Preparation of n-Tetradecane Phase Change Microencapsulated Polyurethane Coating and Experiment on Anti-Icing Performance for Wind Turbine Blades
Abstract
1. Introduction
2. Materials Preparation and Blade Model
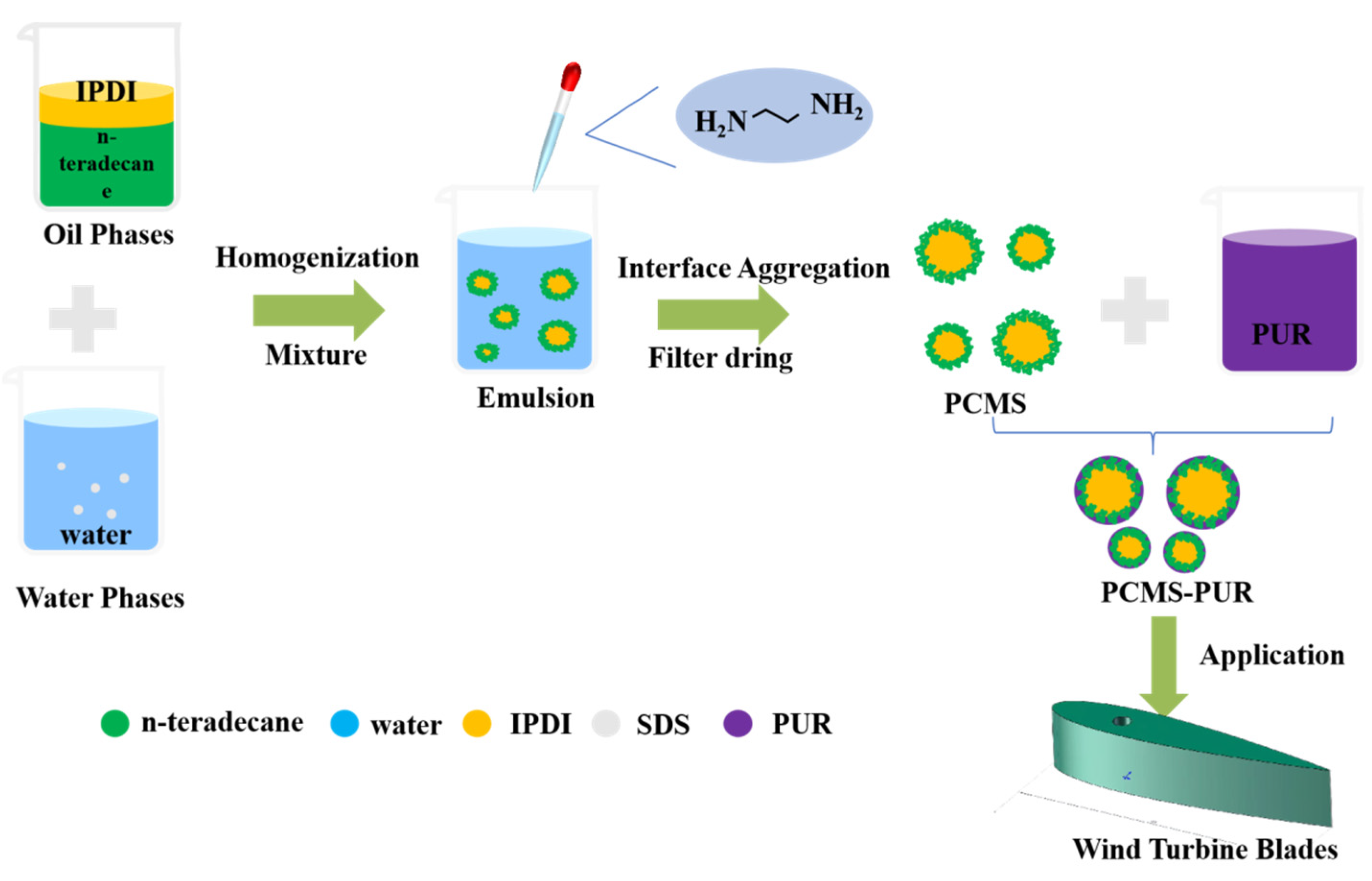
2.1. Preparation of Tetradecane Phase Change Microcapsule Polyurethane Coating
- 1.
- Preparation of Oil Phase Materials;
- Prepare a mixture of 9.9 g of petroleum ether and 0.1 g of dibutyltin dilaurate as the catalyst.
- Weigh 3 g of C14 in a beaker and add 0.02 g of the catalyst and 2 g of IPDI dropwise.
- Seal the beaker with plastic wrap, place it in a heat-collecting constant temperature magnetic stirrer, and heat and stir at 60 °C for 30 min to obtain an oil phase with a core–shell ratio of 6:4.
- 2.
- Preparation of Water Phase Materials;
- Place 0.05 g of sodium dodecyl sulfate (SDS) in a beaker.
- Pour 50 g of deionized water into the beaker and place it in a 60 °C water bath.
- Homogenize for 3 min at 10,000 rpm using a homogenizer to obtain the water phase.
- 3.
- Preparation of PCMS;
- Prepare an aqueous solution of ethylenediamine: add 1.275 g of ethylenediamine to 24.43 g of water.
- Add the oil phase to the water phase and homogenize for 3 min at 60 °C and 10,000 rpm.
- Transfer the mixture to a three-neck flask and react at 60 °C for 6 h.
- During the intense reaction stage in the first 3 hours, add half of the ethylenediamine aqueous solution, and add the remaining half after 3 hours of reaction.
- After 6 h of reaction, filter and dry the product using a filtration drying device to obtain PCMS powder.
- 4.
- Preparation of PCMS-PUR.
- Grind the dried PCMS-PUR into powder.
- Mix the powder with PUR coating to obtain PCMS-PUR coating.
