Corrosion Resistance of Atomically Thin Graphene Coatings on Single Crystal Copper
Abstract
:1. Introduction
2. Material Synthesis and Characterization
2.1. Materials Synthesis
2.2. Characterization
2.3. SRB Cultivation and Growth
2.4. Electrochemical Analysis
- n is the number of exchanged equivalents;
- is the density of the metal (g/cm3);
- a is the atomic weight of the metal (g/mol).
2.5. Molecular Characterization of the SRB
3. Result and Discussion
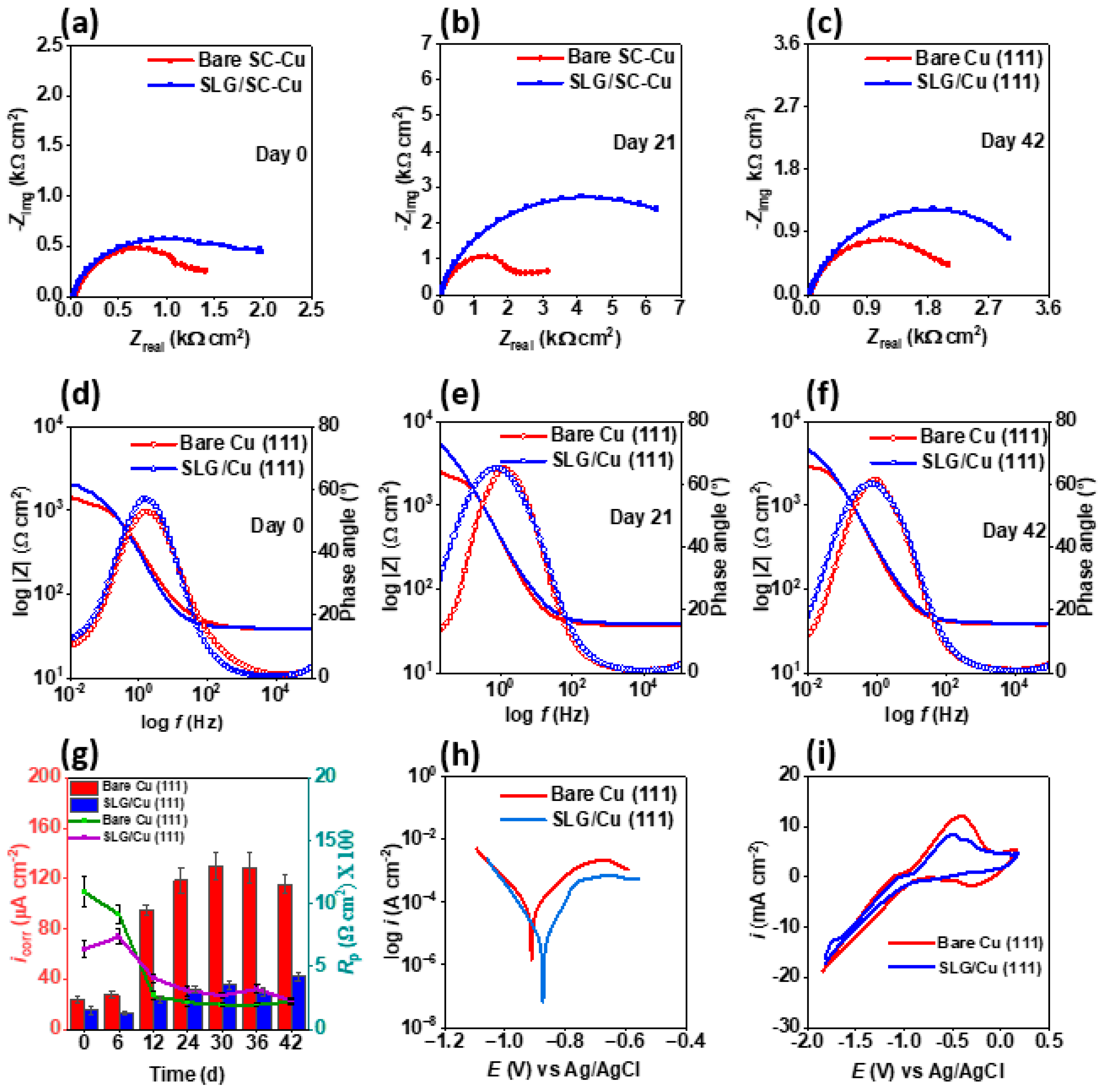
3.1. Corrosion Resistance in an Abiotic Environment
3.2. Corrosion Resistance in the Biotic Environment
3.3. Mechanism of Corrosion Prevention by Graphene on Single Cu (111) Crystals:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coatings, T.I. The Real Cost of Corrosion. 2013. Available online: https://www.thomasindcoatings.com/the-real-cost-of-corrosion/ (accessed on 12 December 2023).
