Research and Development of Online Monitoring Protection Sensors for Paper Books Based on TiO2 NT/MoS2
Abstract
:1. Introduction
2. Preparation and Characterization of Gas-Sensitive Materials
2.1. Preparation of TiO2 NTs
2.2. Preparation of TiO2 NT/MoS2 Nanocomposites
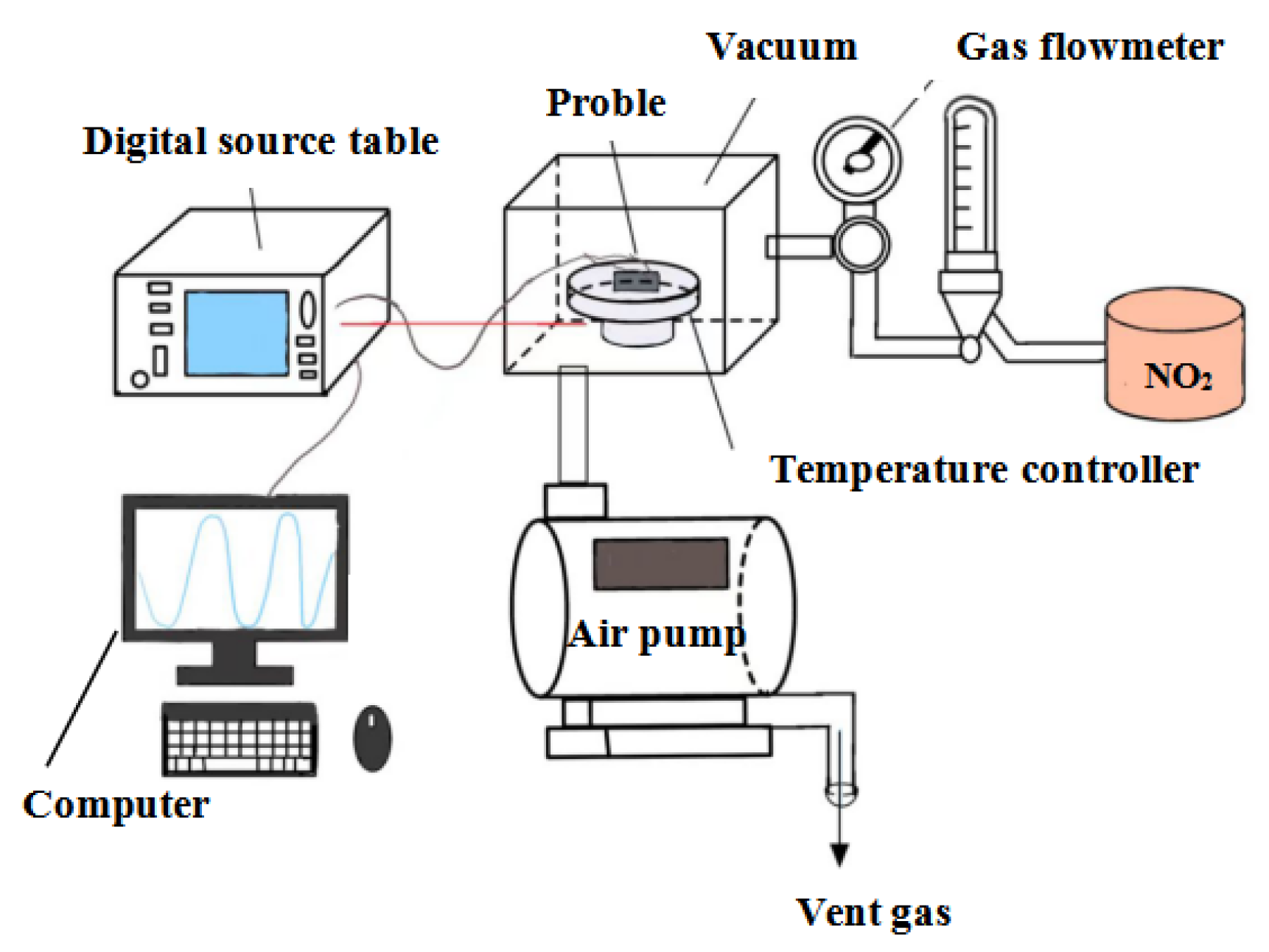
2.3. Preparation of the Online Monitoring Sensor
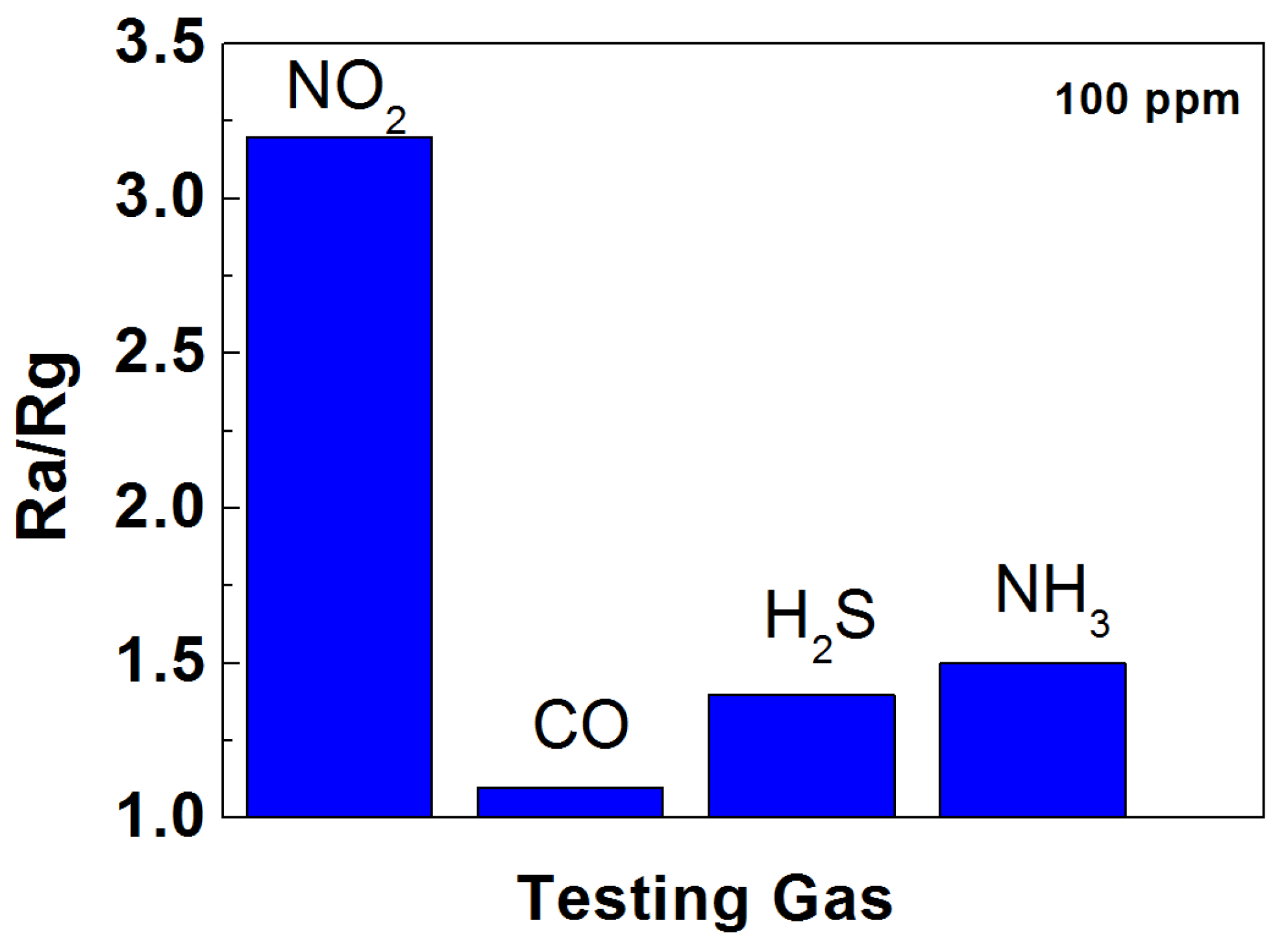
3. Analysis and Discussion
3.1. Phase Analysis of TiO2 NTs and TiO2 NT/MoS2 Composites
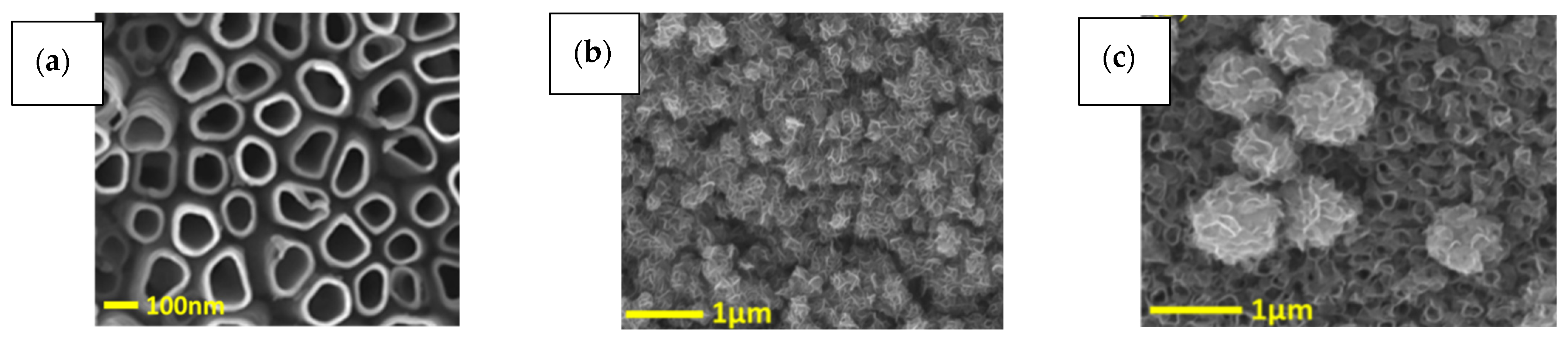
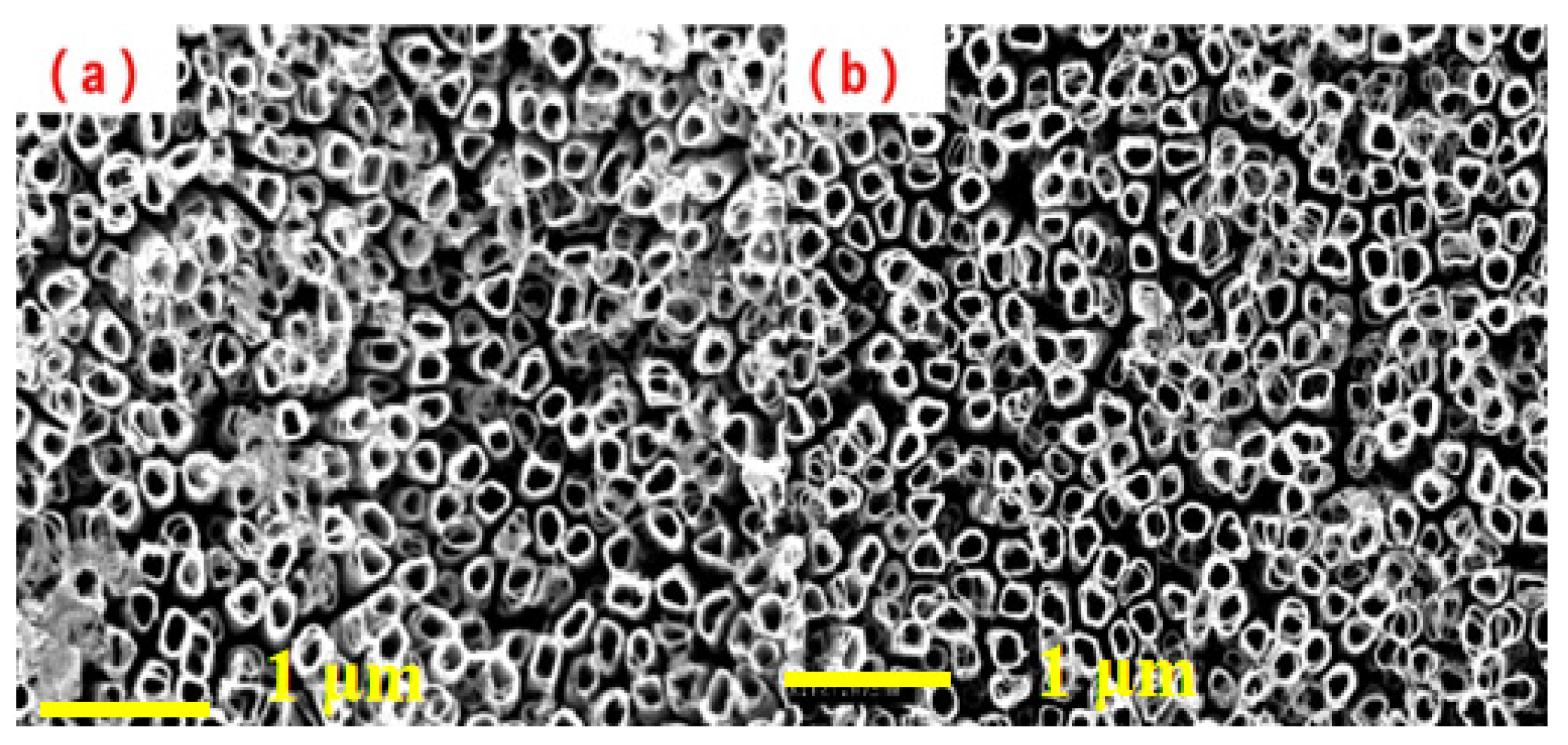
3.2. Morphology and Element Analysis
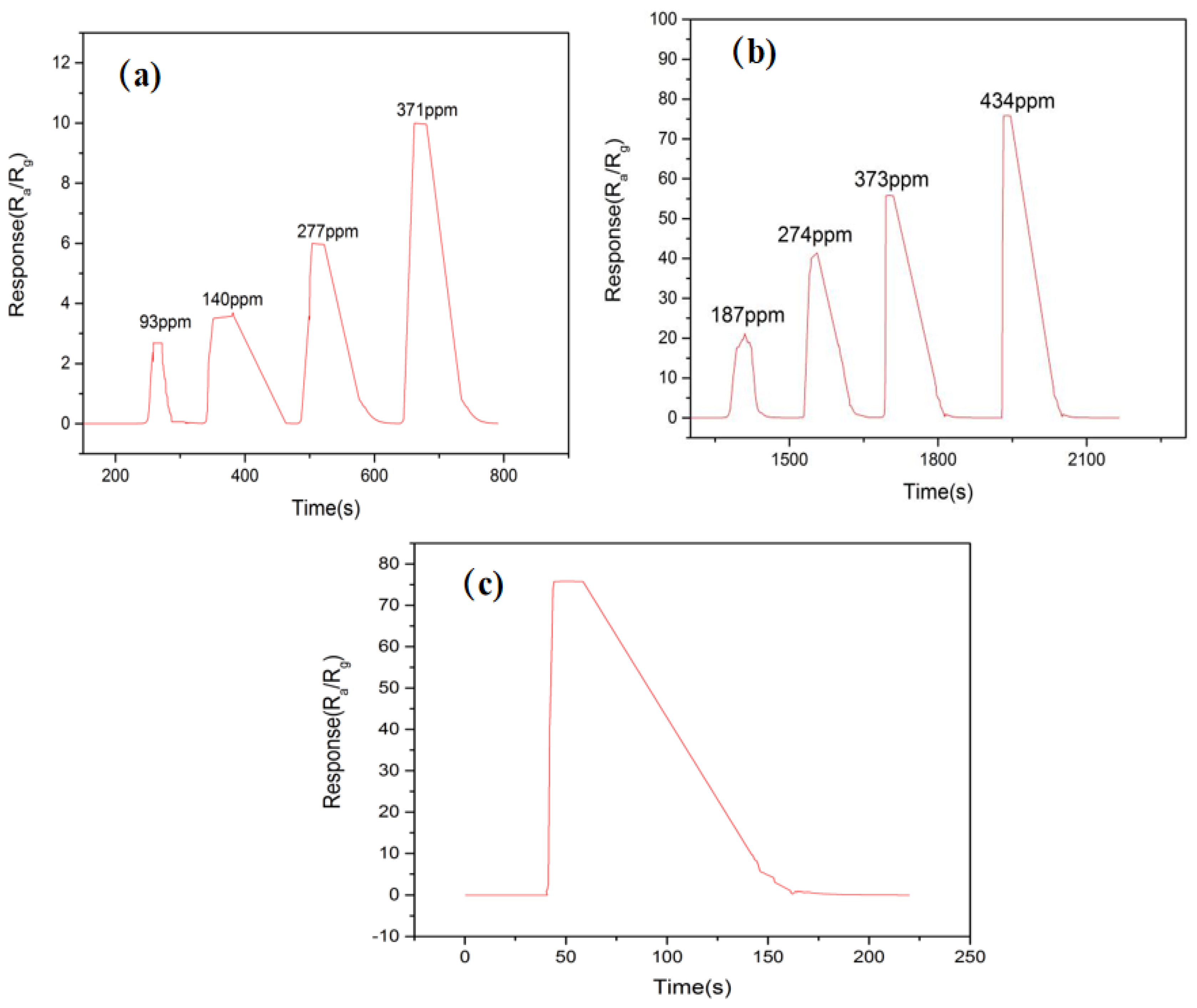
3.3. Gas-Sensitive Performance of TiO2 NT/MoS2
4. Reaction Mechanism for NO2 Monitoring in the Library
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y. Rational display and preventive protection of books and paper. Libr. Res. Work. 2022, 29–54. [Google Scholar]
- Lu, Y. Air pollution and paper document protection-Analysis of the effects of harmful gases on the durability of paper and protective countermeasures. Arch. Commun. 2022, 29, 1–9. [Google Scholar]