2.2. Blade Model
3. Material Characterization and Analysis
3.1. Material Characterization
3.2. Analysis of Material Characterization Results
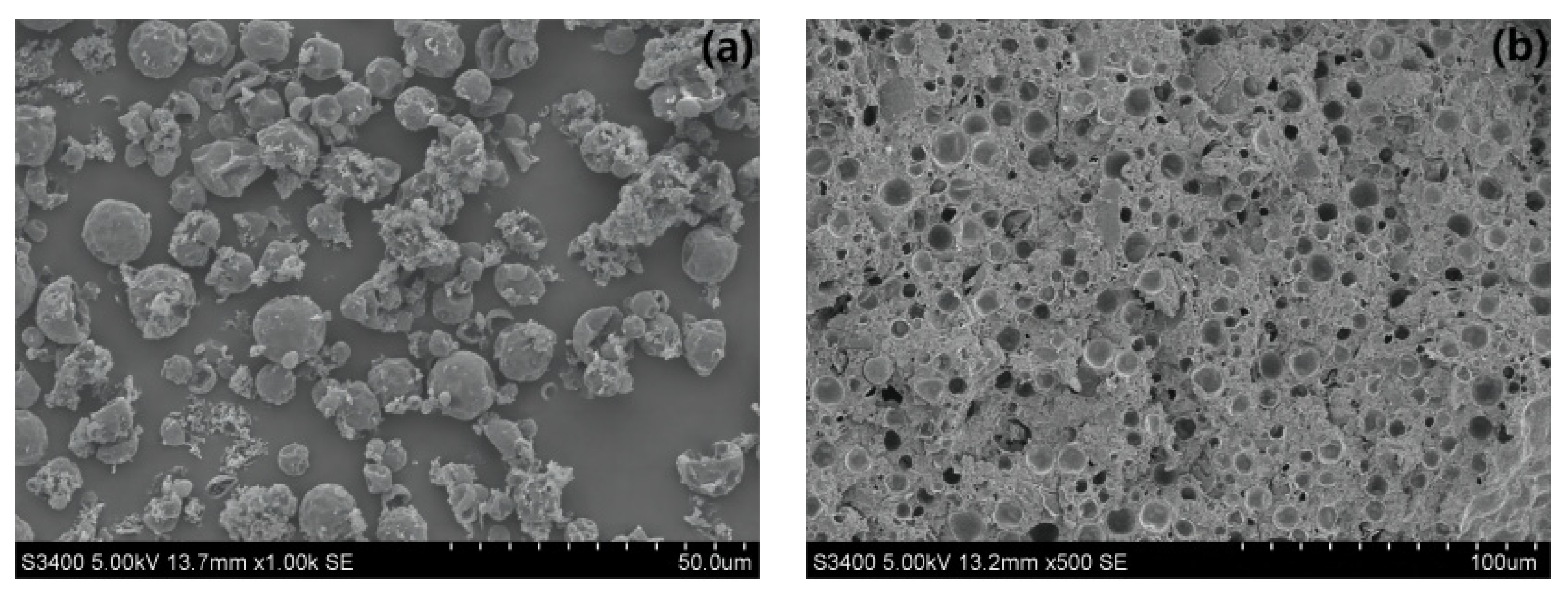
3.2.1. SEM Analysis
3.2.2. DSC Testing and Calculation of Encapsulation Efficiency and Energy Storage Efficiency
- -
- The melting enthalpy (ΔHm) of PCMS at 13% concentration was 3.7 J/g, and the crystallization enthalpy (ΔHc) was 3.3 J/g.
- -
- The melting enthalpy (ΔHm) of PCMS at 26% concentration was 14.7 J/g, and the crystallization enthalpy (ΔHc) was 14.0 J/g.
- -
- The melting enthalpy (ΔHm) of PCMS at 38% concentration was 24.9 J/g, and the crystallization enthalpy (ΔHc) was 27.0 J/g.
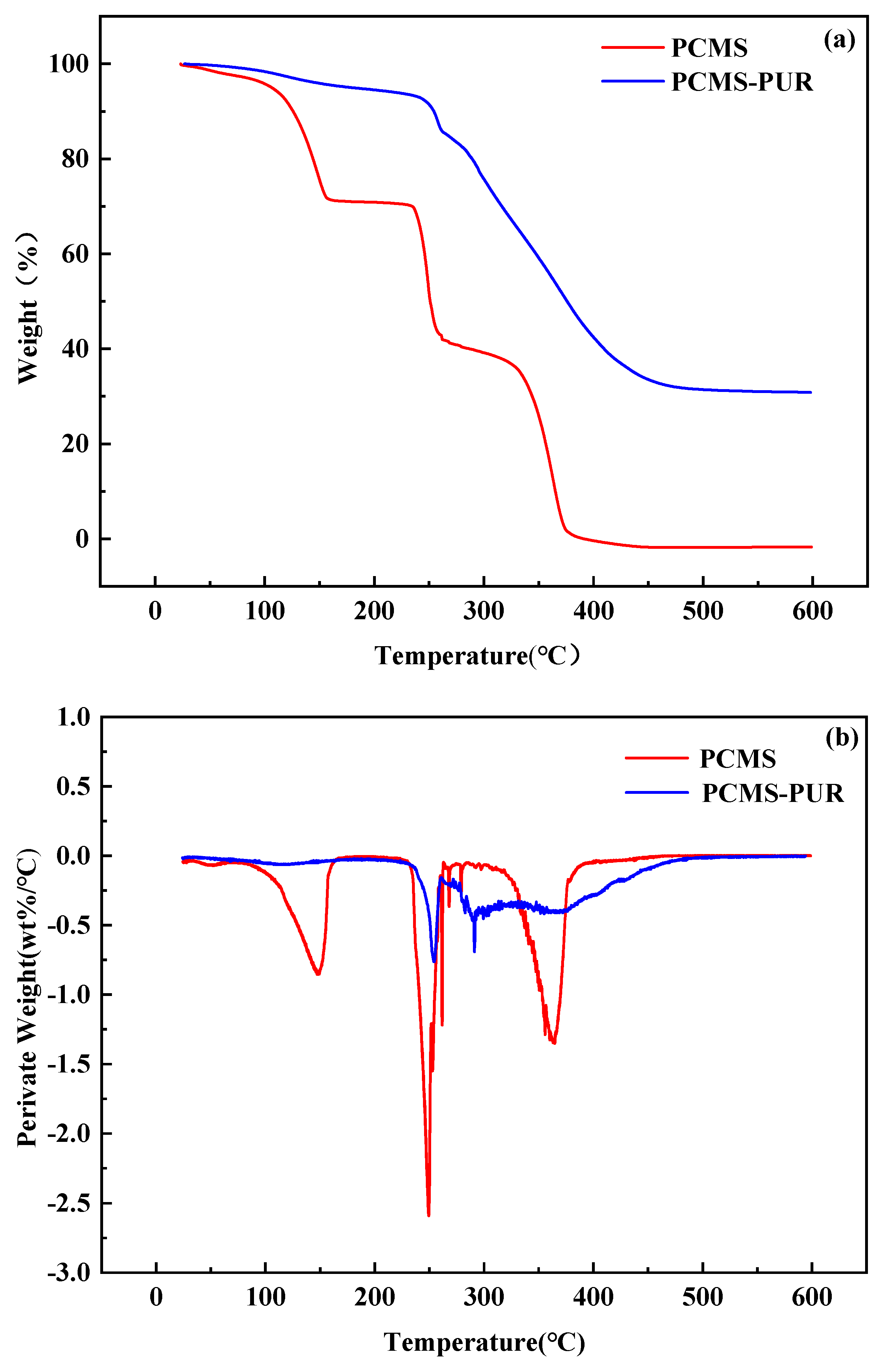
3.2.3. TGA Testing
4. Ice Protection Testing
4.1. Icing Wind Tunnel System
4.2. Dynamic Icing Test Scheme in the Wind Tunnel
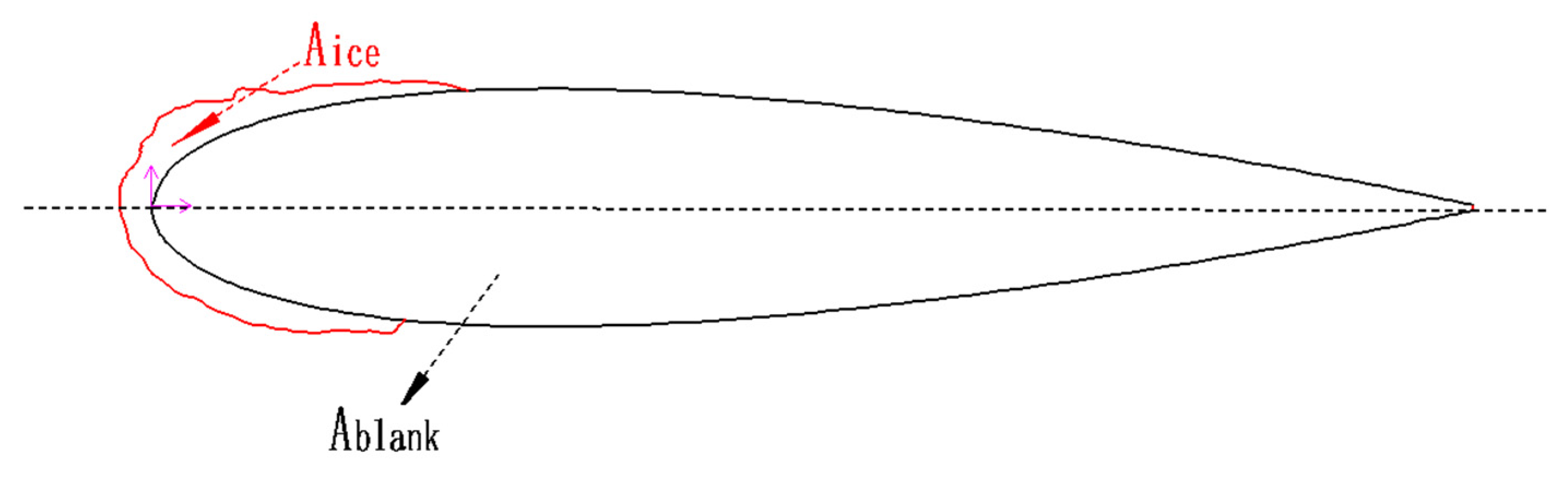
4.3. Evaluation Parameters
- Aice;
- F;
- 3.
- R.
4.4. Results and Discussion
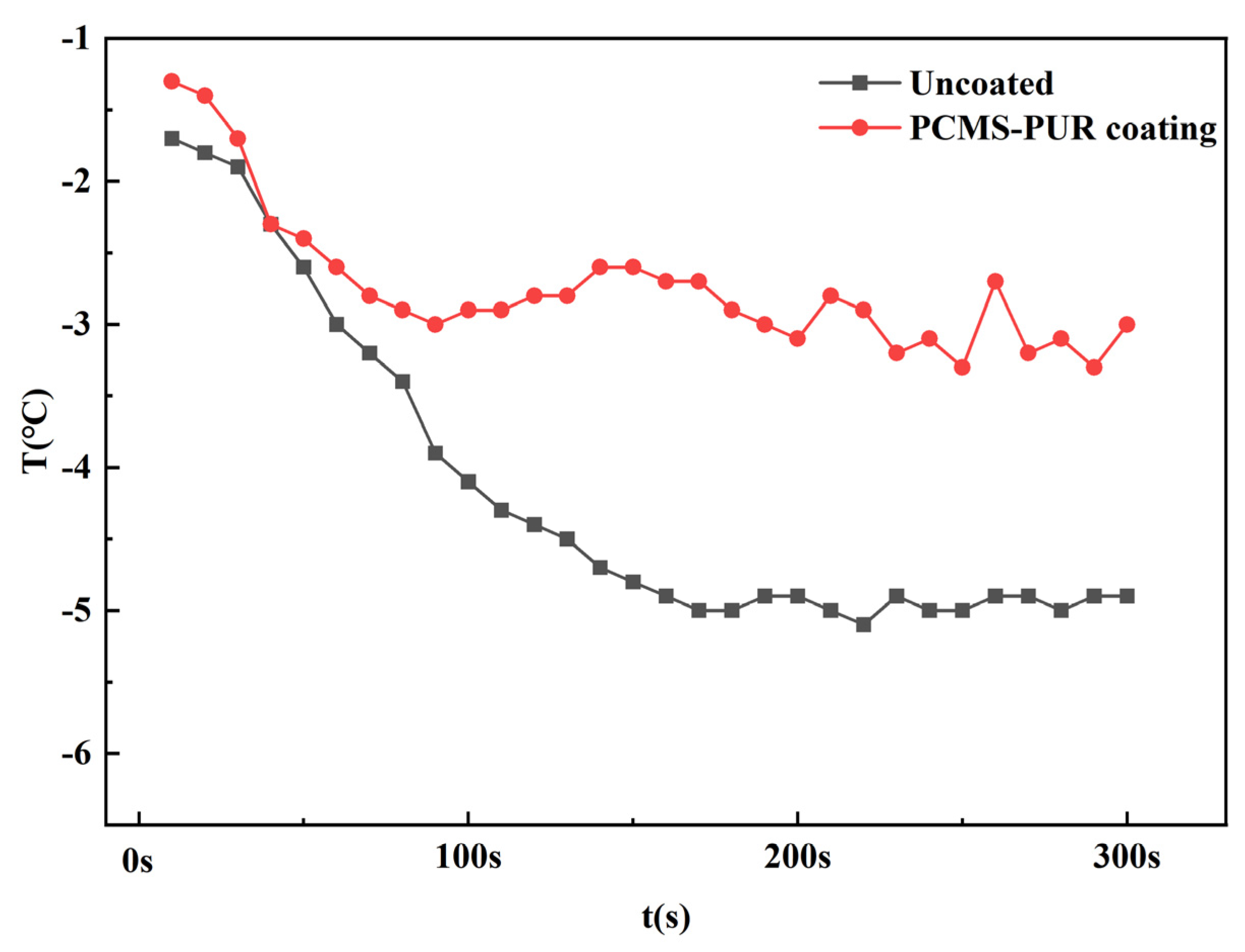
4.4.1. Surface Temperature
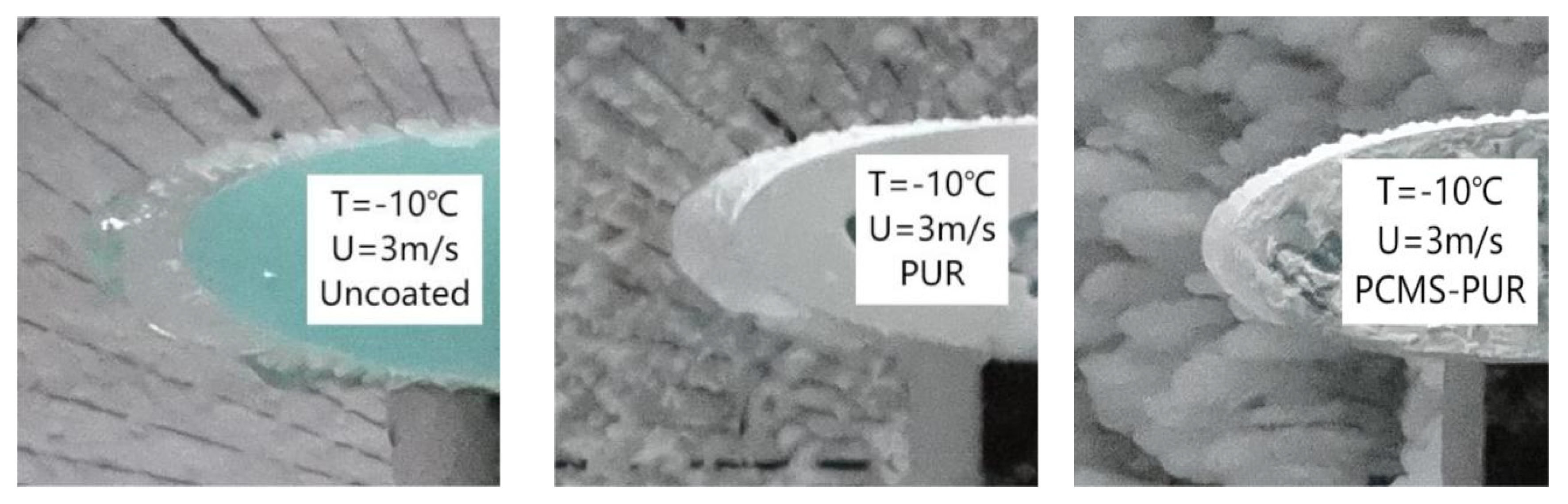
4.4.2. Icing Distribution
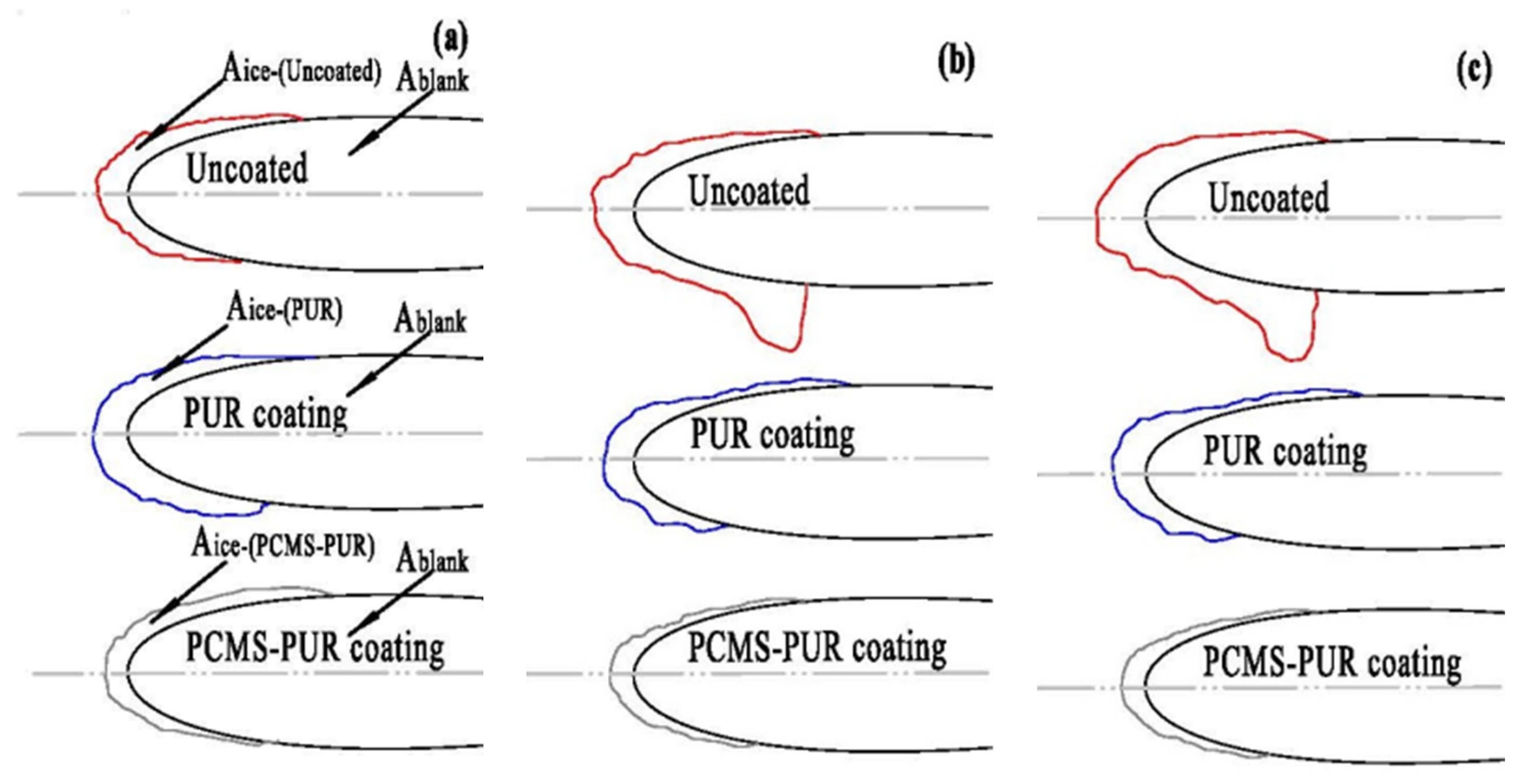
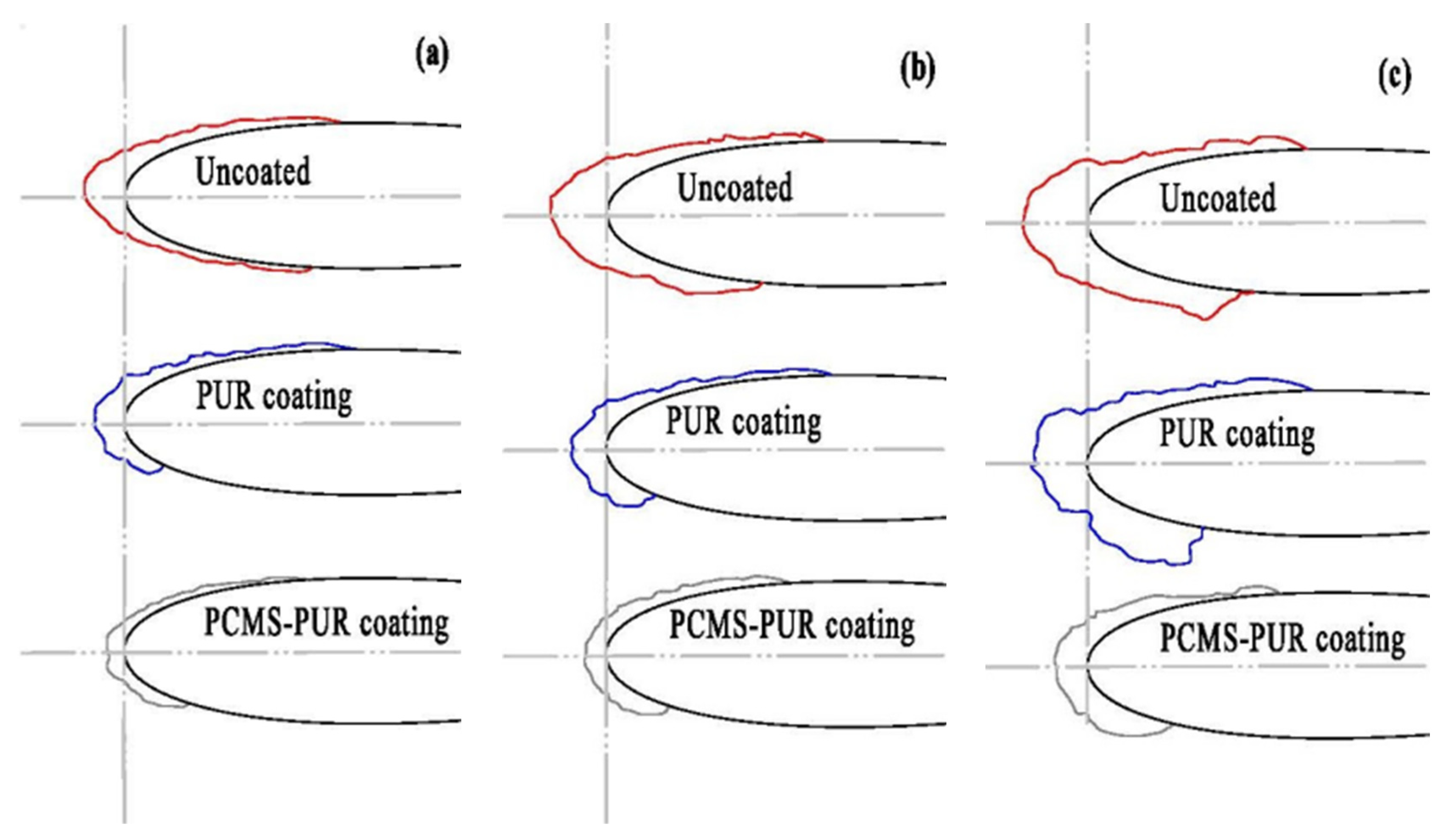
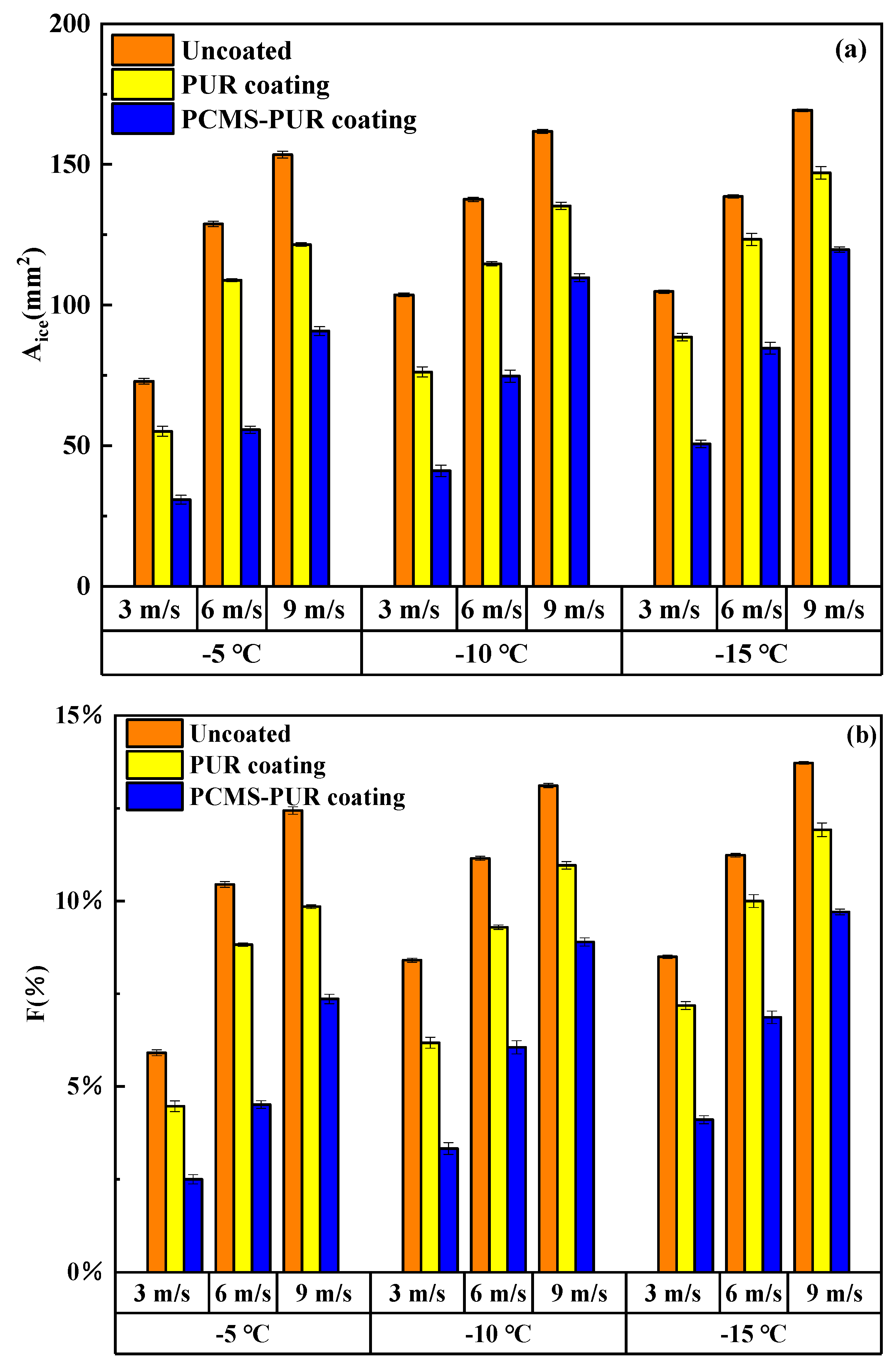
4.4.3. Ice Area Ratio
4.4.4. Anti-Icing Rate Analysis
5. Conclusions and Discussion
- In this study, PCMS were prepared via interfacial polymerization and added into PUR coating to form PCMS-PUR coating, which was applied to wind turbine blades. The enthalpy of melting of the microcapsules was 90.8 J/g, and the enthalpy of crystallization was 96.3 J/g, indicating that they have excellent thermal energy storage capacity.
