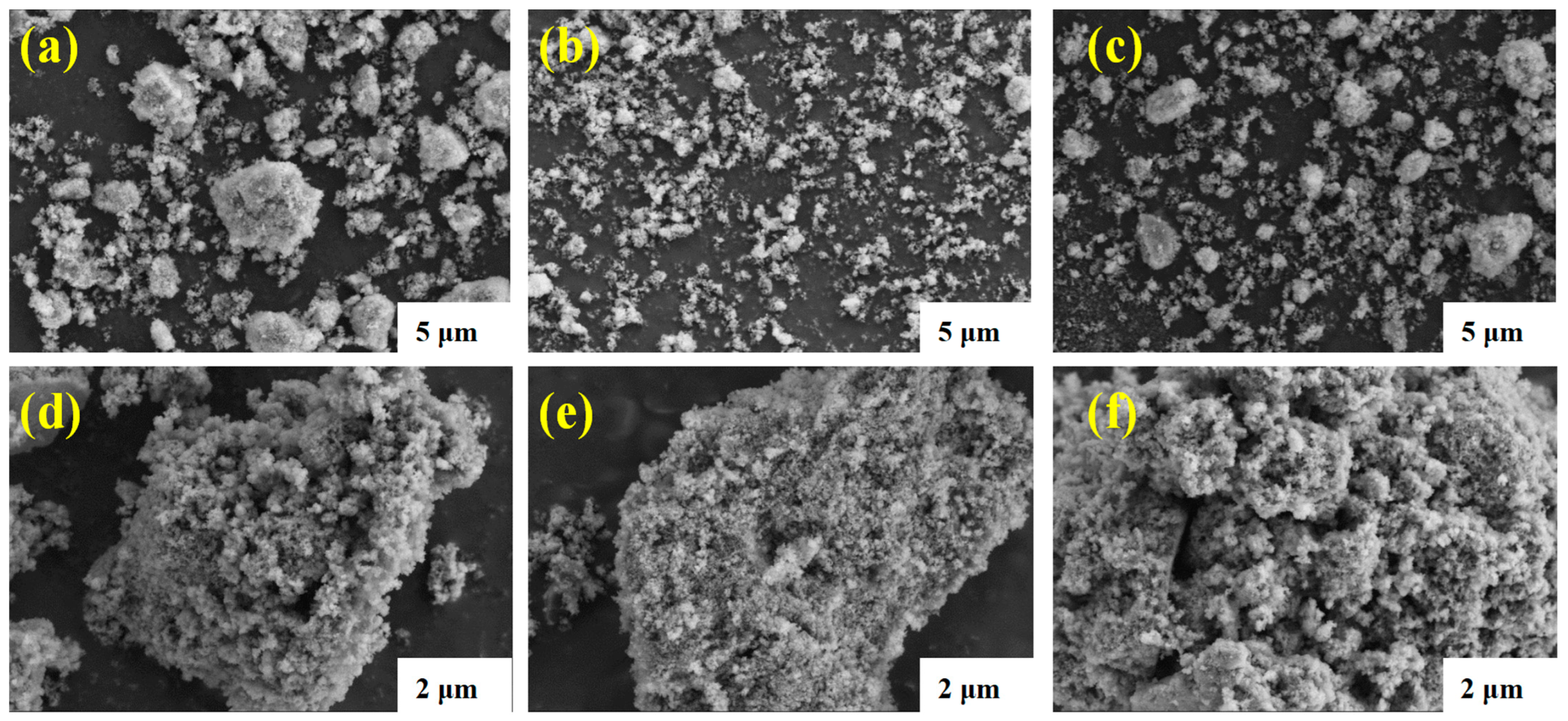
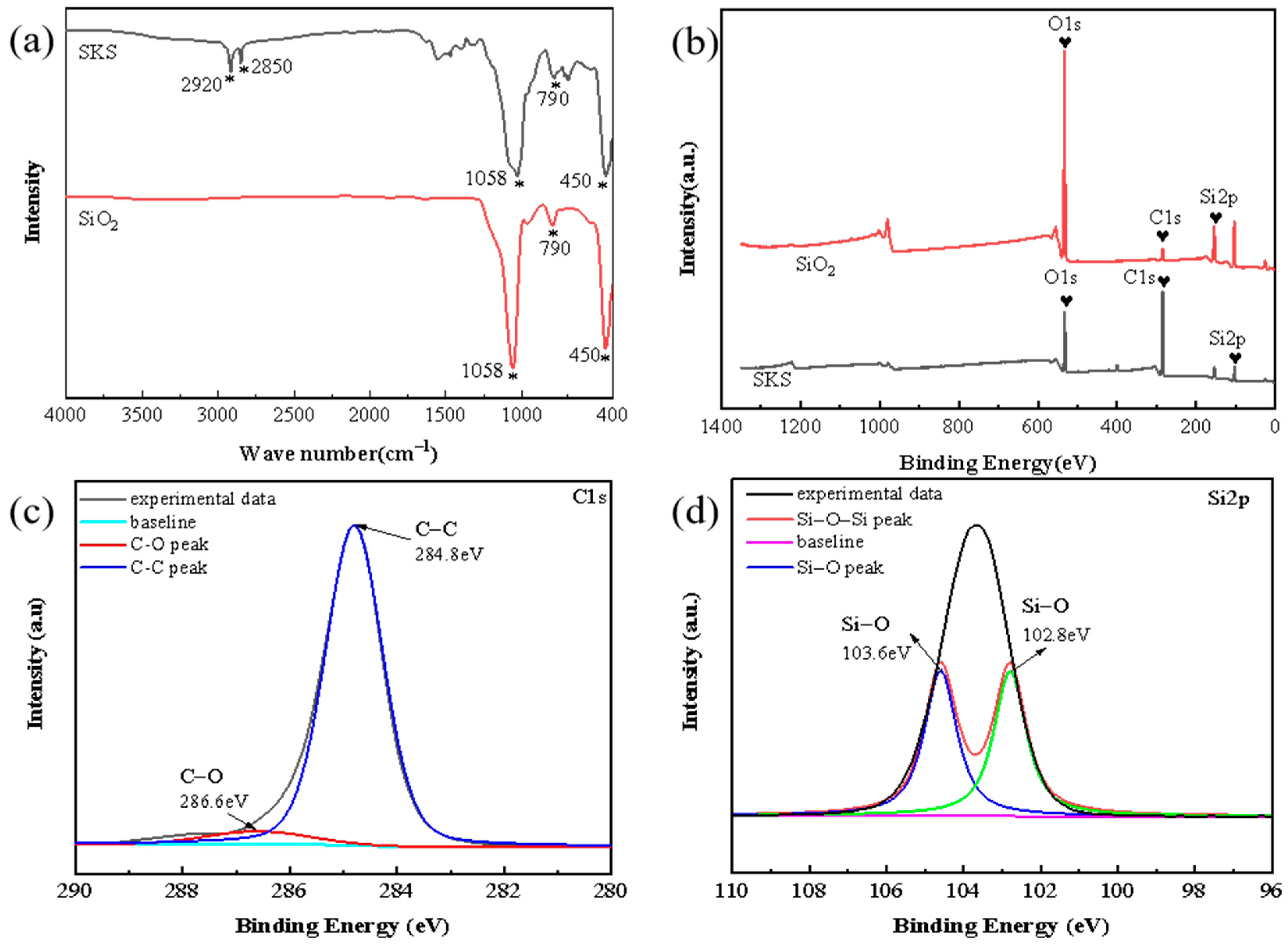
3.1. Surface Characterization
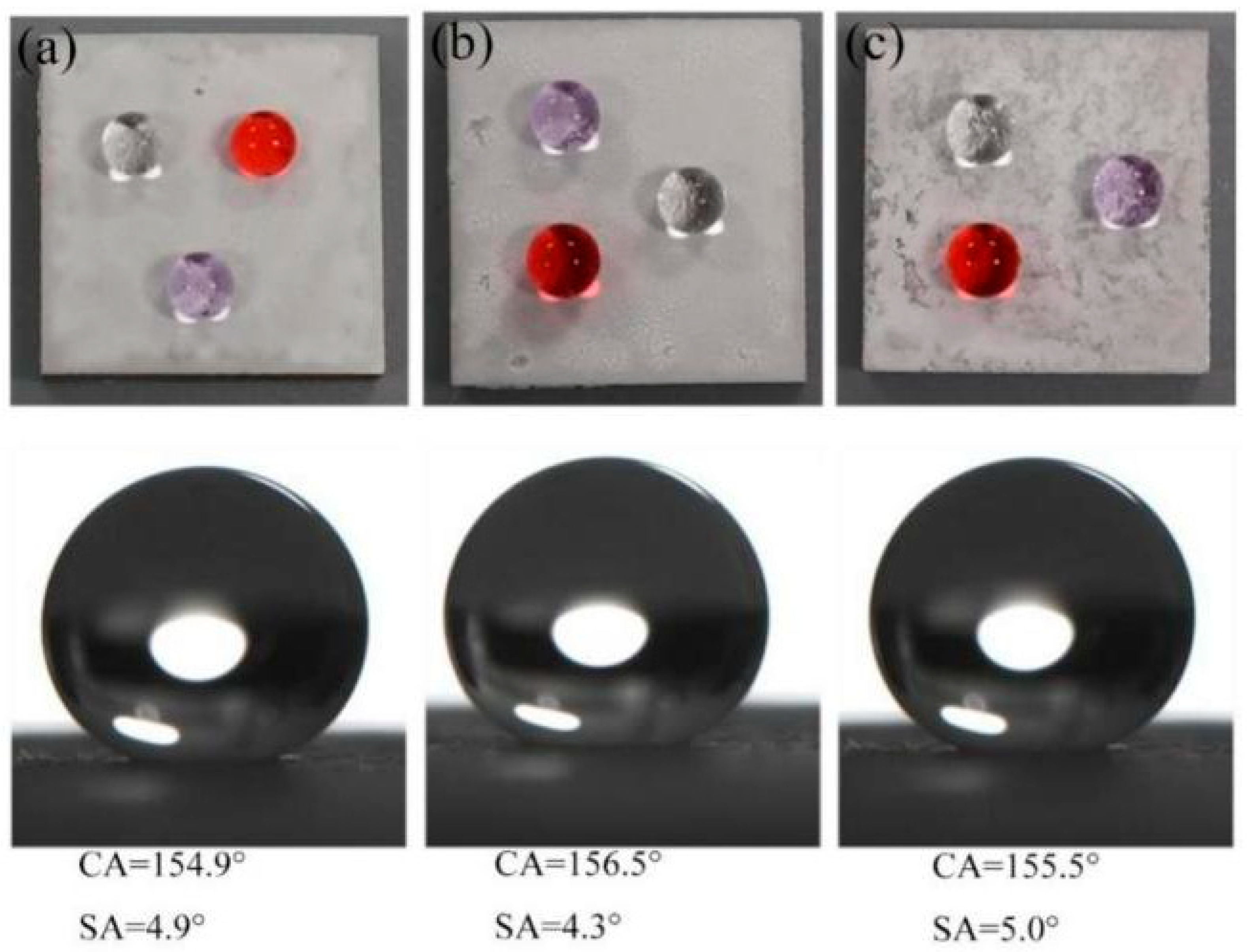
3.2. Surface Functionality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calabrese, L.; Khaskhoussi, A.; Patane, S.; Proverbio, E. Assessment of super-hydrophobic textured coatings on AA6082 aluminum alloy. Coatings 2019, 9, 352. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Cui, L.Y.; Cui, H.Z. Corrosion resistance of superhydrophobic mg-al layered double hydroxide coatings on aluminum alloys. Acta Metall. Sin. Engl. Lett. 2015, 28, 1373–1381. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Tang, A.Q.; Huang, Q.L.; Hu, J.; Mao, J.H.; Bai, G.; Bai, H. Novel strategy in increasing stability and corrosion resistance for super-hydrophobic coating on aluminum alloy surfaces. Appl. Surf. Sci. 2011, 258, 580–585. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. Robust and Chemically Stable Superhydrophobic Aluminum-Alloy Surface with Enhanced Corrosion-Resistance Properties. Int. J. Precis. Eng. Manuf.