- Cao, G. Research progress on the performance of doped metal oxide semiconductor gas sensor. Optoelectron. Technol. Appl. 2020, 35, 15–27. [Google Scholar]
- Yang, J.; Pan, Y.; Qin, M.; Yan, C.C.; Huang, Q.M. Research progress on metal oxide semiconductor gas sensor. Chem. Sens. 2022, 42, 10–18. [Google Scholar]
- Huang, Y.; Zhou, Q.; Yan, J. Research progress in the application of TiO2 nanotube array. Mater. Rev. 2012, 26, 51–54. [Google Scholar]
- Dong, J. Design and Application Research of MoS2/TiO2 Nanotube Array Composites. Ph.D. Thesis, Soochow University, Suzhou, China, 2019. [Google Scholar]
- Feng, H.; Tang, N.; Zhang, S.; Liu, B.; Cai, Q. Fabrication of layered (CdS-Mn/MoS2/CdTe)-promoted TiO2 nanotube arrays with superior photocatalytic properties. J. Colloid Interface Sci. 2017, 486, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Tai, H.; Xie, T.; Yuan, Z.; Liu, C.; Jiang, Y. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B Chem. 2016, 223, 149–156. [Google Scholar] [CrossRef]
- Sennik, E.; Alev, O.; Öztürk, Z.Z. The effect of Pd on the H2 and VOC sensing properties of TiO2 nanorods. Sens. Actuators B Chem. 2016, 229, 692–700. [Google Scholar] [CrossRef]
- Shao, Z.; Liu, W.; Zhang, Y.; Yang, X.; Zhong, M. Insights on interfacial charge transfer across MoS2/TiO2-NTAs nanoheterostructures for enhanced photodegradation and biosensing & gas-sensing performance. J. Mol. Struct. 2021, 1244, 131240. [Google Scholar]
- Zhang, D.; Jiang, C.; Zhou, X. Fabrication of Pd-decorated TiO2/MoS2 ternary nanocomposite for enhanced benzene gas sensing performance at room temperature. Talanta 2018, 182, 324–332. [Google Scholar] [CrossRef]
- Singh, S.; Raj, S.; Sharma, S. Ethanol sensing using MoS2/TiO2 composite prepared via hydrothermal method. Mater. Today Proc. 2021, 46, 6083–6086. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-Dimensional MoS2-Decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloys Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Xiao, Y.; Chen, T.; Xiao, X.; Wang, Y. Pt/MoS2/Polyaniline