- Through the icing wind tunnel experiment, it is found that at a certain temperature and a certain wind speed, the leading edge of the uncoated blade is more likely to be affected by the supercooled water droplets, leading to an increase in the icing amount. The PCMS-PUR coating can release heat at low temperatures, slowing down the sharp decrease in the surface temperature of the blade, which makes the supercooled droplets slow down the speed of icing. Therefore, the increase in icing is slow.
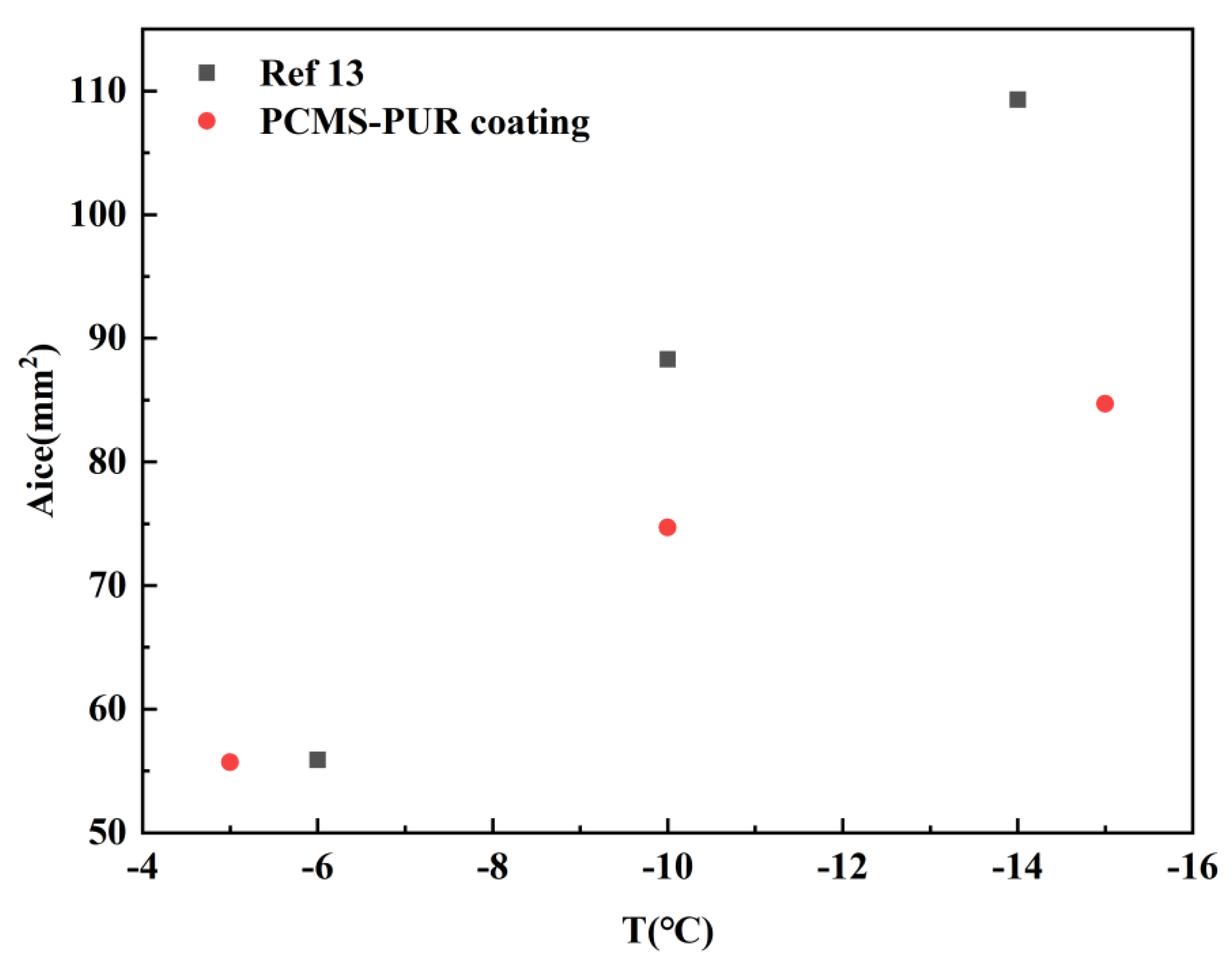
- When the wind speed is certain, the icing area ratio gradually increases as the temperature decreases. When the wind speed is 3 m/s and the temperature is −15 °C, the icing area ratio of the uncoated blade is 8.5%, while the icing area ratio of the PCMS-PUR coating blade is 4.1%. When the temperature is certain, the icing area ratio increases gradually with the increase in wind speed. When the temperature is −15 °C and the wind speed is 6 m/s, the icing area ratio of the uncoated blade is 11.2%, while the icing area ratio of the PCMS-PUR coating blade is 6.9%. In comparison, the icing area ratio of the uncoated blade is higher. This indicates that PCMS-PUR coating is more effective in delaying icing and helps to reduce the degree of blade icing.
- At different wind speeds and the same temperatures, compared with the uncoated blade, the icing area of the blade coated with PCMS-PUR coating is significantly reduced, and the anti-icing rate is as high as 60.41%. At different temperatures and the same wind speed, the anti-icing rate is as high as 57.7%, which indicates that PCMS-PUR coating shows good anti-icing performance at different wind speeds and temperatures, which can effectively reduce the icing on the leading edge of the blade and improve the performance and reliability of the wind turbine.
