Atomic Layer Deposition Growth and Characterization of Al2O3 Layers on Cu-Supported CVD Graphene
Abstract
:1. Introduction
2. Materials and Methods
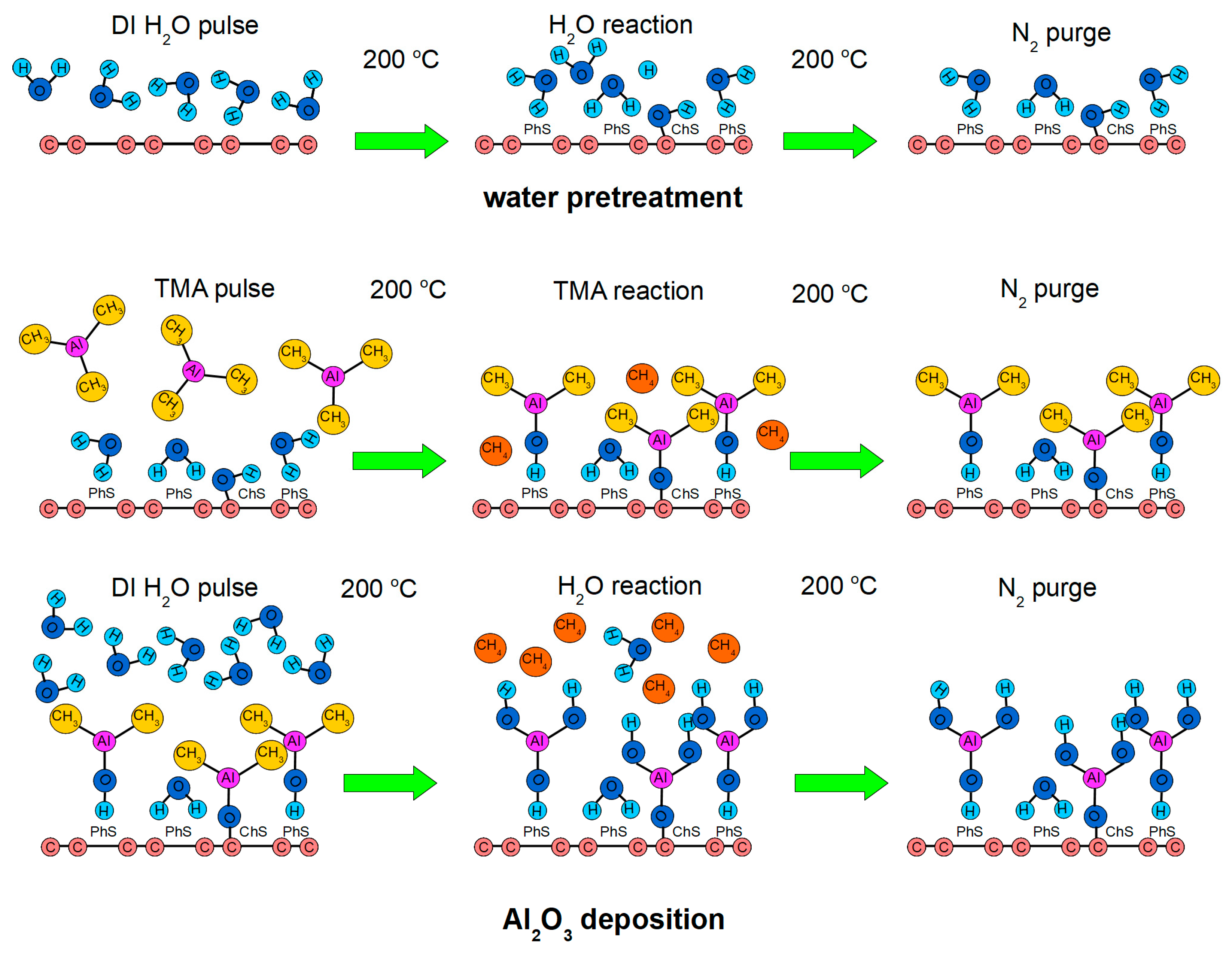
2.1. Synthesis of the Al2O3/Graphene Heterostructure
2.2. Characterization
3. Results and Discussion
3.1. Surface Chemical Composition (XPS Analysis)
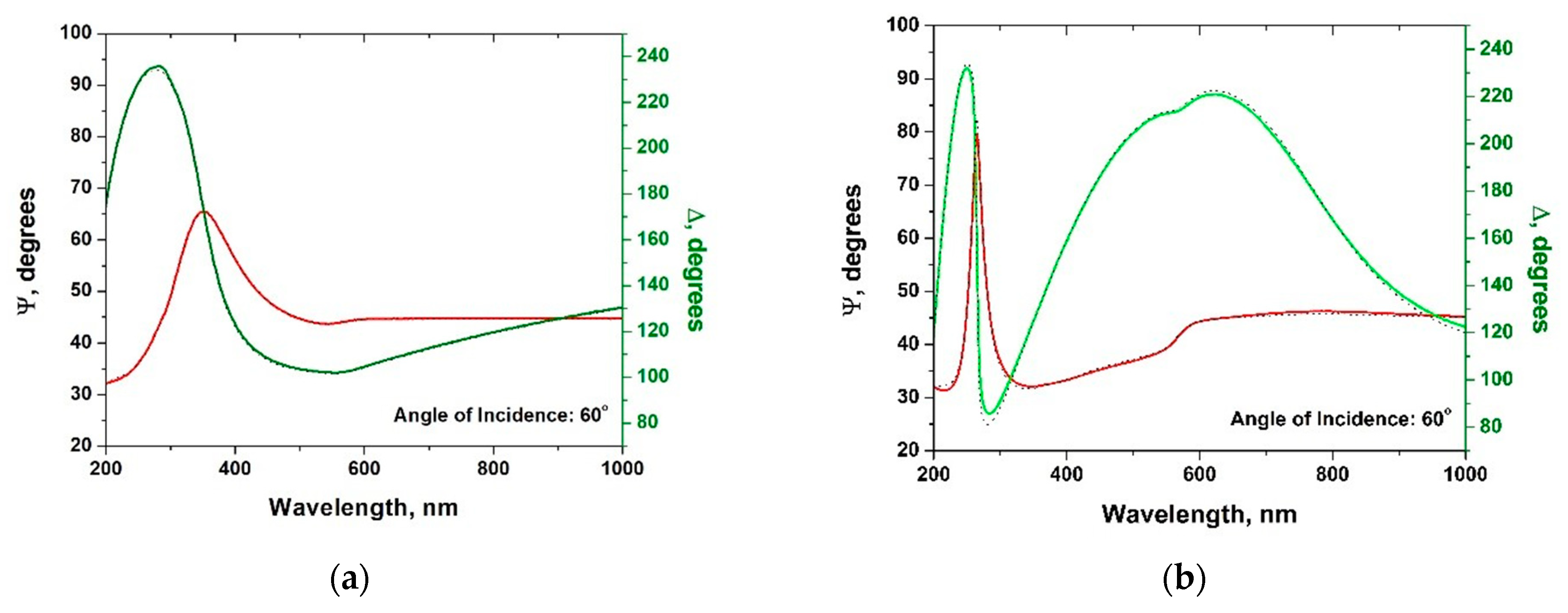
3.2. Study of the Al2O3/Graphene/Cu Heterostructures by Ellipsometry
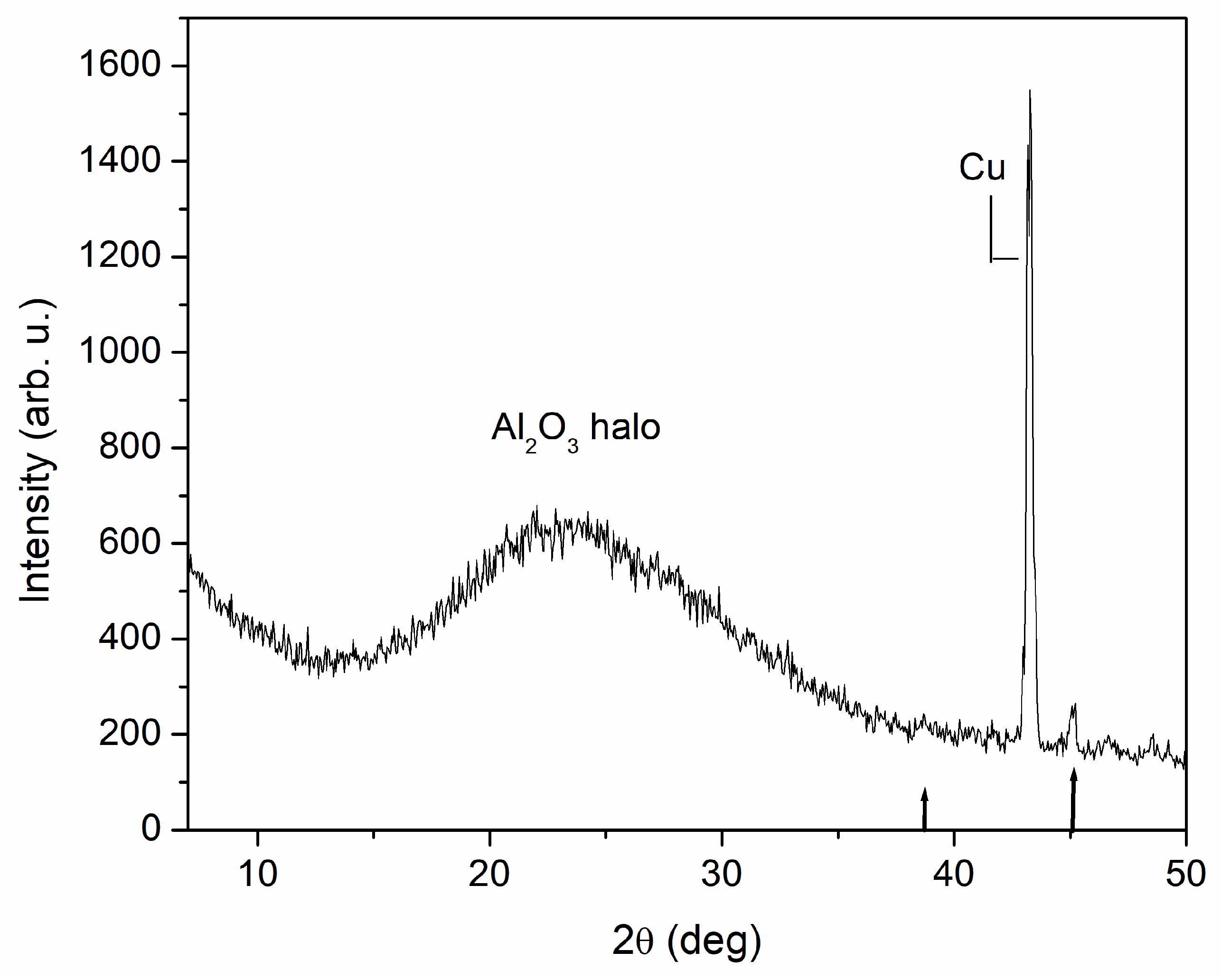
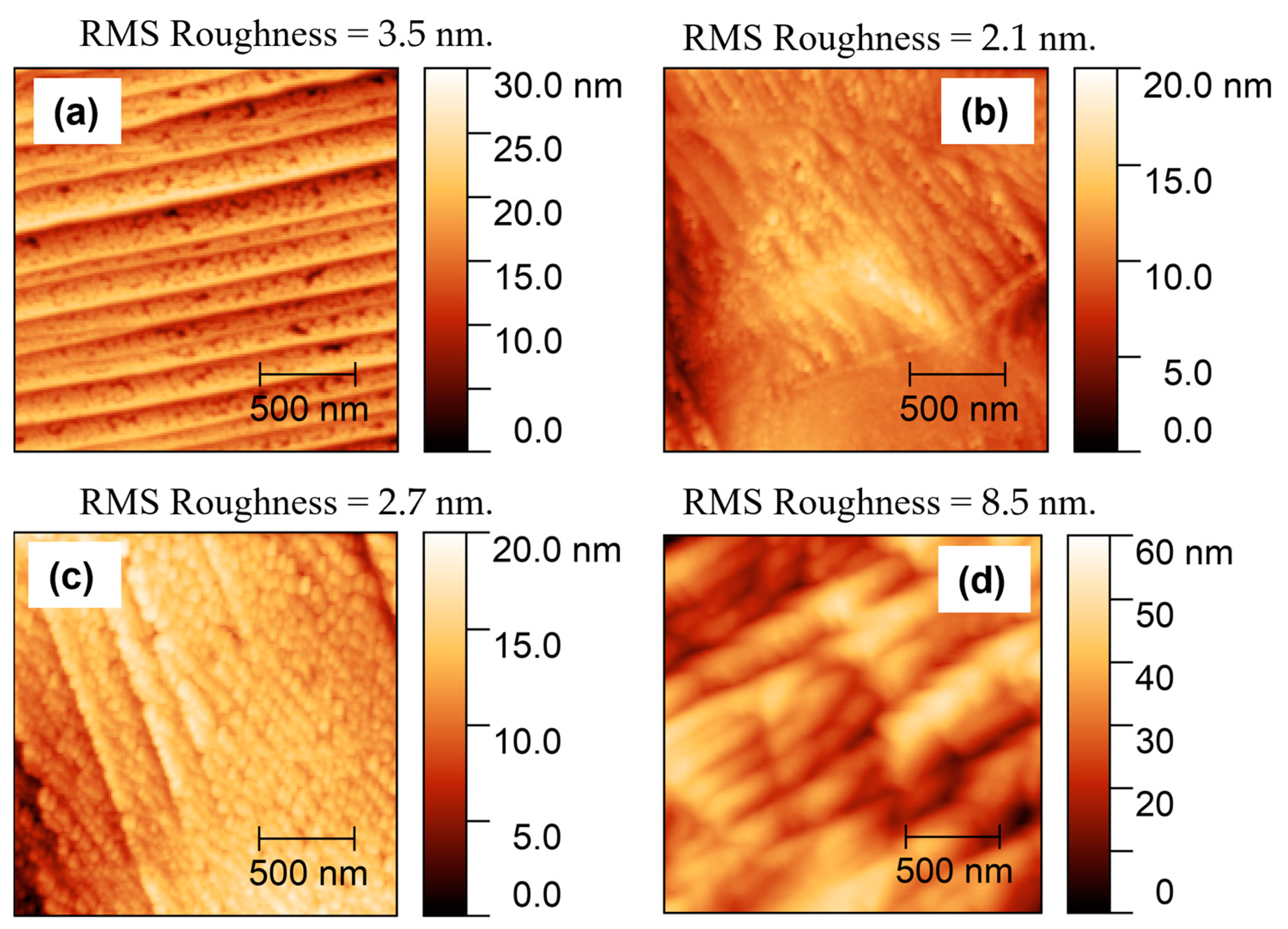
3.3. AFM and SEM Analysis
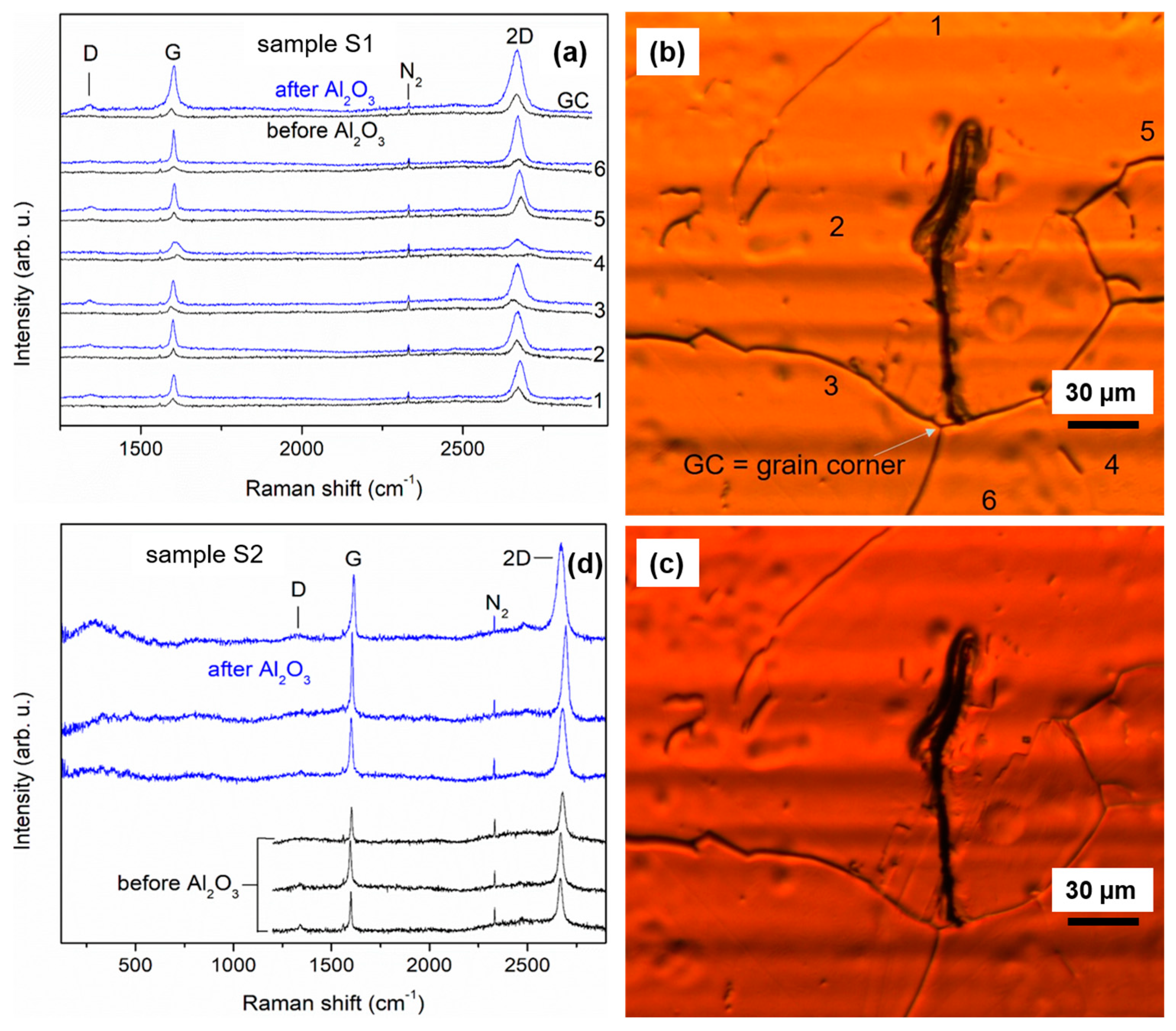
3.4. Raman Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Han, G.H.; Güneş, F.; Bae, J.J.; Kim, E.S.; Chae, S.J.; Shin, H.-J.; Choi, J.-Y.; Pribat, D.; Lee, Y.H. Influence of copper morphology in forming nucleation seeds for graphene growth. Nano Lett. 2011, 11, 4144–4148. [Google Scholar] [CrossRef]
- Wood, J.D.; Schmucker, S.W.; Lyons, A.S.; Pop, E.; Lyding, J.W. Effects of polycrystalline Cu substrate on graphene growth by chemical vapor deposition. Nano Lett. 2011, 11, 4547. [Google Scholar]