-Green Technol. 2020, 7, 481–492. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, H.; Liu, W.; Deng, J.; Yuan, Z.; Liu, Q.; Chen, H. Bacteriostasis of a super-hydrophobic micro-porous aluminum surface by a facile preparation method. Adv. Mater. Res. 2012, 583, 346–349. [Google Scholar] [CrossRef]
- Fahim, J.; Hadavi, S.M.M.; Ghayour, H.; Tabrizi, S.A.H. Cavitation erosion behavior of super-hydrophobic coatings on Al5083 marine aluminum alloy. Wear 2019, 424–425, 122–132. [Google Scholar] [CrossRef]
- Guo, M.; Ding, B.; Li, X.; Wang, X.; Yu, J.; Wang, M. Amphiphobic nanofibrous silica mats with flexible and high-heat-resistant properties. J. Phys. Chem. C 2010, 114, 916–921. [Google Scholar] [CrossRef]
- Jafari, R.; Farzaneh, M. A simple method to create superhydrophobic aluminium surfaces. Mater. Sci. Forum. 2012, 706–709, 2874–2879. [Google Scholar] [CrossRef]
- Esmaeilirad, A.; Rukosuyev, M.V.; Jun, M.B.G.; van Veggel, F.C.J.M. A cost-effective method to create physically and thermally stable and storable super-hydrophobic aluminum alloy surfaces. Surf. Coat. Technol. 