- PCMS is able to absorb and release a large amount of heat during the phase change process and has good heat storage and release ability, which is suitable for regulating and stabilizing the surface temperature of the blades, thus delaying the icing. This study provides basic research data for exploring new anti-icing methods and reveals the great potential of PCMS in delaying the icing of wind turbine blades.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishnan, P.; Shabbir, M.S.; Siddiqi, A.F.; Wang, X. Current status and future prospects of renewable energy: A case study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2698–2703. [Google Scholar] [CrossRef]
- Tong, G.; Li, Y.; Tagawa, K.; Feng, F. Effects of blade airfoil chord length and rotor diameter on aerodynamic performance of straight-bladed vertical axis wind turbines by numerical simulation. Energy 2023, 265, 126325. [Google Scholar] [CrossRef]
- Zheng, C.W.; Li, C.Y.; Pan, J.; Liu, M.Y.; Xia, L.L.J.R.; Reviews, S.E. An overview of global ocean wind energy resource evaluations. Energy Rev. 2016, 53, 1240–1251. [Google Scholar] [CrossRef]
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Abdul Sattar, M. Renewable energy in the 21st century: A review. Mater. Today Proc. 2023, 80, 1756–1759. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Feng, F.; Tagawa, K. A review on numerical simulation based on CFD technology of aerodynamic characteristics of straight-bladed vertical axis wind turbines. Energy Rep. 2023, 9, 4360–4379. [Google Scholar] [CrossRef]
- Rastayesh, S.; Long, L.; Dalsgaard Sørensen, J.; Thöns, S. Risk Assessment and Value of Action Analysis for Icing Conditions of Wind Turbines Close to Highways. Energies 2019, 12, 2653. [Google Scholar] [CrossRef]