- Dlubak, B.; Kidambi, P.R.; Weatherup, R.S.; Hofmann, S.; Robertson, J. Substrate-assisted Nucleation of Ultra-thin Dielectric Layers on Graphene by Atomic Layer Deposition. Appl. Phys. Lett. 2012, 100, 173113. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar]
- Vervuurt, R.H.J.; Karasulu, B.; Verheijen, M.A.; Kessels, W.M.M.; Bol, A.A. Uniform Atomic Layer Deposition of Al2O3 on Graphene by Reversible Hydrogen Plasma Functionalization. Chem. Mater. 2017, 29, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; He, P.; Chen, S.; Chen, Z.; Zang, Y.; Li, Y.; Duan, Y. ALD-Assisted Graphene Functionalization for Advanced Applications. J. Electron. Mater. 2022, 51, 2766–2785. [Google Scholar] [CrossRef]
- Aria, A.I.; Nakanishi, K.; Xiao, L.; Braeuninger-Weimer, P.; Sagade, A.A.; Alexander-Webber, J.A.; Hofmann, S. Parameter Space of Atomic Layer Deposition of Ultrathin Oxides on Graphene. ACS Appl. Mater. Interfaces 2016, 8, 30564. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Musgrave, C.B.; George, S.M. Growth and Characterization of Al2O3 Atomic Layer Deposition Films on sp2-Graphitic Carbon Substrates Using NO2/Trimethylaluminum Pretreatment. ACS Appl. Mater. Interfaces 2015, 7, 12030–12037. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.; Mordi, G.; Lee, B.; Lee, G.; Floresca, C.; Cha, P.R.; Ahn, J.; Wal-lace, R.M.; Chabal, Y.J.; Kim, M.J.; et al. Atomic Layer Deposition of Dielectrics on Graphene Using Reversibly Physisorbed Ozone. ACS Nano 2012, 6, 2722. [Google Scholar] [CrossRef]
- Robinson, J.A.; LaBella, M.; Trumbull, K.A.; Weng, X.; Cavelero, R.; Daniels, T.; Hughes, Z.; Hollander, M.; Fanton, M.; Snyder, D. Epitaxial Graphene Materials Integration: Effects of Dielectric Overlayers on Structural and Electronic Properties. ACS Nano 2010, 4, 2667–2672. [Google Scholar] [CrossRef]
- Canto, B.; Otto, M.; Powell, M.J.; Babenko, V.; O’Mahony, A.; Knoops, H.C.M.; Sundaram, R.S.; Hofmann, S.; Lemme, M.C.; Neumaier, D. Plasma-Enhanced Atomic Layer Deposition of Al2O3 on Graphene Using Monolayer hBN as Interfacial Layer. Adv. Mater. Technol. 2021, 6, 2100489. [Google Scholar] [CrossRef]