2016, 285, 227–234. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid. Interfac. 2022, 304, 102658. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Novel metal-organic super-hydrophobic surface fabricated by nanosecond laser irradiation in solution, Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124343. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, N.; Qi, F.; Zhang, B.; Liao, B.; Ouyang, X. Reinforced superhydrophobic anti-corrosion epoxy resin coating by fluorine–silicon–carbide composites. Coatings 2020, 10, 1244. [Google Scholar] [CrossRef]
- Fu, K.; Lu, C.; Liu, Y.; Zhang, H.; Zhang, B.; Zhang, H.; Zhou, F.; Zhang, Q.; Zhu, B. Mechanically robust, self-healing superhydrophobic anti-icing coatings based on a novel fluorinated polyurethane synthesized by a two-step thiol click reaction. Chem. Eng. J. 2021, 404, 127110. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yuan, S.; Li, Y.; Zhang, X.; Lin, D.; Chen, L.; Zhu, Y. Durable and anti-corrosion superhydrophobic coating with bistratal structure prepared by ambient curing. Prog. Org. Coat. 2020, 149, 105922. [Google Scholar] [CrossRef]
- Quan, Y.Y.; Chen, Z.; Lai, Y.; Huang, Z.S.; Li, H. Recent advances in fabricating durable superhydrophobic surfaces: A review in the aspects of structures and materials. Mater. Chem. Front. 2021, 5, 1655–1682. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Philip, J. Optimal condition for fabricating mechanically durable superhydrophobic titanium surface by rapid breakdown anodization: Self cleaning and bouncing characteristics. Appl. Surf. Sci. 2022, 585, 152628. [Google Scholar]
- Liu, X.L.; Gu, Y.C.; Mi, T.F.; Wang, X.M.; Zhang, X. Dip-Coating Approach to Fabricate Durable PDMS/STA/SiO2 Superhydrophobic Polyester Fabrics. Coatings 2021, 326, 326. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, W.; Fu, W.; Wu, L.; Zhang, D.; Pan, F. Preparing superhydrophobic nanocomposite coating with SiO2 nanoparticles on magnesium alloy. Surf. Eng. 2021, 37, 1231–1238. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Li, B.; Hu, T.; Yang, Y.; Li, L.; Zhang, J. Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J. Colloid Interface Sci. 2019, 542, 8–14. [Google Scholar] [CrossRef]