- Katsaprakakis, D.A.; Papadakis, N.; Ntintakis, I. A Comprehensive Analysis of Wind Turbine Blade Damage. Energies 2021, 14, 5974. [Google Scholar] [CrossRef]
- Kreutz, M.; Ait-Alla, A.; Varasteh, K.; Oelker, S.; Greulich, A.; Freitag, M.; Thoben, K.-D. Machine learning-based icing prediction on wind turbines. Procedia CIRP 2019, 81, 423–428. [Google Scholar] [CrossRef]
- Gao, L.; Hu, H. Wind turbine icing characteristics and icing-induced power losses to utility-scale wind turbines. Proc. Natl. Acad. Sci. USA 2021, 118, e2111461118. [Google Scholar] [CrossRef]
- Swenson, L.; Gao, L.; Hong, J.; Shen, L. An efficacious model for predicting icing-induced energy loss for wind turbines. Appl. Energy 2022, 305, 117809. [Google Scholar] [CrossRef]
- Lopera-Valle, A.; McDonald, A. Flame-sprayed coatings as de-icing elements for fiber-reinforced polymer composite structures: Modeling and experimentation. Int. J. Heat Mass Transf. 2016, 97, 56–65. [Google Scholar] [CrossRef]
- Golewski, P.; Budka, M. The Influence of Flame Exposure and Solid Particle Erosion on Tensile Strength of CFRP Substrate with Manufactured Protective Coating. Materials 2024, 17, 1203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; He, Z. Ice-phobic properties of MoS2-loaded rice straw biogas residue biochar-based photothermal and anti-corrosion coating with low oxygen to carbon ratio. Biochar 2023, 5, 74. [Google Scholar] [CrossRef]
- Wei, X.; Cai, F.; Wang, J. Electrothermal/photothermal superhydrophobic coatings based on micro/nano graphite flakes for efficient anti-icing and de-icing. Prog. Org. Coat. 2023, 182, 107696. [Google Scholar] [CrossRef]