Nanocomposite as a Highly Effective Room Temperature Flexible Gas Sensor for Ammonia Detection. ACS Appl. Mater. Interfaces 2023, 15, 5091110. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Zhao, J.; Qiao, J. Preparation of SnO2-Based Composite Gas-Sensitive Material and Its Effect on the Membrane Treatment Process of Volatile Organic Compounds. J. Nanomater. 2022, 2022, 5091110. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Chen, L.; Ma, J.; Xiong, C. Study on the controllable preparation of TiO2 nanotube arrays by anodic oxidation method. Chem. Eng. 2018, 32, 18–21. [Google Scholar]
- Li, H.; Chen, Y.; Zheng, X.; Zu, G.; Chang, Y.; Wu, Y. Preparation and application of TiO2 nanotubes. Met. Funct. Mater. 2022, 29, 1–9. [Google Scholar]
- Chen, Y.; He, D.; Wang, Y.; Yang, B. Preparation of TiO2-graphene composite photocatalyst by hydrothermal method and its photocatalytic performance. Acta Lumines 2019, 40, 177–182. [Google Scholar]
- Huang, D.; Mo, Z.; Quan, S.; Yang, T.; Liu, Z.; Liu, M. Preparation and photocatalytic reduction performance of graphene/nano-TiO2 composites. J. Compos. 2016, 33, 155–162. [Google Scholar]
- Tan, Z.; Wang, H.; Yang, H.; Zhang, X. Morphology of TiO2 nanotube arrays prepared by anodic oxidation method. J. Mater. Sci. Eng. 2013, 31, 390–394. [Google Scholar]
- Sushmitha, V. Ti@MoS2 incorporated Polypropylene/Nylon fabric-based porous, breathable triboelectric nanogenerator as respiration sensor and ammonia gas sensor applications. Sens. Actuators B Chem. 2023, 380, 133346. [Google Scholar]
- Singh, S.; Sharma, S. Temperature dependent selective detection of ethanol and methanol using MoS2/TiO2 compositeg. Sens. Actuators B. Chem. 2022, 350, 130798. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef] [PubMed]
- Tian, K. Preparation of Metal Oxide Semiconductor Heterojunction and Its Gas Sensing and Photoelectrochemical Properties and Mechanism. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2023. [Google Scholar]
- Bu, X.; Bao, S.; Chu, X.; Liang, S.; Wang, C.; Bai, Y. Preparation and gas sensing properties of MoS2/Cd2SnO4 composites. Acta Inorganica Sin. 2022, 38, 2173–2180. [Google Scholar]
- Zou, C.; Wang, J.; Xie, W. Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 2016, 478, 22–28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ke, L.; Wu, J.; Liang, F.; Xiang, Y. Research and Development of Online Monitoring Protection Sensors for Paper Books Based on TiO2 NT/MoS2. Coatings 2024, 14, 552. https://doi.org/10.3390/coatings14050552
Wang J, Ke L, Wu J, Liang F, Xiang Y. Research and Development of Online Monitoring Protection Sensors for Paper Books Based on TiO2 NT/MoS2. Coatings. 2024; 14(5):552. https://doi.org/10.3390/coatings14050552
Chicago/Turabian StyleWang, Jia, Lifang Ke, Jieling Wu, Feng Liang, and Yanxiong Xiang. 2024. "Research and Development of Online Monitoring Protection Sensors for Paper Books Based on TiO2 NT/MoS2" Coatings 14, no. 5: 552. https://doi.org/10.3390/coatings14050552