- Singh, A.K. Introduction to Corrosion. In Microbially Induced Corrosion and Its Mitigation. SpringerBriefs in Materials; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Dou, W.; Xu, D.; Gu, T. Biocorrosion caused by microbial biofilms is ubiquitous around us. Microb. Biotechnol. 2021, 14, 803–805. [Google Scholar] [CrossRef]
- Chilkoor, G.; Shrestha, N.; Kutana, A.; Tripathi, M.; Robles Hernández, F.C.; Yakobson, B.I.; Meyyappan, M.; Dalton, A.B.; Ajayan, P.M.; Rahman, M.M. Atomic layers of graphene for microbial corrosion prevention. ACS Nano 2020, 15, 447–454. [Google Scholar] [CrossRef] [PubMed]
- BSRIA WMI. Opportunities for Copper in Water and Waste Water Treatment. Available online: https://copperalliance.org/wp-content/uploads/2021/08/fact-sheet-opportunities-for-copper-in-water-and-waste-water-treatment-1.pdf (accessed on 18 December 2023).
- Böhm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Natarajan, B.; Eksik, O.; Ali Shojaee, S.; Lucca, D.A.; Ren, W.; Cheng, H.-M.; Koratkar, N. Superiority of Graphene over Polymer Coatings for Prevention of Microbially Induced Corrosion. Sci. Rep. 2015, 5, 13858. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G.S. Sol–gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Ocón, P.; Cristobal, A.B.; Herrasti, P.; Fatas, E. Corrosion performance of conducting polymer coatings applied on mild steel. Corros. Sci. 2005, 47, 649–662. [Google Scholar] [CrossRef]
- Tan, C.K.; Blackwood, D.J. Corrosion protection by multilayered conducting polymer coatings. Corros. Sci. 2003, 45, 545–557. [Google Scholar] [CrossRef]
- Chen, F.; Liu, P. Conducting Polyaniline Nanoparticles and Their Dispersion for Waterborne Corrosion Protection Coatings. ACS Appl. Mater. Interfaces 2011, 3, 2694–2702. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Li, J.; Lu, J.; Wang, F. Polyaniline for corrosion prevention of mild steel coupled with copper. Electrochim. Acta 2007, 52, 5392–5399. [Google Scholar] [CrossRef]
- González-García, Y.; González, S.; Souto, R.M. Electrochemical and structural properties of a polyurethane coating on steel substrates for corrosion protection. Corros. Sci. 2007, 49, 3514–3526. [Google Scholar] [CrossRef]
- Khorgami, G.; Solaimany, F.; Haddadi, S.A.; Ramezanzadeh, M.; Ramezanzadeh, B. Polyurethanes for Corrosion Protective Coatings. In Polyurethanes: Preparation, Properties, and Applications Volume 1: Fundamentals; ACS Publications: Washington, DC, USA, 2023; pp. 133–159. [Google Scholar]
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- Cai, R.; Sun, M.; Chen, Z.; Munoz, R.; O’Neill, C.; Beving, D.E.; Yan, Y. Ionothermal synthesis of oriented zeolite AEL films and their application as corrosion-resistant coatings. Angew. Chem. Int. Ed. 2008, 47, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Quraishi, M.; Hussain, C.M. Greenly synthesized zeolites as sustainable materials for corrosion protection: Design, technology and application. Adv. Colloid Interface Sci. 2023, 314, 102868. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, R.; Pu, J.; He, Z.; Xiong, L. 2D graphene and h-BN layers application in protective coatings. Corros. Rev. 2021, 39, 93–107. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2 MXene nanosheets toward high-performance corrosion inhibitor for epoxy coating. Prog. Org. Coat. 2019, 135, 156–167. [Google Scholar] [CrossRef]