- Zhuang, A.; Li, C.; Yu, J.; Lu, Y. The Glaze Icing Performance of a Robust Superhydrophobic Film Composed of Epoxy Resin and Polydimethylsiloxane. Coatings 2023, 13, 1271. [Google Scholar] [CrossRef]
- Piscitelli, F.; Chiariello, A.; Dabkowski, D.; Corraro, G.; Marra, F.; Di Palma, L. Superhydrophobic Coatings as Anti-Icing Systems for Small Aircraft. Aerospace 2020, 7, 2. [Google Scholar] [CrossRef]
- Sullivan, A.; Duan, X.; Yang, J. Energy-efficient anti-icing performance of a hybrid superhydrophobic and electrothermal coating on metallic substrates. Mater. Chem. Phys. 2023, 301, 127700. [Google Scholar] [CrossRef]
- Tong, W.; Xiong, D.; Wang, N.; Wu, Z.; Zhou, H. Mechanically robust superhydrophobic coating for aeronautical composite against ice accretion and ice adhesion. Compos. Part B Eng. 2019, 176, 107267. [Google Scholar] [CrossRef]
- Qin, C.; Mulroney, A.T.; Gupta, M.C. Anti-icing epoxy resin surface modified by spray coating of PTFE Teflon particles for wind turbine blades. Mater. Today Commun. 2020, 22, 100770. [Google Scholar] [CrossRef]
- Peng, G.; Dou, G.; Hu, Y.; Sun, Y.; Chen, Z. Phase Change Material (PCM) Microcapsules for Thermal Energy Storage. Adv. Polym. Technol. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar]
- Sinaga, R.; Darkwa, J.; Omer, S.A.; Worall, M. The microencapsulation, thermal enhancement, and applications of medium and high-melting temperature phase change materials: A review. Int. J. Energy Res. 2022, 46, 10259–10300. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Parale, V.; Vhatkara, R.S.; Mahadik, D.B.; Kavale, M.S.; Wagh, P.B.; Gupta, S.; Gurav, J. Superhydrophobic silica coating by dip coating method. Appl. Surf. Sci. 2013, 277, 67–72. [Google Scholar] [CrossRef]