- Li, Q.; Bao, X.; Sun, J.; Cai, S.; Xie, Y.; Liu, Y.; Liu, J.; Xu, G. Fabrication of superhydrophobic composite coating of hydroxyapatite/stearic acid on magnesium alloy and its corrosion resistance, antibacterial adhesion. J. Mater. Sci. 2021, 56, 5233–5249. [Google Scholar] [CrossRef]
- Liu, W.L.; Wang, S.R.; Wang, G.Q.; Zhang, J.P.; Zhou, C. Investigation on the differences of surface cleaning properties of series of superhydrophobic aluminum alloys. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 651, 129614. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Ye, L.; Sun, L.; Xiao, T.X. Guo. Effect of multi-stage aging treatments on the precipitation and mechanical properties of Al-Zn-Mg alloys. Mater. Sci. Eng. A-Struct. 2020, 785, 139394. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.M.I. Newton. An introduction to superhydrophobicity. Adv. Colloid Interface 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Hu, Q.; Suzuki, H.; Gao, H.; Araki, H.; Yang, W.; Noda, T. High-frequency FTIR absorption of SiO2/Si nanowires. Chem. Phys. Lett. 2003, 378, 299–304. [Google Scholar] [CrossRef]
- Rangelova, N.; Radev, L.; Nenkova, S.; Salvado, I.M.M.; Fernandes, M.H.V.; Herzog, M. Methylcellulose/SiO2 hybrids: Sol-gel preparation and characterization by XRD, FTIR and AFM. Cent. Eur. J. Chem. 2011, 9, 112–118. [Google Scholar] [CrossRef]
- Gang, C.; Chen, Q.; Wang, S.; Zhao, J.; Ai, L.; Chen, Q.; Lin, X.; Hu, C. Fabrication of a superhydrophobic SiO2 coating with anticorrosive protection. ChemistrySelect 2023, 8, e202300172. [Google Scholar]

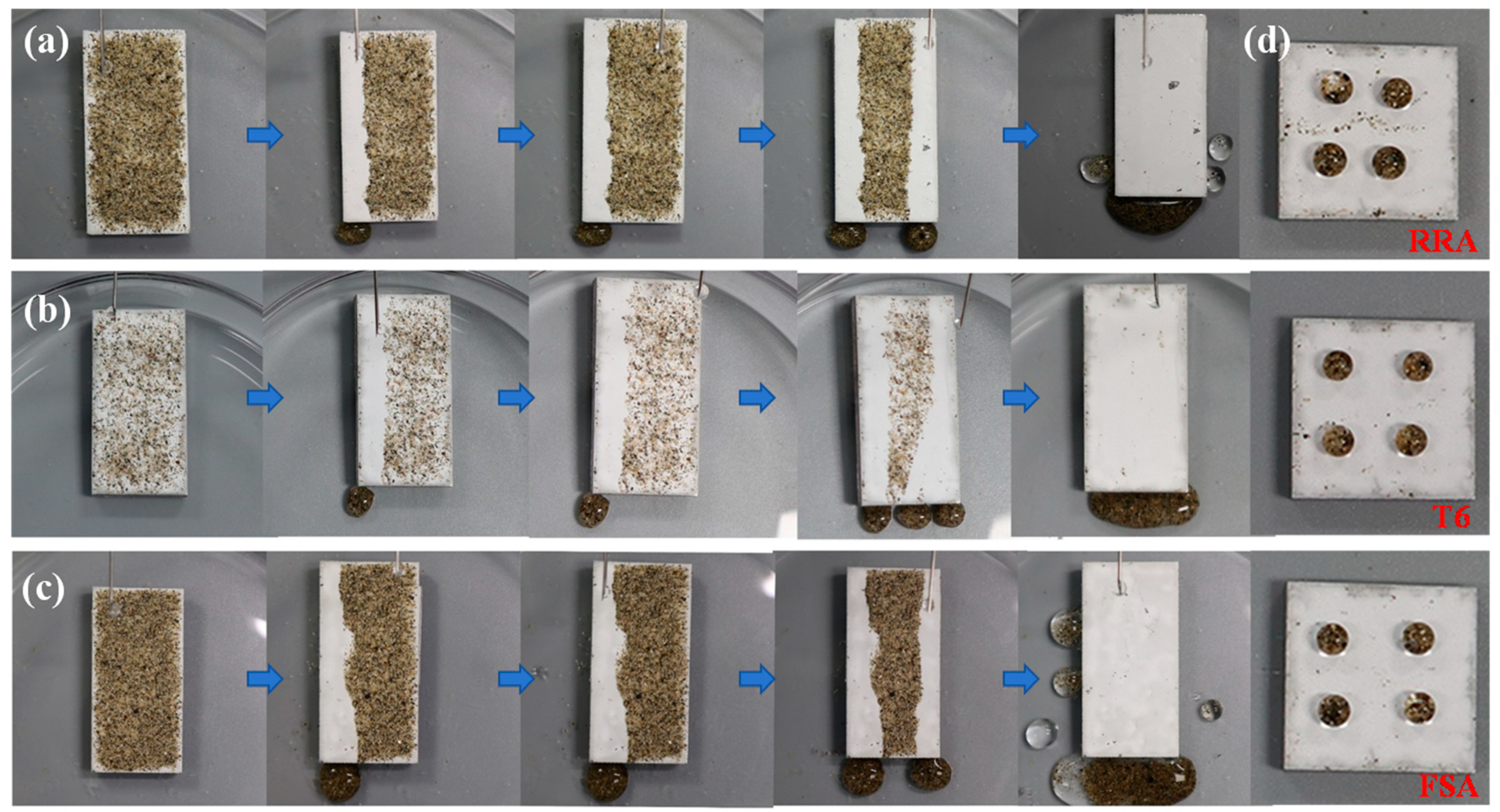

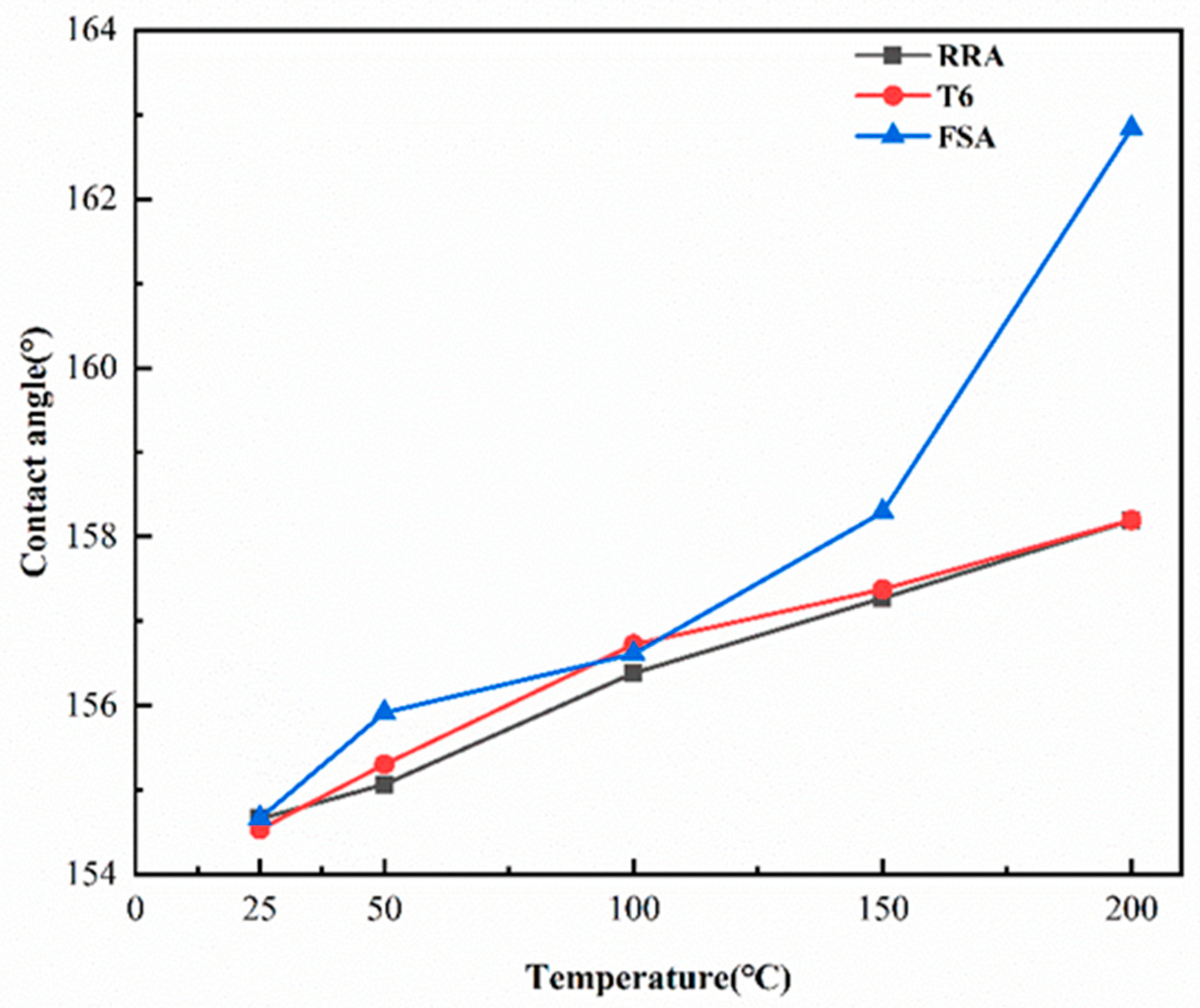

| Element | Si | Fe | Mn | Mg | Cr | Zn | Ti | Zr | Al |
|---|---|---|---|---|---|---|---|---|---|
| Content% | 0.052 | 0.075 | 0.31 | 1.28 | 0.13 | 4.98 | 0.028 | 0.14 | Bal. |
| Samples | Peak Designation | Band (eV) | Atomic (%) |
|---|---|---|---|
| SiO2 powder | C | 284.8 | 7.67 |
| Si | 103.6 | 62.1 | |
| O | 532.8 | 30.24 | |