- Nayfeh, O.M.; Marr, T.; Dubey, M. Impact of plasma-assisted atomic-layer-deposited gate dielectric on graphene transistors. IEEE Electron Device Lett. 2011, 32, 473. [Google Scholar] [CrossRef]
- Liu, L.; Ryu, S.; Tomasik, M.R.; Stolyarova, E.; Jung, N.; Hybertsen, M.S.; Steigerwald, M.L.; Brus, L.E.; Flynn, G.W. Graphene oxidation: Thickness-dependent etching and strong chemical doping. Nano Lett. 2008, 8, 1965. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Cao, Z.-Y.; Li, X.; Wu, D.; Li, A.-D. A facile way to deposit conformal Al2O3 thin film on pristine graphene by atomic layer deposition. Appl. Surf. Sci. 2014, 291, 78–82. [Google Scholar] [CrossRef]
- Zheng, L.; Cheng, X.; Cao, D.; Wang, G.; Wang, Z.; Xu, D.; Xia, C.; Shen, L.; Yu, Y.; Shen, D. Improvement of Al2O3 Films on Graphene Grown by Atomic Layer Deposition with Pre-H2O Treatment. Appl. Mater. Interfaces 2014, 6, 7014–7019. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Z.; Cheng, X.; Xie, H.; Wang, H.; Xie, X.; Yu, Y.; Liu, R. Direct Growth of High-Quality Al2O3 Dielectric on Graphene Layers by Low-Temperature H2O-Based ALD. J. Phys. D Appl. Phys. 2014, 47, 055106. [Google Scholar] [CrossRef]