- Chou, C.-S.; Chou, F.-C.; Kang, J.-Y. Preparation of ZnO-coated TiO2 electrodes using dip coating and their applications in dye-sensitized solar cells. Powder Technol. 2012, 215–216, 38–45. [Google Scholar] [CrossRef]
- Lu, S.; Shen, T.; Xing, J.; Song, Q.; Shao, J.; Zhang, J.; Xin, C. Preparation and characterization of cross-linked polyurethane shell microencapsulated phase change materials by interfacial polymerization. Mater. Lett. 2018, 211, 36–39. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Zhou, H.; Shi, W.; Zhang, Y.; Huang, Y. Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization. Materials 2022, 15, 2479. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.-B.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 21840–21856. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Benes, N.E. Current trends in interfacial polymerization chemistry. Prog. Polym. Sci. 2016, 63, 86–142. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Shen, J.; Wang, B.; Mao, Z.; Xu, H.; Feng, X.; Sui, X. Stable microencapsulated phase change materials with ultrahigh payload for efficient cooling of mobile electronic devices. Energy Convers. Manag. 2020, 223, 113478. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, F.; Li, Y.; Sun, Y.; Tagawa, K. A corncob biochar-based superhydrophobic photothermal coating with micro-nano-porous rough-structure for ice-phobic properties. Surf. Coat. Technol. 2023, 457, 129299. [Google Scholar] [CrossRef]






| Concentration | Melting Process | Crystallization Process | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |
| 13% | 4.7 | 3.7 | −17.3 | 3.3 |
| 26% | 5.4 | 14.7 | −18.8 | 14.0 |
| 38% | 7.8 | 24.9 | −19.5 | 27.0 |