| SiO2-KH550 | C | 284.8 | 57.1 |
| Si | 102.8 | 13.9 | |
| O | 532.2 | 22.62 |
| Samples | (V) | PE% | |
|---|---|---|---|
| Bare (RRA) | −0.93 | 2.24 × 10−4 | - |
| Bare (T6) | −0.95 | 2.31 × 10−4 | - |
| Bare (FSA) | −0.95 | 2.72 × 10−4 | - |
| RRA | −0.66 | 9.57 × 10−5 | 57.3 |
| T6 | −0.64 | 6.05 × 10−5 | 73.8 |
| FSA | −0.89 | 22.80 × 10−5 | 16.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Guo, F.; Li, X.; Xia, P.; Yang, L.; Hu, C. Preparation and Corrosion Resistance of Superhydrophobic Coatings on 7005 Aluminum Alloy. Coatings 2024, 14, 499. https://doi.org/10.3390/coatings14040499
Huang H, Guo F, Li X, Xia P, Yang L, Hu C. Preparation and Corrosion Resistance of Superhydrophobic Coatings on 7005 Aluminum Alloy. Coatings. 2024; 14(4):499. https://doi.org/10.3390/coatings14040499
Chicago/Turabian StyleHuang, Huilan, Feng Guo, Xintao Li, Peng Xia, Li Yang, and Chuanbo Hu. 2024. "Preparation and Corrosion Resistance of Superhydrophobic Coatings on 7005 Aluminum Alloy" Coatings 14, no. 4: 499. https://doi.org/10.3390/coatings14040499