- Tamm, A.; Kahro, T.; Piirsoo, H.-M.; Jõgiaas, T. Atomic-Layer-Deposition-Made Very Thin Layer of Al2O3 Improves the Young’s Modulus of Graphene. Appl. Sci. 2022, 12, 2491. [Google Scholar] [CrossRef]
- Song, Y.; Zou, W.; Lu, Q.; Lin, L.; Liu, Z. Graphene Transfer: Paving the Road for Applications of Chemical Vapor Deposition Graphene. Small 2021, 17, 2007600. [Google Scholar] [CrossRef]
- Cabrero-Vilatela, A.; Alexander-Webber, J.A.; Sagade, A.A.; Aria, A.I.; Braeuninger-Weimer, P.; Martin, M.; Weatherup, R.S.; Hofmann, S. Atomic layer deposited oxide films as protective interface layers for integrated graphene transfer. Nanotechnology 2017, 28, 485201. [Google Scholar] [CrossRef]
- Shivayogimath, A.; Eriksson, L.; Whelan, P.R.; Mackenzie, D.M.A.; Luo, B.; Bøggild, P.; Booth, T.J. Atomic Layer Deposition Alumina-Mediated Graphene Transfer for Reduced Process Contamination. Phys. Status Solidi RRL 2019, 13, 1900424. [Google Scholar] [CrossRef]