| Sample | K (%) | E (%) | Melting Process | Crystallization Process | |||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | Tr (°C) | ΔHc (J/g) | |||
| C14 | 7.1 | 172.9 | 0.8 | 182.2 | |||
| PCMS | 53 | 53 | 7.4 | 90.8 | −16.9 | −1.7 | 96.3 |
| PCMS-PUR | 7.8 | 24.9 | −19.5 | 27.0 | |||
| Working Condition | Coating Type | Temperature (°C) | Wind Velocity (m/s) |
|---|---|---|---|
| 1 | Uncoated | −5 °C/−10 °C/−15 °C | 3 m/s |
| 2 | PUR coating | 3 m/s | |
| 3 | PCMS-PUR coating | 3 m/s | |
| 4 | Uncoated | 6 m/s | |
| 5 | PUR coating | 6 m/s | |
| 6 | PCMS-PUR coating | 6 m/s | |
| 7 | Uncoated | 9 m/s | |
| 8 | PUR coating | 9 m/s | |
| 9 | PCMS-PUR coating | 9 m/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shen, H.; Sun, Z.; Li, Y.; Feng, F. Preparation of n-Tetradecane Phase Change Microencapsulated Polyurethane Coating and Experiment on Anti-Icing Performance for Wind Turbine Blades. Coatings 2024, 14, 645. https://doi.org/10.3390/coatings14050645
Wang Y, Shen H, Sun Z, Li Y, Feng F. Preparation of n-Tetradecane Phase Change Microencapsulated Polyurethane Coating and Experiment on Anti-Icing Performance for Wind Turbine Blades. Coatings. 2024; 14(5):645. https://doi.org/10.3390/coatings14050645
Chicago/Turabian StyleWang, Yiting, He Shen, Zheng Sun, Yan Li, and Fang Feng. 2024. "Preparation of n-Tetradecane Phase Change Microencapsulated Polyurethane Coating and Experiment on Anti-Icing Performance for Wind Turbine Blades" Coatings 14, no. 5: 645. https://doi.org/10.3390/coatings14050645
APA StyleWang, Y., Shen, H., Sun, Z., Li, Y., & Feng, F. (2024). Preparation of n-Tetradecane Phase Change Microencapsulated Polyurethane Coating and Experiment on Anti-Icing Performance for Wind Turbine Blades. Coatings, 14(5), 645. https://doi.org/10.3390/coatings14050645