- Tanjil, M.R.-E.; Jeong, Y.; Yin, Z.; Panaccione, W.; Wang, M.C. Ångström-scale, atomically thin 2D materials for corrosion mitigation and passivation. Coatings 2019, 9, 133. [Google Scholar] [CrossRef]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-Dimensional Nanomaterials for Anticorrosive Polymeric Coatings: A Review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Chilkoor, G.; Karanam, S.P.; Star, S.; Shrestha, N.; Sani, R.K.; Upadhyayula, V.K.; Ghoshal, D.; Koratkar, N.A.; Meyyappan, M.; Gadhamshetty, V. Hexagonal boron nitride: The thinnest insulating barrier to microbial corrosion. ACS Nano 2018, 12, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Mahvash, F.; Eissa, S.; Bordjiba, T.; Tavares, A.; Szkopek, T.; Siaj, M. Corrosion resistance of monolayer hexagonal boron nitride on copper. Sci. Rep. 2017, 7, 42139. [Google Scholar] [CrossRef]
- Husain, E.; Narayanan, T.N.; Taha-Tijerina, J.J.; Vinod, S.; Vajtai, R.; Ajayan, P.M. Marine corrosion protective coatings of hexagonal boron nitride thin films on stainless steel. ACS Appl. Mater. Interfaces 2013, 5, 4129–4135. [Google Scholar] [CrossRef]
- Allen, C.; Aryal, S.; Do, T.; Gautum, R.; Hasan, M.M.; Jasthi, B.K.; Gnimpieba, E.; Gadhamshetty, V. Deep learning strategies for addressing issues with small datasets in 2D materials research: Microbial Corrosion. Front. Microbiol. 2022, 13, 1059123. [Google Scholar] [CrossRef]
- Kalita, G.; Ayhan, M.E.; Sharma, S.; Shinde, S.M.; Ghimire, D.; Wakita, K.; Umeno, M.; Tanemura, M. Low temperature deposited graphene by surface wave plasma CVD as effective oxidation resistive barrier. Corros. Sci. 2014, 78, 183–187. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Liu, C.; Wang, L. A comprehensive review on ultrathin, multi-functionalized, and smart graphene and graphene-based composite protective coatings. Corros. Sci. 2023, 212, 110939. [Google Scholar] [CrossRef]
- Markets, M.A. Graphene Coating Market by Product Type (Solvent-Based and Water-Based), Application (Corrosion-Resistant Coating, Scratch-Resistant Coating, Antifouling Coating, Flame-Retardant Coating), End-Use industry, and Region-Global Forecast to 2028. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/graphene-coating-market-69854439.html (accessed on 12 December 2023).
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Luo, D.; Wang, M.; Li, Y.; Kim, C.; Yu, K.M.; Kim, Y.; Han, H.; Biswal, M.; Huang, M.; Kwon, Y. Adlayer-free large-area single crystal graphene grown on a Cu (111) foil. Adv. Mater. 2019, 31, 1903615. [Google Scholar] [CrossRef]
- Ansari, R.; Motevalli, B.; Montazeri, A.; Ajori, S. Fracture analysis of monolayer graphene sheets with double vacancy defects via MD simulation. Solid State Commun. 2011, 151, 1141–1146. [Google Scholar] [CrossRef]
- Lee, G.-D.; Wang, C.; Yoon, E.; Hwang, N.-M.; Kim, D.-Y.; Ho, K. Diffusion, coalescence, and reconstruction of vacancy defects in graphene layers. Phys. Rev. Lett. 2005, 95, 205501. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hwang, J.H.; Suh, J.; Tongay, S.; Kwon, S.; Hwang, C.; Wu, J.; Young Park, J. Work function engineering of single layer graphene by irradiation-induced defects. Appl. Phys. Lett. 2013, 103, 171604. [Google Scholar] [CrossRef]
- Long, F.; Yasaei, P.; Sanoj, R.; Yao, W.; Král, P.; Salehi-Khojin, A.; Shahbazian-Yassar, R. Characteristic work function variations of graphene line defects. ACS Appl. Mater. Interfaces 2016, 8, 18360–18366. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Louie, S.G. Topological defects in graphene: Dislocations and grain boundaries. Phys. Rev. B 2010, 81, 195420. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Biswas, T.; Ghosh, P.; Suran, S.; Mishra, A.; Mishra, R.; Sachan, R.; Jain, M.; Varma, M.; Pratap, R. Reversible defect engineering in graphene grain boundaries. Nat. Commun. 2019, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Berman, D. Inhibitor or promoter: Insights on the corrosion evolution in a graphene protected surface. Carbon 2018, 126, 225–231. [Google Scholar] [CrossRef]