- Blagoev, B.S.; Delibatov, D.A.; Mehandzhiev, V.B.; Sveshtarov, P.; Terziyska, P.; Avramova, I.; Rafailov, P.M. Optimization of atomic layer deposition of Al2O3 films as possible template for graphene transfer. J. Phys. Conf. Ser. 2022, 2240, 012002. [Google Scholar] [CrossRef]
- Schilirò, E.; Lo Nigro, R.; Roccaforte, F.; Giannazzo, F. Substrate-Driven Atomic Layer Deposition of High-k Dielectrics on 2D Materials. Appl. Sci. 2021, 11, 11052. [Google Scholar] [CrossRef]
- Cazzanelli, E.; De Luca, O.; Vuono, D.; Policicchio, A.; Castriota, M.; Desiderio, G.; De Santo, M.P.; Aloise, A.; Fasanella, A.; Rugiero, T.; et al. Characterization of graphene grown on copper foil by chemical vapor deposition (CVD) at ambient pressure conditions. J. Raman Spectrosc. 2018, 49, 1006–1014. [Google Scholar] [CrossRef]
- Horiba Scientific. Available online: https://www.horiba.com/int/scientific/technologies/raman-imaging-and-spectroscopy (accessed on 10 May 2024).
- XPSPEAK41 Version 4.1 Is Free, Fully Featured, Software for the Analysis of XPS Spectra. Available online: https://xpspeak.software.informer.com (accessed on 14 November 2023).
- Renault, O.; Gosset, L.G.; Rouchon, D.; Ermolieff, A. Angle-resolved X-ray Photoelectron Spectroscopy of Ultrathin Al2O3 Films Grown by Atomic Layer Deposition. J. Vac. Sci. Technol. A 2002, 20, 1867–1876. [Google Scholar] [CrossRef]
- van den Brand, J.; Snijders, P.C.; Sloof, W.G.; Terryn, H.; de Wit, J.H.W. Acid-base Characterization of Aluminum Oxide Surfaces with XPS. J. Phys. Chem. B 2004, 108, 6017–6024. [Google Scholar] [CrossRef]
- Barbosa, C.; Blanc-Pelissiera, D.; Favea, A.; Blanquetb, E.; Criscib, A.; Fourmonda, E.; Albertinia, D.; Sabaca, A.; Ayadia, K.; Girarda, P.; et al. Characterization of Al2O3 thin films prepared by thermal ALD. Energy Procedia 2015, 77, 558–564. [Google Scholar] [CrossRef]
- Rafailov, P.M.; Sveshtarov, P.K.; Mehandzhiev, V.B.; Avramova, I.; Terziyska, P.; Petrov, M.; Katranchev, B.; Naradikian, H.; Boyadjiev, S.; Cserháti, C.; et al. Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces. Molecules 2022, 27, 1789. [Google Scholar] [CrossRef]
- Palik, E. Handbook of Optical Constants of Solids, 1st ed.; Evsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Wu, R.; Gan, L.; Ou, X.; Zhang, Q.; Luo, Z. Detaching graphene from copper substrate by oxidation-assisted water intercalation. Carbon 2016, 98, 138. [Google Scholar] [CrossRef]
- Choi, J.; Koo, S.; Song, M.; Jung, D.; Choi, S.; Ryu, S. Varying electronic coupling at graphene-copper interfaces probed with Raman spectroscopy. 2D Mater. 2020, 7, 025006. [Google Scholar] [CrossRef]
- Debbichi, L.; de Lucas, M.C.M.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Morales, C.; Urbanos, F.J.; del Campo, A.; Leinen, D.; Granados, D.; Rodríguez, M.A.; Soriano, L. Electronic Decoupling of Graphene from Copper Induced by Deposition of ZnO: A Complex Substrate/Graphene/Deposit/Environment Interaction. Adv. Mater. Interfaces 2020, 7, 1902062. [Google Scholar] [CrossRef]