- Wood, J.D.; Schmucker, S.W.; Lyons, A.S.; Pop, E.; Lyding, J.W. Effects of Polycrystalline Cu Substrate on Graphene Growth by Chemical Vapor Deposition. Nano Lett. 2011, 11, 4547–4554. [Google Scholar] [CrossRef]
- Cho, J.; Gao, L.; Tian, J.; Cao, H.; Wu, W.; Yu, Q.; Yitamben, E.N.; Fisher, B.; Guest, J.R.; Chen, Y.P.; et al. Atomic-Scale Investigation of Graphene Grown on Cu Foil and the Effects of Thermal Annealing. ACS Nano 2011, 5, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.D.; Jung, J.; Kim, Y.; Huynh, V.N.; Lee, C. Large-area single-crystal graphene grown on a recrystallized Cu(111) surface by using a hole-pocket method. Nanoscale 2016, 8, 13781–13789. [Google Scholar] [CrossRef] [PubMed]
- Ani, M.H.; Kamarudin, M.A.; Ramlan, A.H.; Ismail, E.; Sirat, M.S.; Mohamed, M.A.; Azam, M.A. A critical review on the contributions of chemical and physical factors toward the nucleation and growth of large-area graphene. J. Mater. Sci. 2018, 53, 7095–7111. [Google Scholar] [CrossRef]
- Skriver, H.L.; Rosengaard, N. Surface energy and work function of elemental metals. Phys. Rev. B 1992, 46, 7157. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Jawaharraj, K.; Sigdel, P.; Gu, Z.; Muthusamy, G.; Sani, R.K.; Gadhamshetty, V. Photosynthetic microbial fuel cells for methanol treatment using graphene electrodes. Environ. Res. 2022, 215, 114045. [Google Scholar] [CrossRef]
- Chilkoor, G.; Shrestha, N.; Karanam, S.P.; Upadhyayula, V.K.; Gadhamshetty, V. Graphene coatings for microbial corrosion applications. In Encyclopedia of Water: Science, Technology, and Society; Wiley: New York, NY, USA, 2019; pp. 1–25. [Google Scholar]
- Seifert, M.; Drieschner, S.; Blaschke, B.M.; Hess, L.H.; Garrido, J.A. Induction heating-assisted repeated growth and electrochemical transfer of graphene on millimeter-thick metal substrates. Diam. Relat. Mater. 2014, 47, 46–52. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical Delamination of CVD-Grown Graphene Film: Toward the Recyclable Use of Copper Catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L.-P.; Zhang, Z.; Fu, Q.; Peng, L.-M. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3, 699. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Shin, D.; Bae, S.; Hong, B.H. Graphene transfer: Key for applications. Nanoscale 2012, 4, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Chilkoor, G.; Jawaharraj, K.; Vemuri, B.; Kutana, A.; Tripathi, M.; Kota, D.; Arif, T.; Filleter, T.; Dalton, A.B.; Yakobson, B.I.; et al. Hexagonal Boron Nitride for Sulfur Corrosion Inhibition. ACS Nano 2020, 14, 14809–14819. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A. Principles and prevention. Corrosion 1996, 2, 168. [Google Scholar]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lipatov, A.; Vorobeva, N.S.; Muratov, D.S.; Sinitskii, A. Photoswitchable monolayer and bilayer graphene devices enabled by in situ covalent functionalization. Adv. Electron. Mater. 2018, 4, 1800021. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.; Kim, S.J.; Jeon, S.; Jung, H.-T. The effects of the crystalline orientation of Cu domains on the formation of nanoripple arrays in CVD-grown graphene on Cu. J. Mater. Chem. C 2013, 1, 7819–7824. [Google Scholar] [CrossRef]
- Xu, M.; Fujita, D.; Sagisaka, K.; Watanabe, E.; Hanagata, N. Single-layer graphene nearly 100% covering an entire substrate. arXiv 2010, arXiv:1006.5085. [Google Scholar]