- Poncharal, P.; Ayari, A.; Michel, T.; Sauvajol, J.-L. Raman spectra of misoriented bilayer graphene. Phys. Rev. B 2008, 78, 113407. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Grebel, H.; Stan, L.; Sumant, A.V.; Liu, Y.; Gosztola, D.; Ocola, L.; Fisher, B. Transfer of Graphene with Protective Oxide Layers. ChemEngineering 2018, 2, 58. [Google Scholar] [CrossRef]
- Neumann, C.; Reichardt, S.; Venezuela, P.; Drögeler, M.; Banszerus, L.; Schmitz, M.; Watanabe, K.; Taniguchi, T.; Mauri, F.; Beschoten, B.; et al. Raman spectroscopy as probe of nanometre-scale strain variations in graphene. Nat. Commun. 2015, 6, 8429. [Google Scholar] [CrossRef]
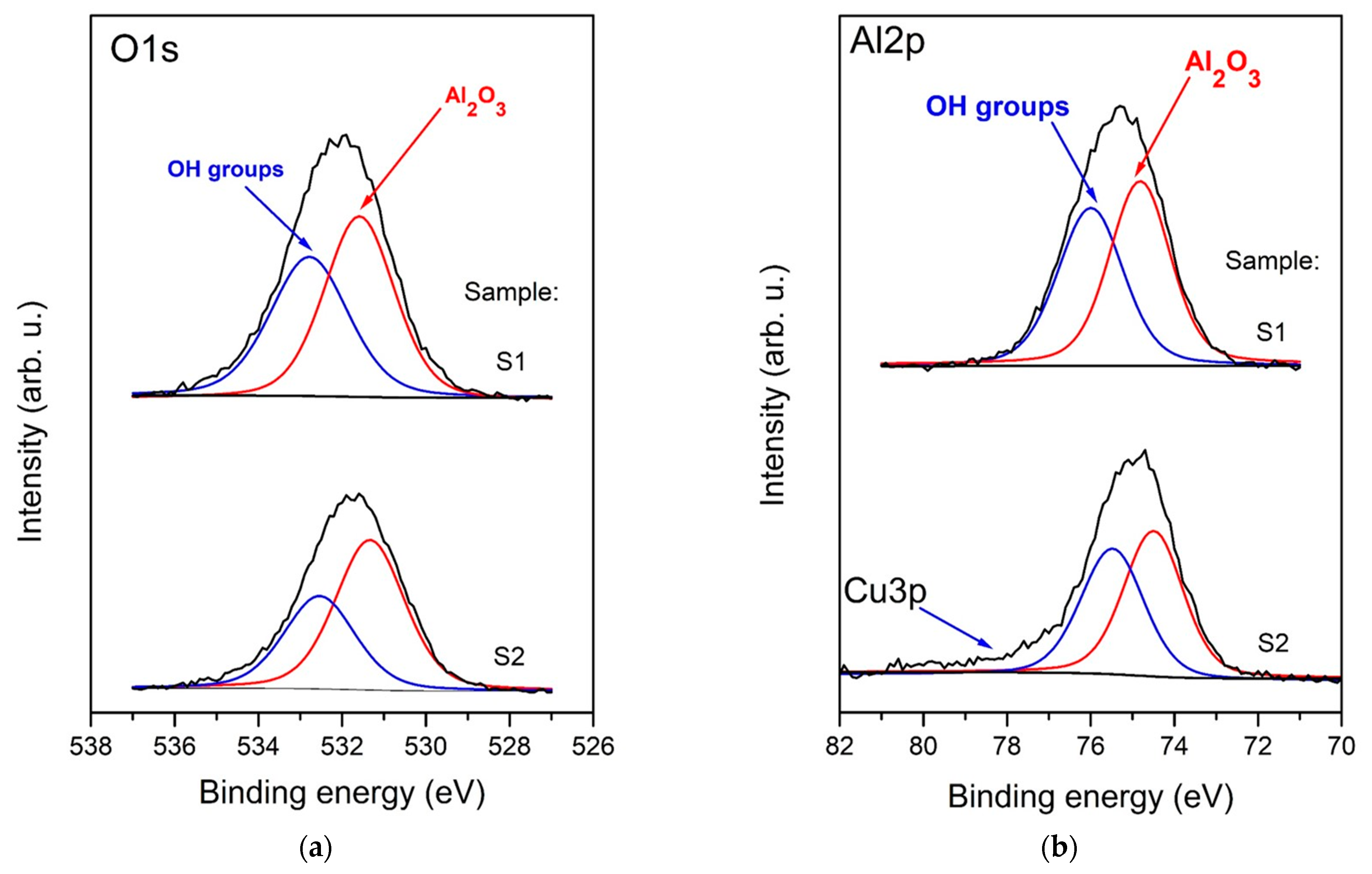


| Sample | C, at. % | O, at. % | Cu, at. % | Al, at. % | Al/O Ratio |
| S1 | 15.4 | 49.0 | - | 35.6 | 0.7 |
| S2 | 29.8 | 40.5 | 0.4 | 29.3 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafailov, P.; Mehandzhiev, V.; Sveshtarov, P.; Blagoev, B.; Terziyska, P.; Avramova, I.; Kirilov, K.; Ranguelov, B.; Avdeev, G.; Petrov, S.; et al. Atomic Layer Deposition Growth and Characterization of Al2O3 Layers on Cu-Supported CVD Graphene. Coatings 2024, 14, 662. https://doi.org/10.3390/coatings14060662
Rafailov P, Mehandzhiev V, Sveshtarov P, Blagoev B, Terziyska P, Avramova I, Kirilov K, Ranguelov B, Avdeev G, Petrov S, et al. Atomic Layer Deposition Growth and Characterization of Al2O3 Layers on Cu-Supported CVD Graphene. Coatings. 2024; 14(6):662. https://doi.org/10.3390/coatings14060662
Chicago/Turabian StyleRafailov, Peter, Vladimir Mehandzhiev, Peter Sveshtarov, Blagoy Blagoev, Penka Terziyska, Ivalina Avramova, Kiril Kirilov, Bogdan Ranguelov, Georgi Avdeev, Stefan Petrov, and et al. 2024. "Atomic Layer Deposition Growth and Characterization of Al2O3 Layers on Cu-Supported CVD Graphene" Coatings 14, no. 6: 662. https://doi.org/10.3390/coatings14060662