- Kondrashov, I.; Komlenok, M.; Pivovarov, P.; Savin, S.; Obraztsova, E.; Rybin, M. Preparation of copper surface for the synthesis of single-layer graphene. Nanomaterials 2021, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Dong, J.; Yi, D.; Niu, J.; Wu, M.; Lin, L.; Yin, R.; Li, M.; Zhou, J. Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci. Bull. 2017, 62, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Habib, K.; Al-Sabti, F. Electrochemical impedence spectroscopy versus optical interferometry techniques during anodization of aluminium. Opt. Lasers Eng. 2001, 35, 225–232. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Gadhamshetty, V.; Nadeau, L.J.; Johnson, G.R. Impedance spectroscopy as a tool for non-intrusive detection of extracellular mediators in microbial fuel cells. Biotechnol. Bioeng. 2009, 104, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, D.; Masia, N. An insight on corrosion resistance ability of biocompatible dental implants through electrochemical impedance spectroscopy. In Corrosion-Fundamentals and Protection Mechanisms; IntechOpen: London, UK, 2021. [Google Scholar]
- Chauhan, P.; Kumari, P.; Kumar, A. An environmentally friendly copper-based superhydrophobic coating on steel substrate via electrodeposition to reduce corrosion. In Proceedings of the Bioinspiration, Biomimetics, and Bioreplication XIII, Long Beach, CA, USA, 12–17 March 2023; pp. 117–121. [Google Scholar]
- Singh, M.K.; Gautam, R.K.; Ji, G. Mechanical properties and corrosion behavior of copper based hybrid composites synthesized by stir casting. Results Phys. 2019, 13, 102319. [Google Scholar] [CrossRef]
- Devadig, R.; Gurung, B.D.S.; Gnimpieba, E.; Jasthi, B.; Gadhamshetty, V. Computational methods for biofouling and corrosion-resistant graphene nanocomposites. A transdisciplinary approach. In Proceedings of the 2023 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Istanbul, Turkey, 5–8 December 2023; pp. 4494–4496. [Google Scholar]
- Huttunen-Saarivirta, E.; Rajala, P.; Carpén, L. Corrosion behaviour of copper under biotic and abiotic conditions in anoxic ground water: Electrochemical study. Electrochim. Acta 2016, 203, 350–365. [Google Scholar] [CrossRef]
- Chen, J.; Qin, Z.; Shoesmith, D. Long-term corrosion of copper in a dilute anaerobic sulfide solution. Electrochim. Acta 2011, 56, 7854–7861. [Google Scholar] [CrossRef]
- Dou, W.; Jia, R.; Jin, P.; Liu, J.; Chen, S.; Gu, T. Investigation of the mechanism and characteristics of copper corrosion by sulfate reducing bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Raya, D.; Shreya, A.; Kumar, A.; Giri, S.K.; Salem, D.R.; Gnimpieba, E.Z.; Gadhamshetty, V.; Dhiman, S.S. Molecular regulation of conditioning film formation and quorum quenching in sulfate reducing bacteria. Front. Microbiol. 2022, 13, 1008536. [Google Scholar] [CrossRef]
- Hasan, M.M.; Sikder, R.; Jasthi, B.K.; Gnimpieba, E.Z.; Gadhamshetty, V. Discovery of 2D Materials with Machine Learning. In Machine Learning in 2D Materials Science; CRC Press: Boca Raton, FL, USA, 2023; pp. 59–88. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Devadig, R.; Sigdel, P.; Lipatov, A.; Avci, R.; Jasthi, B.K.; Gadhamshetty, V. Corrosion Resistance of Atomically Thin Graphene Coatings on Single Crystal Copper. Coatings 2024, 14, 656. https://doi.org/10.3390/coatings14060656
Hasan MM, Devadig R, Sigdel P, Lipatov A, Avci R, Jasthi BK, Gadhamshetty V. Corrosion Resistance of Atomically Thin Graphene Coatings on Single Crystal Copper. Coatings. 2024; 14(6):656. https://doi.org/10.3390/coatings14060656
Chicago/Turabian StyleHasan, Md Mahmudul, Ramesh Devadig, Pawan Sigdel, Alexey Lipatov, Recep Avci, Bharat K. Jasthi, and Venkataramana Gadhamshetty. 2024. "Corrosion Resistance of Atomically Thin Graphene Coatings on Single Crystal Copper" Coatings 14, no. 6: 656. https://doi.org/10.3390/coatings14060656
APA StyleHasan, M. M., Devadig, R., Sigdel, P., Lipatov, A., Avci, R., Jasthi, B. K., & Gadhamshetty, V. (2024). Corrosion Resistance of Atomically Thin Graphene Coatings on Single Crystal Copper. Coatings, 14(6), 656. https://doi.org/10.3390/coatings14060656


.jpg)


